Etherification to improve the performance of lignosulfonate as dye dispersant
Lixuan
Yu
a,
Jue
Yu
a,
Wenjie
Mo
a,
Yanlin
Qin
a,
Dongjie
Yang
*ab and
Xueqing
Qiu
*ab
aSchool of Chemistry and Chemical Engineering, South China University and Technology, Guangzhou, 516640, China. E-mail: cedjyang@scut.edu.cn; xueqingqiu66@163.com
bKey Lab of Pulp and Paper Engineering, South China University of Technology, Guangzhou, 510640, China
First published on 11th July 2016
Abstract
Lignosulfonates (SLs) are widely used as dye dispersants. However, they have some disadvantages, including poor high temperature dispersibility and severe fiber staining resulting from the abundant phenolic hydroxyl content in the SL molecules. In this work, etherified lignosulfonates (ESLs) were obtained by using epichlorohydrin to reduce the content of phenolic hydroxyl while increasing the molecular weight. ESLs with lighter color can reduce fiber staining rate by 52% due to whitening by the epichlorohydrin process. The lower adsorption capacity of ESLs onto the surface of fibers can reduce the fiber staining effect, owing to the lower phenolic hydroxyl content. The ESLs also exhibit superior high temperature stability to SL because of their higher adsorption capacity and more rigid adsorption films.
Introduction
Lignosulfonate (SL) is a by-product in the pulp and paper industry; it is usually recovered from sulfite or bisulfite pulping waste. It can also be obtained by the introduction of sulfonic acid groups into alkali lignin from kraft or sulfate pulping processes. Lignin is commonly considered to be a three dimensional amorphous polymer composed of three phenylpropane units. These are guaiacyl (G), syringyl (S) and hydroxy-phenyl (H). In addition to hydrophobic groups, SLs contain many hydrophilic groups, including sulfonic, phenolic hydroxyl, and alcoholic hydroxyl groups,1 which is advantageous for polymer surfactants.2 Because of its favorable wettability, adsorptivity, dispersibility and other colloidal properties, SL has applications in various industries, such as cement water reducing agents,3 pesticide dispersants,4 CWS dispersants,5 fuel cell membranes6 and dye dispersants.7Currently, anionic surfactants play a major role in dye dispersants, especially lignosulfonates (SL) and naphthalene sulfonates (NS) from the petroleum industry.8 NS, derived from fossil resources, has less fiber staining and good dispersibility in dyes; however, it is expensive and toxic. However, SLs, which are the main component in waste liquid from paper pulping, are abundant, renewable, economical and environmentally friendly. Therefore, the dosage of SL used in dye dispersants is increasing every year. Meanwhile, there are a number of disadvantages in employing SLs as dye dispersants, such as fiber staining and poor dispersive ability;9,10 these limit the application of SLs in the dye industry, especially when they are employed in the dyeing process, where superior performance is required. Numerous studies have revealed that the fiber staining rate of SL is greatly dependent on its phenolic hydroxyl groups,11,12 which can form hydrogen bonds with electronegative groups in fibers.13 Therefore, an adequate modification method is necessary to reduce the phenolic hydroxyl content of SL.
At present, the methods to reduce phenolic hydroxyl content include oxidation, chelation with divalent metal salts, and other chemical reactions. Falkehag14 treated SL with 2-chloroethanol, and the product showed decreased fiber staining. A two-step process was also introduced to block the phenolic hydroxyl groups in lignin followed by oxidation with chlorine dioxide, and a light-colour lignin azo dye dispersant was obtained.15 By reacting divalent metal salts with lignin through chelation and an ester formation mechanism, the amount of dihydroxyl groups in lignin was reduced; therefore, the fiber staining was weakened.16
Etherification by epichlorohydrin is also a useful method to block the phenolic hydroxyl group in lignin molecules. Recently, by epoxidation with epichlorohydrin followed by etherification with polyethylene glycol, alkaline lignin was converted into water-soluble lignin derivates which can enhance the efficiency of enzymatic hydrolysis and ethanol fermentation. However, the molecular weight of the product was only slightly changed.17 Therefore, in our present study, epichlorohydrin was used as a crosslinking agent via a ring-opening reaction in aqueous solution to decrease the content of phenolic hydroxyl groups as well as to increase the molecular weight of the SL molecules.
