Chondroitin sulfate-functionalized polyamidoamine-mediated miR-34a delivery for inhibiting the proliferation and migration of pancreatic cancer†
Yan Duan‡
,
Zhen Xing‡,
Jiebing Yang,
Yudi Wang,
Jiawen Chen,
Yan Zhang,
Wei Shi* and
Quanshun Li*
Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, School of Life Sciences, Jilin University, Changchun 130012, China. E-mail: quanshun@jlu.edu.cn; shiw@jlu.edu.cn; Fax: +86-431-85155200; Tel: +86-431-85155216
First published on 15th July 2016
Abstract
Herein, chondroitin sulfate-functionalized polyamidoamine (CS-PAMAM) was employed as a carrier in miR-34a delivery, and the inhibition of cell proliferation and migration of pancreatic cancer was systematically evaluated, using human pancreatic carcinoma cell line MiaPaCa-2 as a model. Through confocal laser scanning microscopy, an efficient cellular uptake of CS-PAMAM/miR-34a nanoparticles has been demonstrated to be executed in a CD44-dependent manner. After the successful delivery of miR-34a, the cell proliferation could be obviously inhibited due to the activation of cell apoptosis and cell cycle arrest. Meanwhile, CS-PAMAM-mediated miR-34a delivery could realize the suppression of cell migration elucidated by wound healing and transwell migration assays. Thus, the derivative CS-PAMAM could potentially be used as an effective carrier for miR-34a delivery to achieve the tumor gene therapy.
Introduction
Pancreatic cancer is one of the most aggressive malignances in the world, and also the fourth leading cause of cancer-related deaths in the US.1 To date, the clinical outcome of patients with pancreatic cancer remains poor, with 5 year survival of only 7%.2 Thus, it is urgent to develop novel therapeutic strategies for treating pancreatic cancer.In contrast to traditional radiotherapy and chemotherapy, gene therapy exhibits great potential in cancer treatment since it can edit the mutation abnormalities or alter the gene expression.3 Recently, microRNAs (miRNAs) have been identified to be highly associated with the development and progression of cancers,4,5 and thus they have the potential to be employed as tools for achieving effective anti-tumor efficiency. Among them, miR-34a has been observed to be down-regulated in pancreatic cancer due to the epigenetic regulation, which will lead to the cell proliferation, cell cycle progression and migration.6,7 Previous reports have shown that enhanced expression of miR-34a could inhibit the expression level of Bcl-2, survivin and Notch-1,8–11 and thus the carrier-mediated transfection of miR-34a to improve its intracellular level will be an efficient technique for suppressing the proliferation (through the induction of apoptosis and cell cycle arrest) and migration of pancreatic cancer cells. Among the gene carriers, branched polyethylenimine with a weight-average molecular weight of 25 kDa (PEI25K) and its derivatives were widely used to mediate miRNA delivery.12,13 However, compared to PEI25K, amine-terminated polyamidoamine (PAMAM) has been identified to possess relatively lower cytotoxicity and higher cellular uptake rate.14 Meanwhile, it exhibited other unique characteristics for delivering the foreign genes into targeted cells,15–18 e.g., well-defined nanostructure, spherical shape, excellent solubility and high density of surface functionalities. Additionally, it could facilitate the endosomal escape of loading cargos through “proton sponge” effect originated from the high density of tertiary amine groups within its interior.19,20
Tumor targeting is another key issue to be considered in the design of gene carriers. For instance, CD44 has been observed to be over-expressed in many solid tumors,21,22 and hyaluronic acid (HA) and chondroitin sulfate (CS) have been identified to possess favorable affinity with CD44, which could be used as ligands to realize an improved delivery of chemotherapeutics and genes.23–26 In our previous research, CS has been successfully conjugated to PEI25K through Michael addition and the conjugate CS–PEI could promote the endocytosis of nanoparticles in a CD44-mediated manner.27
In the present research, the derivative CS-PAMAM was constructed and employed as a carrier in miR-34a delivery, and the inhibition of cell proliferation and migration of pancreatic cancer was systematically evaluated, using human pancreatic carcinoma cell line MiaPaCa-2 as a model.
