Enhancing the efficiency of desensitizing agents with shockwave treatment – a new paradigm in dentinal hypersensitivity management
Akshay Datey†
abc,
C. S. Adeeb Thaha†d,
Sudhir R. Patild,
Jagadeesh Gopalan*bc and
Dipshikha Chakravortty*ac
aDepartment of Microbiology & Cell Biology, Indian Institute of Science, Bangalore, India. E-mail: dipa@mcbl.iisc.ernet.in
bDepartment of Aerospace Engineering, Indian Institute of Science, Bangalore, India
cCentre for Biosystems Science & Engineering, Indian Institute of Science, Bangalore, India
dDepartment of Periodontics, K.L.E. Society's Institute of Dental Sciences, Bangalore, India
First published on 15th July 2016
Abstract
Dentine sensitivity, characterised by a sharp dental pain is experienced by the population globally. Desensitizing toothpastes are prescribed to treat dentine hypersensitivity. These agents occlude the exposed dentine tubules thereby reducing fluid movement although the effect is not long lived. We have developed a novel system which uses micro-shockwaves in combination with commercially available desensitizing toothpastes to efficiently treat hypersensitivity. This method of treating hypersensitivity strongly blocks dentinal tubules making it resistant to erosion even by acid challenge. We, thus, report a novel method to manage hypersensitivity using the most minimally invasive technique which is potentially translatable to clinics.
1. Introduction
Dentine hypersensitivity (DH) is a common, critical and persistent problem in dentistry. It is caused by the exposure of dentinal tubules to the external environment which allows the movement of dentinal fluid within it causing sharp pain referred to as dentine hypersensitivity.1 Brannstrom's concept of hydrodynamic theory states that when a stimulus of different origin is experienced by the dentin surface, it causes movement of the fluid within the tubules.2 This movement transmits a signal to the underlying network of nerves within the dental pulp eliciting sensitivity to the stimuli. DH is prevalent in conditions like gingival recession, abrasion caused by improper or overzealous tooth brushing, para-functional habits, erosion due to dietary factors, improper positioning of the teeth, chronic periodontal diseases, post-periodontal surgeries, occlusal problems or a combination of these factors.3 Different options for treating hypersensitivity include dietary counselling, correct tooth brushing instructions, occlusal adjustment, application of desensitizing agents, application of adhesive systems and/or restorations, iontophoresis and more recently use of laser irradiation. The most commonly prescribed treatment for hypersensitivity is the use of toothpastes containing desensitizing agents. The chemicals used in these toothpastes range from strontium chloride, calcium hydroxide, fluorides, sodium citrate to potassium oxalate either in combination or alone. The present generation biomimetic agents include novamine (a synthetic a synthetic mineral composed of calcium, sodium, phosphorous and silica), nano-HAP (hydroxyapatite nano particle) etc. These chemicals are known to occlude the exposed dentine tubules, thereby reducing exposure to stimuli and reduction in pain. Although the desensitizing agents may provide instant relief to hypersensitivity, the effect is not long lasting. It is because of the removal or washing away of the occluding agent(s) due to acidic food items and the normal fluid flow in the oral cavity.4 Current literature available shows that no form of treatment or desensitizing agent is effective in providing a permanent or long-lasting solution to dentine hypersensitivity. Studies have shown that sensitivity can be reduced by occluding patent tubules.5 These tubule occlusion characteristics have been studied in vitro either in simulated or un-simulated oral conditions.Shockwaves are high energy waves travelling at velocities higher than the local velocity of sound.6 They are classified as supersonic and hypersonic shockwaves based on their respective Mach number (M). Mach number of a shockwave is calculated as the ratio of its velocity and the velocity of sound in that medium. Shockwaves which have a Mach number of 1.2–5 are classified as supersonic shockwaves whereas the ones with Mach number >5 are called as hypersonic shockwaves. The medium through which shockwaves travel experience an abrupt change in pressure, temperature and density.7 They are known to be the most efficient energy carriers.8 This unique quality of shockwaves has enabled researchers across various disciplines to study and develop applications using shockwaves. Shockwave research has witnessed beginning of a new era when extracorporeal shockwave lithotripsy was developed. Till date extracorporeal shockwave lithotripsy (ESWL) remains the most celebrated application of shockwaves in the field of bioengineering.9 They have also been used to accelerate wound healing, treating tendinitis and avascular necrosis.10 In the past we have been successful in developing micro shockwaves assisted biological applications which include biofilm disruption and clearance,6 needle-less vaccination,11 bacterial transformation12 and drug delivery.13 Till date the use of shockwaves in dentistry is limited to dental biofilm and calculus removal.14 In this article, we report a novel system that uses shockwaves in combination with commercially available desensitizing toothpastes. The efficiency of dentinal tubule occlusion as well as its sustenance were found to be significant.