It has been reported that decreasing the phenolic content may decrease the water solubility of lignin18 and thus decrease its dispersive ability and high temperature stability.19 Compared with NS, SLs have better high temperature stability, probably due to the phenolic hydroxyl group content in the molecules. Therefore, there is a contradiction between the influence of the phenolic hydroxyl group on dispersive ability and on fiber staining. If the phenolic hydroxyl content is decreased in order to reduce fiber staining, the dispersive properties may deteriorate. Therefore, it is highly necessary to develop a method to not only reduce the amount of phenolic hydroxyl groups in lignin but also to prevent the decrease of its heat stability. It has been reported that the high molecular weight of SL makes a great contribution to the dispersive ability and stability of particles;20,21 therefore, increasing the molecular weight and reducing the phenolic hydroxyl content simultaneously become the key factors for improving the dispersive ability while weakening the fiber staining. Fiber staining and the dispersive ability of the dye suspension are also related to the adsorption characteristics of SL on fibers and dyes. Quartz crystal microbalance with dissipation (QCM-D) is a new technique to detect the adsorption characteristics of dispersants at a solid/liquid interface by measuring the change of the third overtone of the frequency shift (Δf) and the dissipation shift (ΔD). The larger the value of |Δf|, the higher the adsorption amount of the dispersant on a quartz chip. ΔD is related to the viscoelastic property of the film on the quartz chip. The smaller the value of ΔD, the stronger the density and rigidity of the film.22 The absolute value of the slope of |ΔD/Δf| reflects the firmness of the adsorption structure. The adsorption structure becomes solid with increasing |ΔD/Δf| value. However, the application of QCM-D in revealing the mechanism of absorption of SL in the dye process has not yet been widely reported. Therefore, in previous work, we developed a method using QCM-D and atomic force microscopy (AFM) to study the absorption characteristics of SL on fiber and dye surfaces.23
In this paper, SL was modified with epichlorohydrin by etherification to reduce the content of phenolic hydroxyl groups while increasing the molecular weight. ESLs with different phenolic hydroxyl contents were obtained by adjusting the pH and adding different amounts of epichlorohydrin. The influences of the phenolic hydroxyl content and the molecular weight on the properties of dye liquor were investigated by determining the fiber staining rate and the particle size of the dye. The absorption characteristics of ESLs on fiber and dye particle surfaces were further examined by QCM and AFM.
Experimental
Materials
Dispersant lignosulfonate (SL), a by-product of aspen wood sulfite pulping, was supplied by Shixian Paper Making Co. Ltd., Jilin, China; it is composed of 70 wt% SL, 11 wt% reductive substances, and other materials, including sugar acids, organic compounds and inorganic salts.Sodium naphthalene sulfonic acid formaldehyde condensation (SNF) (Shangyu Wencai Co., Zhengjiang, China) is a commercial dispersant used for dye dispersion; its purity was greater than 90%.
Ultrazine Na (UNA) (Borregaard Co., Sarpsborg, Norway) is a widely used dispersant lignosulfonate; it was purified by ultrafiltration, and its purity was 95%, with a small amount of inorganic salts.
C.I. disperse blue 79, an azo dye, was supplied by Runtu Co. Ltd., Zhejiang, China. Folin–Ciocalteu phenol reagent (2 mol L−1), vanillin and poly(diallyldimethylammonium chloride) (PDAC, Mw 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 350
000 to 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 20% solution) were supplied by Sigma-Aldrich (Xuhui District, Shanghai, China).