Experimental
Materials
The miR-34a, FAM-labeled miR-34a and scrambled miRNA were synthesized by GenePharma (Suzhou, China) as follows:miR-34a: sense: 5′-UGGCAGUGUCUUAGCUGGUUGU-3′;
antisense: 3′-AACCAGCUAAGACACUGCCAUU-5′;
scrambled: sense: 5′-UUCUCCGAACGUGUCACGUdTdT-3′;
antisense: 3′-ACGUGACACGUUCGGAGAAdTdT-5′.
The amine-terminated G5 PAMAM dendrimer was purchased from Chenyuan Co. (Weihai, China) and used as received. Sodium CS (Mn = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and methacrylic anhydride were obtained from Aladdin (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, USA). The Annexin-V-FITC/PI apoptosis and PI cell cycle detection kits were purchased from Bestbio (Shanghai, China). Lipofectamine2000 were purchased from Invitrogen (Carlsbad, USA), and polyvinylidene fluoride (PVDF) membrane was obtained from Millipore. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 4,6-diamidino-2-phenylindole (DAPI) were purchased from Amersco (Solon, USA). The FITC-labelled anti-CD44 monoclonal antibody, antibodies against procaspase-3, Bcl-2, Notch-1 and β-actin, horseradish peroxidase (HRP)-labelled goat anti-rabbit IgG and HRP-labelled goat anti-mouse IgG were obtained from Abcam.
000 g mol−1) and methacrylic anhydride were obtained from Aladdin (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, USA). The Annexin-V-FITC/PI apoptosis and PI cell cycle detection kits were purchased from Bestbio (Shanghai, China). Lipofectamine2000 were purchased from Invitrogen (Carlsbad, USA), and polyvinylidene fluoride (PVDF) membrane was obtained from Millipore. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 4,6-diamidino-2-phenylindole (DAPI) were purchased from Amersco (Solon, USA). The FITC-labelled anti-CD44 monoclonal antibody, antibodies against procaspase-3, Bcl-2, Notch-1 and β-actin, horseradish peroxidase (HRP)-labelled goat anti-rabbit IgG and HRP-labelled goat anti-mouse IgG were obtained from Abcam.
Synthesis and characterization of CS-PAMAM
The derivative CS-PAMAM was synthesized through Michael addition according to a similar method previously reported.27 Briefly, CS (40 mg) was dissolved in 2 mL of distilled water, and methacrylic anhydride (0.7 g) was added into CS solution. Subsequently, the system was stirred at room temperature for 2 h and at 4 °C for another 24 h, during which the pH of system was kept at 8.0 using NaOH solution (5 M). Afterwards, 50 mL of ethanol was added into the system, and the precipitate CSMA was obtained via filtration, washed with ethanol three times and dried in a vacuum oven at room temperature. For the synthesis of CS-PAMAM, CSMA solution (1 mg mL−1) was added into PAMAM solution (1 mg mL−1) dropwise at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and the mixture was stirred at room temperature for 48 h. The product CS-PSAMAM was purified by dialysis in distilled water for 48 h (molecular weight cut-off: 50
10, and the mixture was stirred at room temperature for 48 h. The product CS-PSAMAM was purified by dialysis in distilled water for 48 h (molecular weight cut-off: 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), and then obtained by lyophilization.
000 Da), and then obtained by lyophilization.
The structure of CSMA and CS-PAMAM was characterized by 1H NMR on an AVANCE DMX 500 spectrometer at 500 MHz in D2O. Transmission electron microscopy (TEM) images were collected using a HITACHI-H800 microscope operating at 200 kV. The hydrodynamic size and zeta potential of CS-PAMAM/miR-34a nanoparticles were measured by dynamic light scattering (DLS) on a Malvern Nano ZS90 Zetasizer (Malvern, UK). For the serum stability analysis, CS-PAMAM/miR-34a nanocomplex was prepared by gently mixing the carrier CS-PAMAM and miR-34a at a mass ratio of 40 and incubating them at room temperature for 30 min, and then treated with 10% FBS for different time. Afterwards, the mixture was assessed by 2% agarose gel electrophoresis in Tris–acetate–EDTA buffer solution (80 V, 10 min).