2. Experimental
2.1 Dental sample collection
Teeth were extracted under local anesthesia by a registered oral surgeon at KLE Society's Institute of Dental Sciences, Bangalore, India (Recognized by Dental Council of India). All the procedures were done according to the hospital's ethical guidelines. Extractions were done from patients suffering from either gingival disease, supernumery teeth, impacted teeth or from patients undergoing any form of orthodontic treatment. The extracted teeth were intact without any decay or abnormality.2.2 Preparation of dentin specimens
Freshly extracted human premolars and molars were cleaned with hydrogen peroxide (6% w/v) to remove debris and blood. The specimens were stored in 10% formalin till further use. A double sided diamond disk operated on micro-motor with water-cooled mechanism (Aseptico-M4B-27755, USA) with a straight handpiece (Uniq – Kavo, Germany) was used to prepare approximately 1.5 mm thick dentin slices from teeth crown below the dentino-enamel junction. The dentin discs were then polished on one side using 600-grit silicon carbide paper for 30 seconds to create uniform surface. The polished specimens were transferred to a polycarbonate tube containing deionized water and were sonicated at 30 kHz for 60 seconds to remove the polishing abrasive. After sonication, the dentin specimens were thoroughly rinsed with deionized water. The dentin specimens were placed in a fresh polycarbonate tube containing 37% ortho-phosphoric acid solution and were sonicated for 30 seconds to ensure opening of the dentinal tubule. After etching, the specimens were rinsed with Milli-Q water and stored in phosphate buffered saline (PBS, pH = 7.4).2.3 Hand held shockwave generator
The hand held shockwave generator consists of a polymer tube coated with HMX – octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine. Upon ignition, combustion of the chemical contained in the polymer tube causes the generation of shockwaves at the other end. The other end of the polymer tube is placed inside a polycarbonate tube (sample holding tube) which contains the dentine disc in saline. The shockwave hits the liquid surface and is ultimately encountered by the dentine discs.2.4 Experimental groups
The dentin specimens were equally distributed into 4 groups.Group 1: etched specimens without any treatment.
Group 2: etched specimens brushed with desensitizing agent.
Group 3: etched specimens exposed to micro-shockwaves.
Group 4: etched specimens brushed with desensitizing agent followed by application of micro-shockwaves.
2.5 Desensitizing agent and shockwave treatment
Double-sided carbon tape was used to secure the dentine discs onto a glass slide with the polished side facing up. Group 2 and 4 specimens were wetted with PBS and brushed with desensitizing agent majorly containing calcium carbonate, sorbitol, arginine bicarbonate, sodium lauryl sulphate and benzyl alcohol, using a powered toothbrush (Colgate 360° whole mouth clean) operated at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914 oscillations per minute for 120 seconds. Group 4 samples were then exposed to shockwaves using the hand held shockwave generator. A single shot of shockwave was operated for each dentine disc. The samples were left undisturbed for 5 min at room temperature. Next, the specimens were gently rinsed with Milli-Q water to ensure removal of excess tooth paste from the surface. The samples were stored in vacuum until use.
914 oscillations per minute for 120 seconds. Group 4 samples were then exposed to shockwaves using the hand held shockwave generator. A single shot of shockwave was operated for each dentine disc. The samples were left undisturbed for 5 min at room temperature. Next, the specimens were gently rinsed with Milli-Q water to ensure removal of excess tooth paste from the surface. The samples were stored in vacuum until use.