000, 20% solution) were supplied by Sigma-Aldrich (Xuhui District, Shanghai, China).
Etherification of SL
30 wt% SL solution was prepared and the pH was adjusted to 10.8 or 12.0 with NaOH solution. The solution was heated to 90 °C, and epichlorohydrin was added dropwise by peristaltic pump. After 3 h, etherified SL (ESL) was obtained. Four samples with different etherification degrees were selected for further study, named ESL-15, ESL-36, ESL-45 and ESL-81; the etherification degrees of these samples are 15%, 36%, 45% and 81%, respectively.Structural characteristics of dispersants
Structural characteristics of the dispersants
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.67
4.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and the pH was adjusted to 5.5 with acetic acid. A 30 wt% dye slurry was obtained by ball-milling at a milling rate of 400 rpm for 8 h.
20, and the pH was adjusted to 5.5 with acetic acid. A 30 wt% dye slurry was obtained by ball-milling at a milling rate of 400 rpm for 8 h.
| K/S = (1 − R2)/2R |
Then, the staining rate was calculated using the following equation:
| Fiber staining rate/% = [(R0 − Ri)]/R0 × 100 |
Adsorption of dispersants on dye and fiber
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to prepare a 10 g L−1 solution.
1) to prepare a 10 g L−1 solution.
Results and discussion
Effects of etherification on Ph-OH and Mw
It has been demonstrated that the dispersive ability of a surfactant is greatly affected by its molecular weight.25 A previous study has shown that lignosulfonates with medium molecular weights have favorable dispersive ability and high temperature stability. Lignosulfonates with high molecular weights display stronger steric hindrance and will prevent the aggregation of dye particles, while those with low molecular weights can absorb on dye particles easily but provide weaker steric hindrance.26 The fouling of lignin onto fiber is mainly produced by the dark colour of lignin and the hydrogen bonding forces between the phenolic hydroxyl and carboxyl groups of lignin and the amide bonds of the fiber. Meanwhile, lignosulfonate with high molecular weight contains fewer phenolic carboxyl and phenolic hydroxyl groups than those with lower molecular weights, resulting in less fiber staining.5 Hence, it is necessary to improve the molecular weight of lignosulfonate while reducing the content of phenolic hydroxyl to achieve superior dispersive ability and weaker fiber staining.A series of ESLs were prepared by modification with epichlorohydrin. The structural characteristics of the ESLs depended on the amount of ECH added and the pH value. By adjusting the amounts of epichlorohydrin added at pH 10.8 and 12.0, ESLs with different molecular weights were prepared. The Ph-OH content and the mass average of the molecular weight (Mw) of the ESLs are shown in Fig. 1. With increasing amount of epichlorohydrin and increasing pH, the Ph-OH content decreased and the Mw increased gradually. At pH 12.0, the Ph-OH content and Mw changed more significantly as increasing amounts of epichlorohydrin were added, from 0.5 to 2.5 mmol g−1. The degree of change is described by the degree of etherification and polymerization and can be calculated as follows:
where Ci is the Ph-OH content of ESLs, C0 is the Ph-OH content of SL, Mw(ESL) is the mass average of the molecular weight of ESL, and Mw(SL) is the mass average of the molecular weight of SL.
The fitting relationships between the degrees of etherification and polymerization are shown in Fig. 2, where y is the degree of polymerization and x is the etherification degree; the slope reflects the degree of change. At pH 10.8, the equation is y = 0.0203x + 1.0085, R2 = 0.9939, and at pH 12.0, the equation is y = 0.0336x + 0.9314, R2 = 0.9626. With increasing etherification rate, the degree of polymerization increases more obviously at pH 12.0 than at pH 10.8, which indicates that under high pH conditions, SL molecules could be more readily linked together by epichlorohydrin.