Cellular uptake of CS-PAMAM/miR-34a nanoparticle
Briefly, MiaPaCa-2 cells were seeded in 6-well plates with 2 mL DMEM containing 10% FBS (1.0 × 105 cells per well), and the sterilized coverslips were placed into the wells. The cells were cultured at 37 °C for 24 h, and subjected to miR-34a transfection for pre-determined time (36 nM of miR-34a-FAM). The cells were washed with phosphate buffer saline (PBS) twice and fixed with 75% ethanol, and nuclei were stained with DAPI. Finally, the coverslips were analysed using LSM 710 confocal laser scanning microscope (CLSM, Carl Zeiss Microscopy LLC, Jena, Germany). In the assay, pre-treatment with 10 mg mL−1 CS for 1 h before miR-34a transfection was used to block the CD44 receptor of cell surface.Determination of CD44 expression level
The murine preadipocyte 3T3 and MiaPaCa-2 cells were seeded in 6-well plates at a density of 1.0 × 106 cells per well and incubated at 37 °C for 24 h. The cells were harvested, washed with PBS three times and re-suspended in PBS. The samples were incubated with FITC-labelled anti-CD44 monoclonal antibody (1 μg mL−1) for 30 min, and then the expression level of CD44 was determined by FACS Calibur instrument (BD Bioscience Mountain View, USA).Inhibition of cell proliferation by CS-PAMAM/miR-34a nanoparticle
The cytotoxicity of CS-PAMAM and the inhibition of cell proliferation by CS-PAMAM/miR-34a nanoparticle were evaluated by MTT assay. The MiaPaCa-2 cells were seeded in 96-well plates with 200 μL DMEM containing 10% FBS at a density of 5.0 × 103 cells per well. After incubating at 37 °C overnight, the cells were directly treated with DMEM harboring CS-PAMAM (0–80 μg mL−1) for 24 h in the cytotoxicity assay, or washed with PBS twice and subjected to miR-34a transfection in 100 μL serum-free DMEM for 6 h (2 μg mL−1 of miR-34a). Following this, the cells were cultured in 10% FBS-containing DMEM (200 μL) for further 72 h. Subsequently, MTT solution (5 mg mL−1 in PBS, 20 μL) was added to each well, and the plate was incubated for an additional 4 h. After removing MTT solution from each well, the formazan crystals were dissolved by 150 μL DMSO, and the plates were incubated for 10 min and analysed on a GF-M3000 microplate reader (Shandong, China) to record the absorbance at 492 nm. The cell viability (%) was calculated as the ratio of Asample and Acontrol, which were the absorbance values of the treated and untreated groups, respectively.Cell apoptosis and cell cycle arrest assays
The MiaPaCa-2 cells were seeded in 6-well plates at a density of 2.0 × 105 cells per well and incubated at 37 °C for 24 h, and then subjected to miR-34a transfection for 6 h (final concentration of 2 μg mL−1 in 2 mL DMEM). After removing the medium, the cells were cultured in 2 mL DMEM containing 10% FBS for 72 h, harvested and washed with PBS twice. For the apoptosis assay, the cells were re-suspended in binding buffer provided by the manufacturer and incubated with Annexin-V-FITC and PI for 10 min in the dark, while for the cell cycle arrest, the cells were re-suspended in 0.5 mL solution containing 10 μL RNase A (25 μg mL−1) and 10 μL PI (50 μg mL−1), and incubated at 37 °C for an additional 30 min in the dark. The assays were then conducted by analysing 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gated cells using FACS caliber (BD Bioscience Mountain View, USA).
000 gated cells using FACS caliber (BD Bioscience Mountain View, USA).
Western blotting assay
The cell culture and miR-34a transfection were performed as described above. Afterwards, the cells were collected, washed with PBS twice and treated with lysis buffer on ice for 2 h. Then the supernatants were obtained through centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, and an equal amount of protein was analysed by SDS-PAGE and transferred to PVDF membrane by electroblotting. The membrane was then blocked with PBS containing 5% non-fat milk and 0.1% Tween-20 (PBST) at room temperature for 1 h, incubated with desired antibodies at 4 °C overnight and washed with PBST twice. Finally, HRP-labelled secondary antibody was used for 1 h incubation, and specific proteins were detected by ChemiScope 3600 chemiluminescence imaging system (Clinx Science Instrument, Shanghai, China).