2.6 Sample preparation for scanning electron microscopy
Following treatment, the discs were mounted on stubs and were gold sputter coated (JEOL JFC 1100E Ion sputtering device). SEM analysis by field emission was done using FEI-SIRION (Eindhoven, Netherlands) at an operating voltage of 15 kV. The scanning electron micrographs were recorded at 2000×, 4000× and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnifications.
000× magnifications.
2.7 Acid challenge
The samples from each group were immersed in 4 mL of 6% citric acid (pH 2) or carbonated beverage (pH 4.4) in a 35 mm Petri dish. Samples immersed in PBS served as control. The samples were left undisturbed for 60 seconds, followed by rinsing with PBS and preparation for SEM analysis.2.8 Tubule occlusion scoring system
The scanning electron micrographs were assessed (on a categorical scale of 1–5) to score the level of tubule occlusion. They were rated as follows (1) completely occluded (100% of tubules occluded); (2) mostly occluded (50–<100% of tubules occluded); (3) partially occluded (25–<50% of tubules occluded); (4) mostly un-occluded (<25% of tubules occluded) and (5) completely un-occluded (0%, no tubule occlusion). The analysis was performed by 3 independent blinded reviewers to minimize subjectivity. The mean score was considered for statistical analysis.2.9 Statistical analysis
Data sets were analyzed using Graph Pad Prism 5. Following statistical tests were used and are mentioned in the appropriate results unpaired t test, one way and two way ANOVA and Mann Whitney U post-test. Significance values were calculated as ***p < 0.0001, **p < 0.001 and *p < 0.05.3. Results
3.1 Generation of shockwaves using hand held device
For this particular study, shock waves were generated using a hand-held shock wave generator (Fig. 1a).11,12,15 When the polymer tube is ignited at one end, a combustion front travels through the length of the tube and shock waves are generated at the other end (Fig. 1b). A typical pressure signal measured head on at a distance of 20 mm from the end of the tube is shown in (Fig. 1c). The energy in the shock wave emanating from the open end of the polymer tube is estimated to be around 1.25 J.16 This mode of shock wave generation is ideal for in vitro study as it is a simple way to handle this portable device and focus the shock waves generated in a particular region.3.2 Dentin disc preparation
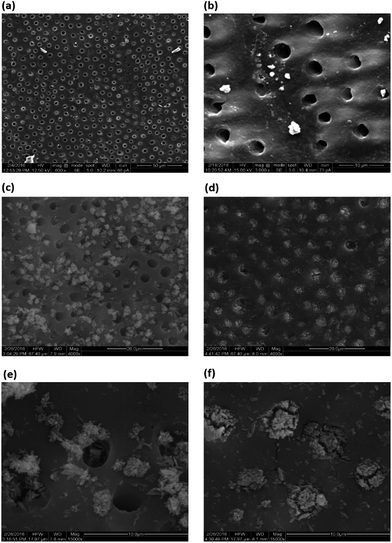
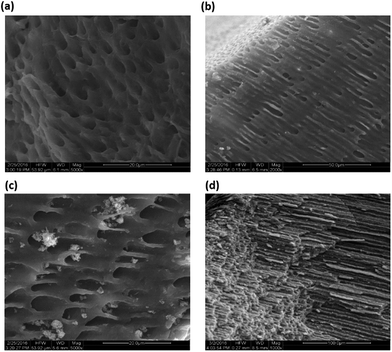
Dentin discs prepared and etched with 37% ortho-phosphoric acid solution resembled the natural dentine structure under SEM. Dense and homogeneous dentine in the Peri-tubular region was observed which suggested that the natural dental architecture was intact (Fig. 2a). The discs were suitable for studying dentinal hypersensitivity as the size of the opening of the tubules was similar to the tubules found in physiologically relevant dentinal hypersensitivity cases. Exposure to shockwaves had no deleterious effect on the structure of dentine (Fig. 2b). The size and the integrity of the dentinal tubule remained unaffected as can be seen in SEM micrographs. Desensitizing agent treatment & effect of shockwaves on occlusion. Brushing with desensitizing agent alone on the etched dentin caused plug formation over the tubules (Fig. 2c and d). Scoring by 3 independent blinded reviews revealed that maximum number of dentine tubules were partially or un-occluded. Precipitates of the desensitizing agent were seen in the Peri-tubular spaces and the presence of completely occluded tubules were significantly rare.A few patent tubules were also seen. SEM micrographs recorded at higher magnifications (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×) showed crystal like deposits in the periphery of the tubule openings. Deep seated depositions of the desensitizing agents were rarely observed in this field (Fig. 2e). Group 4 which consisted of specimens treated with DS followed by shockwave exposure, showed maximum number of completely occluded tubules. The desensitizing agents were found to be completely occluding the tubules. Traces of the precipitates were seen in the per-tubular spaces indicating a highly focused occlusion of tubules. At 15
000×) showed crystal like deposits in the periphery of the tubule openings. Deep seated depositions of the desensitizing agents were rarely observed in this field (Fig. 2e). Group 4 which consisted of specimens treated with DS followed by shockwave exposure, showed maximum number of completely occluded tubules. The desensitizing agents were found to be completely occluding the tubules. Traces of the precipitates were seen in the per-tubular spaces indicating a highly focused occlusion of tubules. At 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification compact and densely occluded tubules were observed (Fig. 2f). Statistical analysis by Mann–Whitney test showed a significant difference in the occlusion between DS and DS-SW samples (p < 0.0001) as indicated in Table 1.