It is suspected that two routes exist in the etherification reaction between lignin and epichlorohydrin, as shown in Fig. 3. Therefore, it is supposed that when a relatively small amount of epichlorohydrin is added or the pH is lowered, product (2) is mainly obtained, and the molecular weight changes only slightly after the reaction; when the amount of epichlorohydrin added is increased or the pH is higher, product (1) is predominant and the molecular weight increases obviously.
The molecular weight distributions are shown in Fig. 4. The Mw, the numerical average of molecular weight (Mn), and the polydispersibility index (Mw/Mn) as well as the functional group contents are given in Table 1. As the etherification degree increased from 15% to 45%, the molecular weight increased from 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 Da to 19
285 Da to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 Da and was much higher than that of SL. The polydispersibility index decreased with increasing etherification degree, indicating that the smaller molecular weight lignin was linked together by epichlorohydrin. It was known to us that the solubility and hydrophilicity of ESL-81 would be too weak for it to serve as a dye dispersant when the molecular weight of lignosulfonate was too high;27 therefore, ESL-81 will not be discussed later.
261 Da and was much higher than that of SL. The polydispersibility index decreased with increasing etherification degree, indicating that the smaller molecular weight lignin was linked together by epichlorohydrin. It was known to us that the solubility and hydrophilicity of ESL-81 would be too weak for it to serve as a dye dispersant when the molecular weight of lignosulfonate was too high;27 therefore, ESL-81 will not be discussed later.
| Sample | M w (Da) | M n (Da) | M w/Mn | Ph-OH content (mmol g−1) | Surface charge (eq. kg−1) |
|---|---|---|---|---|---|
| SL | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001 001 |
5264 | 1.90 | 1.90 | 1.07 |
| ESL-15 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 285 |
8252 | 1.61 | 1.61 | 1.02 |
| ESL-36 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227 227 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023 023 |
1.20 | 1.20 | 0.95 |
| ESL-45 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 261 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
1.04 | 1.04 | 0.89 |
| UNA | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
4780 | 2.14 | 0.71 | — |
| SNF | 8050 | 3180 | 2.53 | 0.42 | — |
UV spectra
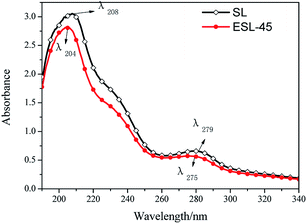
The benzene ring structure and chromophoric groups in SL absorb UV light strongly and are considered to be characteristic absorption peaks. The peak at 210 nm is related to the unsaturated chains, while that at 280 nm corresponds to unconjugated Ph-OH groups and the aromatic moiety of the lignin molecule.28 The conjugated structure and hydrogen bonds of SL are regarded as the key factors in the UV absorption spectrum. The UV spectra of SL and ESL-45 are represented in Fig. 5. Compared with SL, the UV absorption spectrum of ESL-45 shows a blue shift; the peaks appear at 204 nm and 275 nm, and the absorbance decreases. This spectroscopic blue shift at 210 nm indicates that the extent of the conjugated structure in SL decreased. With regard to the absorption peak at 280 nm, the spectroscopic blue shift illustrates that the content of Ph-OH in the benzene ring decreased; that is, the etherification was successfully carried out.The performance of ESL in fiber staining and dispersive ability
As shown in Fig. 6(a), with increasing etherification degree, the fiber staining rate decreases gradually. At 25 °C, the particle sizes of the dye are all less than 0.6 µm, and little change is observed. However, after high-temperature treatment at 130 °C, the particle size of the dye decreased significantly as the etherification rate increased, as shown in Fig. 6(b). | ||
| Fig. 6 Effects of the dispersants on fiber staining (a) and particle size of the dye bath at 25 and 130 °C (b). | ||
It is worth noting that the fiber staining of SL depends on the color of the SL and the adsorption characteristics of SL on the fiber surface and inside the fibers, whilst the dispersive ability and heat stability of a dye are related to the adsorption characteristics of SL on the dye.