000 rpm for 10 min, and an equal amount of protein was analysed by SDS-PAGE and transferred to PVDF membrane by electroblotting. The membrane was then blocked with PBS containing 5% non-fat milk and 0.1% Tween-20 (PBST) at room temperature for 1 h, incubated with desired antibodies at 4 °C overnight and washed with PBST twice. Finally, HRP-labelled secondary antibody was used for 1 h incubation, and specific proteins were detected by ChemiScope 3600 chemiluminescence imaging system (Clinx Science Instrument, Shanghai, China).
Wound healing assay
The MiaPaCa-2 cells were seeded in 6-well plates with 2 mL DMEM containing 10% FBS (density of 1.0 × 106 cells per well) and cultured to 90% confluence. The mechanical scratch wound was generated through a sterile pipette tip on the cell monolayer. The cells were washed with PBS twice, treated with miR-34a transfection samples (2 μg mL−1 of miR-34a) in serum-free DMEM for 6 h, and incubated in 10% FBS-containing DMEM for 6, 12 and 36 h. The images of wound area were recorded by an IX71 fluorescence microscopy (Olympus, Tokyo, Japan).Transwell migration assay
The cell culture and miR-34a transfection were performed as described above. The cell migration assay was conducted using transwell chamber (Costar, Corning, NY, USA) as follows: 1.0 × 106 cells were suspended in 100 μL of serum-free DMEM and placed in the upper chamber with 8 μm pores, and 500 μL DMEM was placed in the lower chamber. After 24 h, non-migrating cells were carefully removed from the top of membrane by mechanical wiping, and the cells migrating to the lower surface of membrane were fixed with 75% ethanol, stained with 0.1% crystal violet (15 min) and assayed using an IX71 fluorescence microscopy (Olympus, Tokyo, Japan).Statistical analysis
All data were presented as the mean value ± SD. Statistical significance between two groups was determined by an unpaired student's t-test and multiple comparisons one-way analysis of variance (ANOVA), and p < 0.05 was considered to be the statistical significance. The result in each figure was a representative of at least three independent experiments.Results and discussion
Synthesis and characterization of CS-PAMAM
Through Michael addition, CS-PAMAM was synthesized and structurally characterized by 1H NMR. The peaks at 5.31 and 5.63 ppm were ascribed to the two protons of double bonds on CSMA, and the peak at 1.79 ppm could be attributed to the methyl group on CSMA (Fig. S1A†). Through the peak areas at 1.79 and 1.94 ppm, the degree of methacrylation on the CS was calculated to be ca. 30.3%. The double bonds of CSMA almost disappeared and a new peak at 1.20 ppm (d) appeared in the spectra of CS-PAMAM (Fig. S1B†), indicating the successful grafting of CS on the dendrimer PAMAM. Based on the peak area of c and d, the number of PAMAM conjugated to the CS was determined to be ca. 0.09 per CS unit. Notably, weak double bonds could still be observed in the spectra of CS-PAMAM probably owing to the un-modified double bonds in the product CS-PAMAM or the residue of CSMA which was difficult to be completely removed by lyophilization. Compared to the partial degradation of free miR-34a in serum, the presence of 10% FBS did not influence the complexation of CS-PAMAM and miR-34a, and CS-PAMAM could efficiently protect miR-34a from digestion in serum (Fig. S2†). TEM images showed that compared with no regular size of CS-PAMAM, CS-PAMAM/miR-34a exhibited a relatively homogeneous spheric structure and a non-aggregative state with particle size of <100 nm (Fig. 1). The hydrodynamic size and polydispersity index (PDI) of CS-PAMAM/miR-34a nanocomplex at a mass ratio of 40 were measured to be 178.0 nm and 0.056, respectively (Fig. S3†). These values were much lower than CS-PAMAM (383.6 nm, PDI of 1.000) due to the assembly of these two components to form tight nanoparticles. In addition, the zeta potential of CS-PAMAM/miR-34a nanocomplex at a mass ratio of 40 was measured to be +25.2 mV.Cellular uptake of CS-PAMAM/miR-34a nanoparticle