000× magnification compact and densely occluded tubules were observed (Fig. 2f). Statistical analysis by Mann–Whitney test showed a significant difference in the occlusion between DS and DS-SW samples (p < 0.0001) as indicated in Table 1.
| Sample | Sub-group | Occlusion | Occlusion after challenge | ||
|---|---|---|---|---|---|
| PBS | Carbonated beverage | Citric acid | |||
| DS | 100% | 5.60 ± 2.41 | 2.33 ± 2.51 | 0.66 ± 0.57 | 0 |
| 50–99% | 10.47 ± 3.87 | 11.33 ± 10.69 | 3.33 ± 1.52 | 0 | |
| 25–49% | 14.73 ± 7.26 | 13.00 ± 11.27 | 5.00 ± 2.00 | 0 | |
| <25% | 23.60 ± 6.56 | 28.00 ± 3.60 | 13.00 ± 3.60 | 1.66 ± 1.52 | |
| 0% | 6.53 ± 5.02 | 42.33 ± 3.21 | 100.0 ± 14.00 | 112 ± 24.33 | |
| DSSW | 100% | 71.00 ± 14.03 | 9.00 ± 5.292 | 22.33 ± 7.09 | 13.67 ± 4.16 |
| 50–99% | 7.26 ± 7.78 | 2.33 ± 0.577 | 15.67 ± 2.88 | 10.67 ± 0.577 | |
| 25–49% | 4.00 ± 2.61 | 1.33 ± 0.577 | 10.33 ± 2.51 | 9.66 ± 3.78 | |
| <25% | 4.26 ± 3.65 | 7.66 ± 2.08 | 14.67 ± 3.51 | 18.67 ± 4.16 | |
| 0% | 0.53 ± 1.06 | 10.33 ± 3.05 | 13.33 ± 2.88 | 10.33 ± 6.65 | |
The longitudinal sections of the group 4, DS followed by SW application, demonstrated complete occlusion and significantly deep seated plugging of the tubules by the desensitizing agents (Fig. 3d).