The quinoid structure of SL is the key cause of its colour, as well as some chromophore and auxochrome groups, such as double bonds, carboxyl groups, carbonyl groups and hydroxyl groups.29 It has been stated that the quinoid structure and chromophore groups can account for the significant UV absorption at 450 nm (A450). All the A450 values of the ESLs are lower than that of SL, which is believed to be the result of the reduced Ph-OH content, except ESL-36. This is because under basic conditions, the catechol structure of SL will be oxidized to the quinone structure, resulting in a dark colour. With increasing etherification degree, more phenolic hydroxyl groups are blocked, which inhibits the formation of quinoid structures and results in a lighter colour. ESL-36 is obtained at pH 12.0, and the amount of ECH added is 0.5 mmol g−1 in the etherification process. Thus, the quinoid structure is produced extremely readily, leading to a dark colour. The A450 values of UNA and SNF are all lower than that of SL; thus, they exhibit less fiber staining than SL. After etherification, on the one hand, the colour of the lignin is lighter; on the other hand, the phenolic hydroxyl content declines significantly, from 1.90 mmol g−1 to 1.04 mmol g−1, which can distinctly lessen the hydrogen bond force between the dispersants and the fiber. Furthermore, the molecular weight also increased as the etherification degree increased. SL with low Mw is believed to embed into the fiber interspace more easily, especially during the high temperature dying process, because the fiber has more pores. Thus, the fiber staining rate decreased to a value close to that of UNA when the etherification degree was increased. Compared with SL, the particle size of the dye with ESLs decreases greatly at 130 °C, while it changes slightly at 25 °C. According to Table 1, with increasing etherification degree, the molecular weight increases from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001 Da to 19
001 Da to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 Da. It is believed that SL with high Mw provides sufficient steric hindrance to disrupt the agglomeration of dye particles and maintain its stability. The Mw of ESL-45 is much greater than those of UNA (Mw = 10
261 Da. It is believed that SL with high Mw provides sufficient steric hindrance to disrupt the agglomeration of dye particles and maintain its stability. The Mw of ESL-45 is much greater than those of UNA (Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250) and SNF (Mw = 8050); therefore, it exhibits much better high temperature dispersive ability and stability, especially compared to SNF.
250) and SNF (Mw = 8050); therefore, it exhibits much better high temperature dispersive ability and stability, especially compared to SNF.
In summary, ESL-45 shows superior performance both in fiber staining and high temperature stability due to its desirable molecular weight and low amount of phenolic hydroxyl groups. As is proposed above, the performance of a dispersant mainly results from its adsorption characteristics on fibers and dyes; therefore, it is of great importance to find a visual method to measure the absorption characteristics to further verify the above results.
Adsorption characteristics of dispersants on fiber
The adsorption characteristics of dispersants on the surface of a fiber can be detected by QCM-D; however, those of dispersants inside the fibers still cannot be measured. A higher value of |Δf| indicates a larger amount of absorption; a higher |ΔD/Δf| value indicates that the conformation of the absorption layer is softer and more viscoelastic. Contrastingly, a smaller slope value indicates that the absorption layer is a rigid and homogeneous structure. As is shown in Fig. 7, for the ESLs, the value of |Δf| of the fiber surface decreases. Moreover, all the values of |ΔD/Δf| are slightly greater than that of SL, which means that the adsorbed layers on the fiber surface are softer and more viscous than that of SL; that is, the absorption layer can be more readily washed off the fiber surface. Etherification reduces the Ph-OH content, thereby decreasing the hydrogen bonding; therefore, the adsorption amounts of ESLs are lower than that of SL.The adsorption characteristics of SL on internal fibers are mainly related to Mw. During the dying process, the fiber expands and many pores appear. SL with low Mw can more readily diffuse inside the fiber voids and cannot be washed away easily, while SL with high Mw cannot easily enter the fibers due to steric hindrance.26,30 After etherification, the Mw of SL increases as a result of crosslinking, which results in less fiber staining. In accordance with the above description, the adsorption model of dispersants on fiber is depicted in Fig. 8.