The FAM-labelled miR-34a was first used to detect the CS-PAMAM/miR-34a transfection through CLSM. As shown in Fig. S4,† green fluorescence intensity exhibited an increasing tendency with the elongation of transfection time, indicating the high efficiency of CS-PAMAM/miR-34a after 4 h transfection in MiaPaCa-2 cells. Meanwhile, enhanced green fluorescence could be achieved with an increasing mass ratio especially at a mass ratio of 40, much higher than free miR-34a (Fig. 2). However, CS-PAMAM showed relatively lower transfection efficiency than PAMAM at a mass ratio of 20 (B and F), mainly due to the unfavorable effect on the endocytosis induced by the introduction of hydrophilic CS segment. Notably, after pre-treatment with 10 mg mL−1 CS for 1 h before the CS-PAMAM/miR-34a transfection to block CD44, the intracellular fluorescence intensity of FAM-labelled miR-34a was remarkably weakened (D and E), while there were no obvious changes in the PAMAM/miR-34a transfection groups (F and G). These results suggested that the CS pre-treatment could inhibit the internalization of CS-PAMAM/miR-34a nanoparticle, and the cellular uptake was facilitated in a CS-dependent manner, owing to the high affinity between CS and CD44 receptor over-expressed in MiaPaCa-2 cells (Fig. S5†). Thus, it could be concluded that selective tumor targeting and efficient cellular uptake of CS-PAMAM/miR-34a nanoparticle were achieved in a CD44-mediated endocytosis manner.Inhibition of cell proliferation by CS-PAMAM/miR-34a nanoparticle
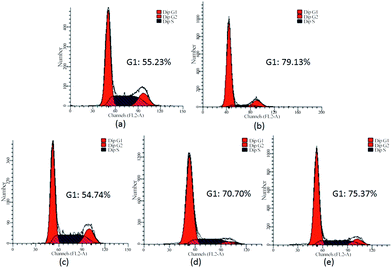
The cytotoxicity analysis showed that the derivative CS-PAMAM possessed low cytotoxicity in the studied concentrations, with cell viability of ca. 84% at a concentration of 80 μg mL−1 (Fig. S6†). For CS–PEI, only ca. 70% of cell viability could be obtained at the same concentration.27 Then the effect of miR-34a transfection on cell proliferation was assessed through the treatment with different miR-34a transfection nanocomplexes (Fig. 3). Similar to the control group, free miR-34a and CS-PAMAM-mediated transfection of scrambled sequence (mock) did not inhibit the cell proliferation. In contrast, miR-34a transfection mediated by lipofectamine2000, PAMAM and CS-PAMAM could achieve obvious inhibitory effects on the cell proliferation, with a similar inhibitory level. Due to the intrinsic cytotoxicity, the commercial transfection reagent lipofectamine2000 could only be used in experimental analysis and not be employed in gene delivery, especially for in vivo study. In contrast to PAMAM, due to the introduction of hydrophilic tumor-targeting ligand CS, the derivative CS-PAMAM could potentially overcome the limited circulation time and side effects, which made it a promising carrier for achieving in vivo miR-34a delivery. As miR-34a could trigger the apoptosis and cell cycle arrest of tumor cells accounting for the tumor suppressor function,28 the apoptosis and cell cycle arrest after miR-34a transfection were analysed by FACS using Annexin-V-FITC/PI staining and PI staining, respectively. As shown in Fig. 4, in contrary to CS-PAMAM/mock (7.15%), CS-PAMAM/miR-34a transfection could induce the early apoptosis of tumor cells at a high level (21.29%), stronger than PAMAM/miR-34a transfection (17.46%) and similar to lipofectamine2000/miR-34a transfection (22.02%). Similar tendency could also be observed in the cell cycle arrest analysis (Fig. 5): CS-PAMAM/miR-34a transfection could trigger a significant increase in the G1 phase (75.37%), higher than PAMAM/miR-34a transfection (70.70%) and similar to lipofectamine2000/miR-34a transfection (79.13%). The results were consistent with the fact that miR-34a has been found to inhibit the cell growth by holding the cell cycle at the G1/S phase.9 Overall, CS-PAMAM can be employed as a promising tumor-targeting carrier for miR-34a delivery, which will lead to the anti-proliferation of tumor cells through inducing both cell apoptosis and cell cycle arrest.To get a deeper insight into the anti-proliferative mechanism, the expression levels of proteins associated with cell apoptosis (procaspase-3 and Bcl-2) were investigated after miR-34a transfection. As shown in Fig. 6, the expression levels of procaspase-3 and Bcl-2 showed a decreased state after miR-34a transfection, indicating the activation of caspase-3 and the inhibition of Bcl-2. Bcl-2 is usually over-expressed in various cancers, which is highly associated with the anti-apoptosis of tumors and resistance of chemotherapeutics.29 Thus, the down-regulation of Bcl-2 level mediated by CS-PAMAM/miR-34a transfection could be a potential strategy for improving the susceptibility of cancer cells to chemotherapeutics and enhancing the chemotherapy efficacy.
