A highly sensitive fluorescent probe for detection of hydrazine in gas and solution phases based on the Gabriel mechanism and its bioimaging†
Abstract
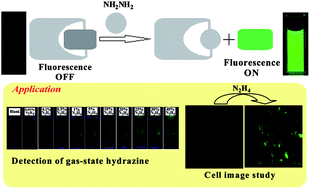
A new probe 2-benzo[1,2,5]thiadiazol-4-yl-isoindole-1,3-dione (BTI) based on the Gabriel reaction mechanism was synthesized and characterized for the specific detection of hydrazine with high selectivity against other amines in an organo-aqueous solution. Upon hydrazinolysis of BTI in the presence of hydrazine in a H2O–DMSO (4 : 6, v/v) solution (10 mM HEPES buffer, pH 7.4) at room temperature, the chemosensor produces fluorescent aminobenzthiadiazole with a maximum emission at 498 nm along with a color change from colorless to green, allowing selective colorimetric and fluorometric detection of hydrazine by the naked eye. Probe BTI was also successfully applied in vapor phase hydrazine detection into a solid state over other interfering volatile analytes. Furthermore, the probe BTI coated with silica gel TLC plates could act as a visual and fluorimetric probe for hydrazine vapor detection. The experimental detection limit of hydrazine is 2.9 ppb, which is well below the accepted limit (10 ppb) for hydrazine set by the U.S. Environmental Protection Agency (EPA). DFT and TDDFT calculations were performed in order to demonstrate the sensing mechanism and the electronic properties of probe and hydrazinolysis products. Additionally, probe BTI could also be applied for the imaging of hydrazine in living cells.

 Please wait while we load your content...
Please wait while we load your content...