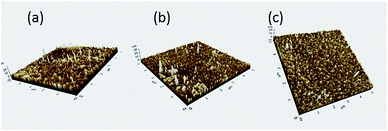
The adsorption characteristic of dispersant on dye
As shown in Fig. 9, with increasing etherification degree, the value of |Δf| increases gradually, indicating that the adsorption amount of the dispersants on the dye increases. Furthermore, the value of |ΔD/Δf| decreases with increasing etherification degree, which further indicates that the conformation of the absorption film becomes rigid and homogenous. As seen in the AFM image given in Fig. 10, after etherification, the absorption film is less rough and become more homogenous. The adsorption characteristics of the dispersant on the dye mainly depend on steric hindrance31 and hydrophobic interactions. After etherification, the Mw of the dispersant increases, leading to a significant increase of steric hindrance between the dispersant and the dye, so that the adsorption amount increases and a thicker, more viscous layer is formed compared with SL.The surface charges of the dispersants are shown in Table 1. The surface charge decreases from 1.07 eq. kg−1 to 0.80 eq. kg−1 with increasing etherification degree, which indicates that the hydrophilic property of the ESLs weakens. This is because the Ph-OH content of ESL decreases after etherification. In addition, the ESL molecules adopt a curling conformation due to the increase of Mw, causing some charged groups, including sulfonic groups, carboxyl groups and Ph-OH, to be wrapped inside the molecule.32
Therefore, when the dye suspension is placed under high temperature conditions, the particles assemble readily. Hence, the dispersant with optimum molecular weight shows a favorable performance. In accordance with the above description, the adsorption model of SL and ESL-45 on dye particles is illustrated in Fig. 11. Compared with SL, ESL-45 has higher Mw and lower phenolic hydroxyl group content; therefore, it can strongly adsorb on the dye surface by hydrophobic effects. In addition, with higher Mw, the 3-D molecular structure of ESL-45 is more flexible and curly, which can disrupt the agglomeration of dye particles by steric hindrance.
Conclusions
A series of ESLs with different Ph-OH contents and molecular weights were prepared by adjusting the dosage of ECH and the pH of the solution. After etherification, the ESLs samples, especially ESL-45, which has optimal molecular weight and phenolic hydroxyl group content, exhibit superior fiber staining and high temperature properties.With increasing etherification degree, the adsorption capacity of ESLs on the fiber surface decreases due to the gradual decrease in hydrogen bonding forces. Therefore, the fiber staining became weaker with increasing Mw of the ESLs. Also, the adsorption amount of ESLs on the dye increased and the adsorption structure became denser because of the enhanced hydrophobic interactions between the ESLs and the dye. Consequently, the high temperature stability improved significantly.
Acknowledgements
The authors would like to acknowledge the financial support of International S&T Cooperation Program of China (2013DFA-41670), the National Natural Science Foundation of China (21436004, 21576106) and the Fundamental Research Funds for the Central Universities (2014ZP0003).Notes and references
- X. P. Ouyang, X. Q. Qiu and P. Chen, Colloids Surf., A, 2006, 489, 282–283 Search PubMed.
- M. S. Zhou, W. L. Wang, D. J. Dong and X. Q. Qiu, RSC Adv., 2015, 5, 2441–2448 RSC.
- L. H. Grierson, J. C. Knight and R. Maharaj, Cem. Concr. Res., 2005, 35(4), 631–636 CrossRef CAS.
- Z. P. Li, Y. X. Pang, Y. Y. Ge and X. Q. Qiu, J. Ind. Eng. Chem., 2012, 18(1), 532–537 CrossRef CAS.
- D. J. Yang, X. Q. Qiu, M. S. Zhou and H. M. Lou, Energy Convers. Manage., 2007, 48(9), 2433–2438 CrossRef CAS.