Inhibition of cell migration by CS-PAMAM/miR-34a nanoparticle
The wound healing and transwell migration assay were conducted to determine the effect of miR-34a transfection on the cell migration, which is an important reason for the cancer-associated mortality. Compared to the control and mock, wound size showed a profound reduced trend after miR-34a transfection mediated by lipofectamine2000, PAMAM and CS-PAMAM, demonstrating the weakened cell migration induced by miR-34a delivery (Fig. 7 and S7†). However, relatively higher inhibition ability of cell proliferation in lipofectamine2000/miR-34a group was observed, which was probably caused by the high cytotoxicity of lipofectamine2000. Furthermore, transwell migration assay was used to measure the effect of miR-34a transfection on the cell migration, in which representative images of migrated cells at the bottom of membrane were stained with crystal violet. As shown in Fig. 8, CS-PAMAM/miR-34a transfection realized the enhanced suppression of cell migration, with much less in the number of migrated cells. Except for the inhibition of Bcl-2 associated with the cell migration, Notch-1 could also be detected in a decreased expression level (Fig. 6), which was probably another key protein mediating the inhibition of cell migration. In a word, CS-PAMAM-mediated miR-34a delivery provided a novel platform for inhibiting the cell proliferation and migration in a tumor-targeting way.Conclusions
In conclusion, a tumor-targeting carrier CS-PAMAM has been successfully used in the miR-34a delivery. Through an efficient miR-34a transfection in a CD44-dependent manner, the anti-proliferative and anti-migration efficiency could be achieved in pancreatic cancer cells. Thus, the nanocarrier with CS as a ligand could be used as a promising miR-34a delivery system to construct a therapeutic strategy for cancers.The in vivo evaluation of CS-PAMAM-mediated miR-34a delivery is still underway in our laboratory.
Acknowledgements
The research was supported by Natural Science Foundation of China (81373344 and 51473060), Science & Technology Department of Jilin Province (20140101140JC) and Education Department of Jilin Province (2015469).Notes and references
- Y. Li and F. H. Sarkar, Int. J. Biol. Sci., 2016, 12, 326 CrossRef CAS PubMed.
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 5 CrossRef PubMed.
- D. Cross and J. K. Burmester, Clin. Med. Res., 2006, 4, 218 CrossRef CAS PubMed.
- D. M. Dykxhoorn, Cancer Res., 2010, 70, 6401 CrossRef CAS PubMed.
- D. P. Bartel, Cell, 2004, 116, 281 CrossRef CAS PubMed.
- M. Vogt, J. Munding, M. Gruner, S. T. Liffers, B. Verdoodt, J. Hauk, L. Steinstraesser, A. Tannapfel and H. Hermeking, Virchows Arch., 2011, 458, 313 CrossRef PubMed.
- N. B. Jamieson, D. C. Morran, J. P. Morton, A. Ali, E. J. Dickson, C. R. Carter, O. J. Sansom, T. R. Evans, C. J. McKay and K. A. Oien, Clin. Cancer Res., 2012, 18, 534 CrossRef CAS PubMed.
