Investigating the metabolic fingerprint of term infants with normal and increased fetal growth†
Abstract
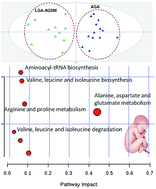
An NMR metabolomic approach was employed to highlight the metabolic changes underlying prenatal disorders and determine metabolites that could serve as potential markers in relation to large for gestational age (LGA) newborns. In this holistic study, multivariate data analysis elicited information from the NMR spectra and probed to metabolic signatures of macrosomic fetuses. Moreover, metabolic trends that characterize LGA from mothers diagnosed with gestational diabetes mellitus (LGA-GDM), as well as LGA from mothers not diagnosed with GDM (LGA-NGDM) were framed. Results obtained from maternal and umbilical cord (UC) samples indicated that LGA fetuses present alterations especially in the aminoacid metabolism as compared to Appropriate for Gestational Age (AGA) cases. Clear discrimination of LGA-NGDM from LGA-GDM was achieved both in maternal and in UC samples' blood. The role of glutamine and alanine together with four essential (valine, leucine, isoleucine, threonine) aminoacids, as well as the role of glycerol and glucose is emphasized for the case of maternal LGA samples' differentiation. Glycine and histidine only contributed to the differentiation of UC samples, the former characterized the AGA cases, while the latter was ascribed to both LGA-GDM and LGA-NGDM cases. Interestingly, both UC and maternal LGA-GDM samples were characterized by increased levels of N-acetylglutamic and acetoacetic acids. The OPLS-DA models were validated with permutation testing and ROC curves. In conclusion, this study indicates that NMR metabolomics may enable the detection of metabolic changes associated with LGA prenatal disorders.

 Please wait while we load your content...
Please wait while we load your content...