- X. Zhang, A. Glüsen and G. Ricard, J. Membr. Sci., 2006, 276(1–2), 301–307 CAS.
- P. Dilling, G. S. Samaranayake and S. L. Waldrop, US5972047, 1999.
- P. Dilling, US5980589, 1999.
- P. Dilling, US4486346, 1984.
- P. Dilling, US551151, 1985.
- S. I. Falkehag, US3763139, 1973.
- H. L. Hintz, US3769272, 1973.
- G. Telysheva, T. Dizhbite, A. Kizima, A. Volpertsl and E. Lazareva, Recent Advances in Environmentally Compatible Polymers, Woodhead Publishing Ltd, England, 2001, pp. 167–172 Search PubMed.
- S. I. Falkehag, H. H. Moorer and C. W. Bailey, US3672817, 1972.
- P. Dilling and P. T. Sarjeant, US4454066, 1984.
- P. Dilling and G. Prazak, US4355996, 1982.
- C. Z. Chen, M. Q. Zhu, M. F. Li, Y. M. Fang and R. C. Sun, Biotechnol. Biofuels, 2016, 9(87) DOI:10.1186/s13068-016-0499-9.
- Y. Qian, Y. H. Deng, H. Li and X. Q. Qiu, Ind. Eng. Chem. Res., 2014, 53(24), 10024–10028 CrossRef CAS.
- S. Lin, US4184845, 1980.
- Y. Y. Ge, Z. P. Li, Y. X. Pang and X. Q. Qiu, Int. J. Biol. Macromol., 2013, 52, 300–304 CrossRef CAS PubMed.
- Y. X. Pang, W. Gao, H. M. Lou and X. Q. Qiu, Colloids Surf., A, 2014, 441, 664–668 CrossRef CAS.
- M. Norgren, L. Gärdlund, S. M. Notley and L. Wågberg, Langmuir, 2007, 23(7), 3737–3743 CrossRef CAS PubMed.
- Y. L. Qin, X. Q. Qiu, W. S. Liang and D. J. Yang, Ind. Eng. Chem. Res., 2015, 54(49), 12313–12319 CrossRef CAS.
- R. J. A. Gosselink, A. Abächerli, H. Semke, R. Malherbe, P. Käuper, A. Nadif and J. E. G. Van Dam, Ind. Crops Prod., 2004, 19(3), 271–281 CrossRef CAS.
- X. L. Lin, M. S. Zhou, S. Y. Wang, H. M. Lou, D. J. Yang and X. Q. Qiu, ACS Sustainable Chem. Eng., 2014, 2(7), 1902–1909 CrossRef CAS.
- Y. L. Qin, D. J. Yang and X. Q. Qiu, ACS Sustainable Chem. Eng., 2015, 3(12), 3239–3244 CrossRef CAS.
- H. Schott, J. Pharm. Sci., 1995, 84(10), 1215–1222 CrossRef CAS PubMed.
- C. A. Lekelefac, N. Busse, M. Herrenbauer and P. Czermak, Int. J. Photoenergy, 2014, 501, 137634 Search PubMed.
- F. Imsgard, S. I. Falkehag and K. P. Kringstad, Tappi Tech. Ass. Pulp Pap. Indus., 1971, 54(10), 1680–1684 CAS.
- D. J. Yang, H. J. Li, Y. L. Qin, R. S. Zhong, M. X. Bai and X. Q. Qiu, J. Dispersion Sci. Technol., 2015, 36, 532–539 CrossRef CAS.
- Y. L. Qin, W. M. Mo, L. X. Yu, D. J. Yang and X. Q. Qiu, Holzforschung, 2016, 70(2), 109–116 CrossRef CAS.
- Y. H. Deng, Y. Wu, Y. Qian, X. P. Ouyang, D. J. Yang and X. Q. Qiu, BioResources, 2010, 5(2), 1178–1196 CAS.
| This journal is © The Royal Society of Chemistry 2016 |