- F. Chen and S. Hu, J. Biochem. Mol. Toxicol., 2012, 26, 79 CrossRef CAS PubMed.
- S. Shi, L. Han, T. Gong, Z. Zhang and X. Sun, Angew. Chem., Int. Ed., 2013, 52, 3901 CrossRef CAS PubMed.
- X. Deng, M. Cao, J. Zhang, K. Hu, Z. Yin, Z. Zhou, X. Xiao, Y. Yang, W. Sheng, Y. Wu and Y. Zeng, Biomaterials, 2014, 35, 4333 CrossRef CAS PubMed.
- D. Cosco, F. Cilurzo, J. Maiuolo, C. Federico, M. T. D. Martino, M. C. Cristiano, P. Tassone, M. Fresta and D. Paolino, Sci. Rep., 2015, 5, 17579 CrossRef CAS PubMed.
- S. Gao, H. Tian, Y. Guo, Y. Li, Z. Guo, X. Zhu and X. Chen, Acta Biomater., 2015, 25, 184 CrossRef CAS PubMed.
- Y. Li, H. Tian, J. Ding, L. Lin, J. Chen, S. Gao and X. Chen, Adv. Healthcare Mater., 2015, 4, 1369 CrossRef CAS PubMed.
- F. P. Seib, A. T. Jones and R. Duncan, J. Controlled Release, 2007, 117, 291 CrossRef CAS PubMed.
- E. R. Gillies and J. M. J. Frechet, Drug Discovery Today, 2005, 10, 35 CrossRef CAS PubMed.
- C. C. Lee, J. A. MacKay, J. M. Frechet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517 CrossRef CAS PubMed.
- F. Wang, Y. Wang, H. Wang, N. Shao, Y. Chen and Y. Cheng, Biomaterials, 2014, 35, 9187 CrossRef CAS PubMed.
- L. Li, C. Wei and C. Gong, J. Biomed. Nanotechnol., 2015, 11, 739 CrossRef CAS PubMed.
- M. Wang, H. Liu, L. Li and Y. Cheng, Nat. Commun., 2014, 5, 3053 Search PubMed.
- Y. Wang, L. Li, N. Shao, Z. Hu, H. Chen, L. Xu, C. Wang, Y. Cheng and J. Xiao, Acta Biomater., 2015, 14, 115 CrossRef PubMed.
- T. Chanmee, P. Ontong, K. Kimata and N. Itano, Front. Radiat. Oncol., 2015, 5, 180 Search PubMed.
- H. Yin, F. Zhao, D. Zhang and J. Li, Int. J. Pharm., 2015, 483, 169 CrossRef CAS PubMed.
- Y. L. Lo, K. H. Sung, C. C. Chiu and L. F. Wang, Mol. Pharm., 2013, 10, 664 CrossRef CAS PubMed.
- Y. S. Liu, C. C. Chiu, H. Y. Chen, S. H. Chen and L. F. Wang, Mol. Pharm., 2014, 11, 1164 CrossRef CAS PubMed.
- F. Dosio, S. Arpicco, B. Stella and E. Fattal, Adv. Drug Delivery Rev., 2016, 97, 204 CrossRef CAS PubMed.
- Y. L. Lo, H. L. Chou, Z. X. Liao, S. J. Huang, J. H. Ke, Y. S. Liu, C. C. Chiu and L. F. Wang, Nanoscale, 2015, 7, 8554 RSC.
- S. Liang, Y. Duan, Z. Xing, H. Han, A. Zhang, L. Li, Y. Yang and Q. Li, Colloids Surf., B, 2015, 136, 577 CrossRef CAS PubMed.
- H. Hermeking, Cell Death Differ., 2009, 17, 193 CrossRef PubMed.
- H. D. Um, Oncotarget, 2016, 7, 5193 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: CLSM images of CS-PAMAM/miR-34a transfection, CD44 expression level, cytotoxicity of CS-PAMAM and wound size analysis. See DOI: 10.1039/c6ra15716e |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2016 |