3.3 Challenge with acid and carbonated beverage to test effect of SW on efficacy of DS
Following acid (carbonated beverage and citric acid) challenge, the dentine surface was similar to that of the acid-etched specimens of control group (Fig. 4). Specimen were subjected to washings using phosphate buffered saline (pH 7.4). Partial removal of the desensitizing agent was observed in both DS and DS-SW specimen (Fig. 4a and b). Exposure to citric acid among group 3 (desenitizing agent alone) caused complete removal the DS with 100% open tubules, however some amount occlusion was still seen on exposure to carbonated beverage (Fig. 4c and e). In contrast a similar occlusion pattern could be seen among group 4 specimens (desensitizing agent followed by shockwave treatment) on exposure to both carbonated beverage and citric acid which was statistically significant (p < 0.001) (Fig. 4d and f, Table 2). However, the superficial plug was washed off and the penetrated DS within the tubules could be appreciated from the SEM micrographs of this group.| DS vs. DSSW | PBS | Carbonated beverage | Citric acid | |
|---|---|---|---|---|
| 100% | 0.0001 | 0.2 | 0.0765 | |
| 50–99% | 0.0317 | 0.35 | 0.1 | |
| 49–25% | 0.0001 | 0.1 | 0.1 | |
| <25% | 0.0001 | 0.1 | 0.7 | 0.1 |
| 0% | 0.0001 | 0.1 | 0.1 | 0.1 |
4. Discussion
Dentinal hypersensitivity is characterized by the presence of exposed dentinal tubules caused by abrasion and consumption of acidic foods and beverages. The exposure of these tubules to certain stimuli which include hot, cold or sour items causes movement of the fluid in the dentinal tubules causing sharp pain. This condition causes extreme discomfort and if left untreated, leads to worsening of the condition. Dentists all over the globe prescribe tooth pastes containing desensitizing agents like salts of calcium, potassium, strontium etc. These salts occlude the exposed dentinal tubules causing a temporary isolation of the tubules from the environmental stimuli. The occlusion provides a temporary relief to the patient but with the passage of time, regular brushing and the normal fluid flow in the oral cavity, the occluding material is washed away. To overcome this, the patient is advised to repeat the treatment on a regular basis. In this report we have demonstrated the successful use of micro-shockwaves in combination with commercially available desensitizing tooth pastes to treat dentine hypersensitivity in a significantly improved way. Dentine discs from extracted molars and premolars were prepared using a rotary diamond disc. Acid etching was done to clear away the smear layer and other debris. These discs were analyzed using SEM and was found that its natural architecture was retained after etching. These discs were used for further experiments. The dentine discs were randomly divided into 4 groups. Group 1 was the control where the discs were not exposed to any treatment. Group 2 was exposed to shockwaves alone. This was done to evaluate whether shockwaves were safe for the specimen or not. SEM analysis revealed that shockwaves had not affected the natural dental architecture and hence we could conclude that the shockwaves generated by the device were safe when tested in vitro. Group 3 was treated with the desensitizing agent alone whereas group 4 was treated with desensitizing agent followed by a single exposure to shockwave generated by the hand held shockwave generator. SEM analysis revealed a significantly high level of dentine tubule occlusion in specimens from group 4. Longitudinal sections also showed a higher depth of penetration of the occluding material in the tubules. These results suggest that the combinatorial use of shockwaves with desensitizing tooth pastes is potent enough to occlude dentinal tubules with greater depth of penetration. A complete occlusion scenario is a full proof evidence of the increased efficiency of tubular occlusion. The occlusion plugs formed are sensitive to challenges like chewing and brushing force, acids present in foods as well as carbonated beverages. To test the tolerance of the DS-SW treated dentinal discs, we subjected the treated specimens to a strong acid test using citric acid at pH 2 as well as a mild acid test using carbonated beverage (cola) which is the most commonly encountered challenge in day to day life. The DS-SW specimen could withstand the strong citric acid as well as the cola test very well. SEM micrographs were recorded to score the occlusion left after the respective challenge. It was found that a significant level of tubule occlusion was retained in both citric acid as well as cola challenge. The occluding plug formed was found not only densely packed but also much more deeply penetrated and no erosion was observed on the dentine disc surface. Statistically, all the occlusion data was found to be significant with p < 0.0001. Thus, we propose that shockwaves can be used for treatment of dentinal hypersensitivity along with desensitizing agents in a significantly superior approach and can provide long lasting relief to the patient without any deleterious effect. This study opens up a new area of research which involves shockwaves in the treatment of conditions associated with exposed dentinal tubules. Moreover, the use of such controlled shockwaves generated at a small scale can be easily incorporated in clinics for therapeutic purposes.5. Conclusions
In this study, micro-shockwaves have been shown to be effective in enhancing the efficiency of tubule occlusion by desensitizing agents. The number of dentine tubules occluded by shockwaves applied after the desensitizing agent was significantly higher than the occluded tubules after application of desensitizing agents alone. The plugs formed in the shockwave treated specimen were resistant to acid and carbonated drink challenges. Thus, micro-shockwaves can be used for effectively treating dentinal hypersensitivity along with available desensitizing agents.Acknowledgements
Authors acknowledge Electron Microscopy facility, Biological Sciences, IISc. DC acknowledges Department of Atomic Energy (DAE) and Department of Biotechnology-IISc Partnership Programme for funds. AD acknowledges Department of Biotechnology for senior research fellowship.Notes and references
- R. Orchardson and D. G. Gillam, Managing dentin hypersensitivity, J. Am. Dent. Assoc., JADA, 2006, 137, 990–998 CrossRef PubMed.
- M. Brannstrom and A. Astrom, The hydrodynamics of the dentine; its possible relationship to dentinal pain, Int. Dent. J., 1972, 22, 219–227 CAS.
- N. X. West, Dentine hypersensitivity: preventive and therapeutic approaches to treatment, J. Periodontol., 2008, 48, 31–41 CrossRef PubMed.
- D. G. Kerns, M. J. Scheidt, D. H. Pashley, J. A. Horner, S. L. Strong and T. E. Van Dyke, Dentinal tubule occlusion and root hypersensitivity, J. Periodontol., 1991, 62, 421–428 CrossRef CAS PubMed.
- T. Y. Ling, D. G. Gillam, P. M. Barber, N. J. Mordan and J. Critchell, An investigation of potential desensitizing agents in the dentine disc model: a scanning electron microscopy study, J. Oral Rehabil., 1997, 24, 191–203 CrossRef CAS PubMed.
- D. P. Gnanadhas, M. Elango, S. Janardhanraj, C. S. Srinandan, A. Datey, R. A. Strugnell, J. Gopalan and D. Chakravortty, Successful treatment of biofilm infections using shock waves combined with antibiotic therapy, Sci. Rep., 2015, 5, 17440 CrossRef CAS PubMed.
- G. Jagadeesh, Industrial applications of shock waves, Proc. Inst. Mech. Eng., Part G, 2008, 222, 575–583 CrossRef.
- G. Jagadeesh, Application of shock waves in pencil manufacturing industry, Shock Waves, 2009, 2, 847–850 Search PubMed.
- M. Kuwahara and S. Orikasa, Microexplosive ESWL, Nippon Geka Gakkai Zasshi, 1989, 47, 2798–2802 CAS.
- C. J. Wang, J. H. Cheng, C. C. Huang, H. K. Yip and S. Russo, Extracorporeal shockwave therapy for avascular necrosis of femoral head, Int. J. Surg., 2015, 24, 184–187 CrossRef PubMed.
- G. Jagadeesh, G. D. Prakash, S. G. Rakesh, U. S. Allam, M. G. Krishna, S. M. Eswarappa and D. Chakravortty, Needleless vaccine delivery using micro-shock waves, Clin. Vaccine Immunol., 2011, 18, 539–545 CrossRef CAS PubMed.
- G. Divya Prakash, R. V. Anish, G. Jagadeesh and D. Chakravortty, Bacterial transformation using micro-shock waves, Anal. Biochem., 2011, 419, 292–301 CrossRef CAS PubMed.
- D. P. Gnanadhas, M. Elango, M. Ben Thomas, J. Gopalan and D. Chakravortty, Remotely triggered micro-shock wave responsive drug delivery system for resolving diabetic wound infection and controlling blood sugar levels, RSC Adv., 2015, 5, 13234–13238 RSC.
- P. Muller, B. Guggenheim, T. Attin, E. Marlinghaus and P. R. Schmidlin, Potential of shock waves to remove calculus and biofilm, Clin. Oral. Investig., 2011, 15, 959–965 CrossRef PubMed.
- S. G. Rakesh, D. P. Gnanadhas, U. S. Allam, K. N. Nataraja, P. K. Barhai, G. Jagadeesh and D. Chakravortty, Development of micro-shock wave assisted dry particle and fluid jet delivery system, Appl. Microbiol. Biotechnol., 2012, 96, 647–662 CrossRef CAS PubMed.
- I. O. Samuelraj, G. Jagadeesh and K. Kontis, Micro-blast waves using detonation transmission tubing, Shock Waves, 2013, 23, 307–316 CrossRef.
Footnote |
| † Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |