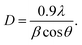Lignin-assisted solid-phase synthesis of nano-CuO for a photocatalyst with excellent catalytic activity and high performance supercapacitor electrodes†
Xiaohong Wang*a,
Fangsheng Wua,
Yawei Duana,
Yingying Wanga,
Chen Hao*a and
Cunwang Geb
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang, Jiangsu 212013, China. E-mail: chhao@ujs.edu.cn; xhwang@ujs.edu.cn; Fax: +86 511 88791800; Tel: +86 511 88791800
bSchool of Chemistry and Chemical Engineering, Nantong University, Nantong, Jiangsu 226019, China
First published on 5th July 2016
Abstract
CuO nanostructures were successfully synthesized using NaOH and Cu(NO3)2 as starting materials by an aminated lignin (AL)-assisted solid-phase method. The results of X-ray diffraction (XRD) analyses indicate that the prepared CuO nanoparticles have a monoclinic crystal structure with an abundance of mesopores. The emission scanning electron microscopy (SEM) images exhibit noticeable changes in morphology and size of the CuO samples with different content of AL. The CuO nanostructures were applied into the photocatalytic degradation of methylene blue (MB) and methyl orange (MO) under UV light irradiation. Meanwhile, the best photocatalytic activity with an AL dosage of 0.5 g and the calcination temperature at 400 °C shows almost complete degradation (∼97.83%) for MB and 66.76% for MO under UV light irradiation for 1.5 h, respectively. The supercapacitor behavior was characterized by cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy techniques. The maximum specific capacitances of CA3.0-400 are found to be 644.8 F g−1, much higher than other samples. However, it exhibits slightly worse cyclic stability compared with CA0.5-400.
Introduction
In recent years, the fabrication of high-efficiency photocatalysts and energy storage devices has received increased attention in the field of environmental remediation and renewable energy resources. Photocatalysts with well-defined morphologies, have great potential in the applications of degradation of harmful pollutants from industrial wastewater. In particular, the coloured dye emissions of textile and paper industries such as MB, acid red 14, remazol red RR, reactive blue 19 and MO, can easily lead to severe environmental contamination because of their toxicity and non-biodegradation.1,2 However, conventional biological treatment methods cannot be used efficiently for the degradation of dyes and organic pollutants.3 Hence, photocatalytic treatment of organic pollutants from water and air using semiconductors as photocatalysts has increased more and more extensive research due to their excellent decolorization and degradation efficiency.4–8 Additionally, supercapacitors (SCs), known as electrochemical capacitors, are recognized as promising energy storage devices because of its high power density, long cycle life, rapid charge/discharge rate and low maintenance cost than batteries.9,10Copper oxide (CuO) as an p-type important semiconductor with a narrow band-gap,11–13 has been studied extensively over recent decades due to its unique physicochemistry properties, which include excellent chemical stability, low toxicity, low cost, desirable optical and environmentally friendly.14,15 The morphology and size of CuO nanoparticles have a significant effect on their chemical and physical properties.16,17 Thus CuO has received widespread attention and has potential applications in magnetic storage media,18 photocatalysts,19 electro-optical devices,20 gas sensors,21 electrode materials,22 and nanodevices.23
A novel and simple solid-state reaction in the presence of AL has been developed to synthesize mesoporous CuO nanoparticles because of the following reasons: good homogeneity, ease of composition control, low processing temperature, large area coatings, low equipment cost, low pollution and good optical properties.24 Particularly, the solid-phase processes are efficient in preparing mesoporous copper oxide in accordance with the process route of our proposal.
Lignin is the third most abundant biopolymer on earth, exceeded only by cellulose and hemicelluloses.25–28 It can be acquired from wood during wood pulping and paper-making and has been used primarily as a bioresources for the raw material of the synthesis of environmentally compatible polymers.29 The comprehensive utilization of lignin not only economizes natural resources, but also helps preserve the ecological environment, which attracts enormous research interest. Amination modification has been considered as one important method of the conversion of lignin. The more reactive amino groups can be introduced onto lignin by an amination reaction, which can make the anionic lignin converted into the cationic lignin amine.30,31 Ion performance of AL is enhanced after ammonification. Ion dissociation from AL can produce competitive inhibition on the formation of the copper hydroxide precursor in the reaction system. Additionally, the addition of AL in reaction system can control the size of CuO nanoparticle effectively.32 As reported, the calcined AL also can be applied as the electrode material in lithium ion batteries,33 supercapacitors,34–36 and display outstanding electrochemical performance.
A facile synthesis method for CuO nanoparticles has been applied. In this approach, we directly prepare CuO precursor by the solid-state reaction between NaOH and Cu(NO3)2 with an AL template. Accordingly, the best experimental condition were acquired for the CuO catalysts. The CuO nanoparticles exhibited a good electrochemical performance in KOH electrolyte as well higher degradation efficiency than pure CuO. Therefore, the cationic lignin amine can be seen as a better alternative to the prevailing surfactants used in the synthesis of CuO.
Experimental section
Materials
In the preparation of CuO nanostructures, the following materials were used: sodium hydroxide (NaOH, AR), cupric nitrate (Cu (NO3)2·3H2O, AR), MB (AR), MO (AR) and deionized water; lignin was purchased from Guizhou Straw plant and purified by acidulate and coagulate using sulfate. AL was prepared by the grafting reaction of lignin and hexamethylenediamine. Additionally, the Ni foam was also cleaned by sonication in the acetone, ethanol and deionized water in sequence for 30 min, respectively, to be used for the substrate of the working electrode.Preparation of AL
The AL was prepared on the basis of our previous reports.30,31 In a typical step, 4 g of sodium lignin was dispersed in 5.5 mL of 0.4 M sodium hydroxide under vigorous stirring at room temperature, following by addition of 1.5 mL methanal and 4.8 g of hexamethylenediamine. After being stirred vigorously for 20 min, the obtained solution was refluxed at 65 °C for 3 h, then allowed to cool to room temperature and filtered. 10% potassium ferricyanide was used to precipitate the lignin absolutely, next, the as-formed precipitates were filtered and washed repeatedly with water and ethanol to remove the excess potassium ferricyanide, brown products can be obtained and later be dried in a vacuum oven at 90 °C for 10 h.Preparation of CuO
Mesoporous CuO nanoparticles were prepared using AL as the template. Briefly, 0.05 mol Cu(NO3)2·3H2O and 0.10 mol NaOH were ground for 15 min in an agate mortar respectively, then mixed together, afterwards, 0.5 g of AL was added into the mixture and were kept for further grinding, subsequently black colored precipitates were formed immediately, and which were washed with deionized water and ethanol several times and dried in an vacuum oven at 60 °C for 10 h. Finally, the CuO nanocomposites obtained were calcined at 300 °C, 400 °C and 500 °C for 2 h in muffle furnace (calcination temperatures mainly depend on photocatalyst active).The resultant samples were defined as CA0.5-300, CA0.5-400 and CA0.5-500, respectively. According to the different doping content of AL (0.0, 0.5, 1.0, 2.0 and 3.0 g) used during the same process, the products calcined at 400 °C were labeled as C-400, CA0.5 (unsintered), CA0.5-400, CA1.0-400, CA2.0-400 and CA3.0-400 sequentially. In addition, the defination of other resultant samples was the similar as the above and the formation mechanism of CA sample was also supplemented (shown in Fig. S1†).
Characterization of mesoporous CuO nanostructures
Fourier transform infrared spectroscopy (FT-IR) analysis was performed using Nicolet-470 UV-vis spectrophotometer. The crystallinity and crystal phases of CuO nanoparticles were determined by XRD using D8 Advance diffractometer equipped with Cu Kα radiation (λ = 0.154 nm, 2θ = 20–80°), operating at a generator voltage of 40 kV and a current of 250 mA. The morphology and chemical composition of CuO nanoparticles was analyzed by SEM (JEOL JSM-6390LV). Nitrogen adsorption–desorption experiments were carried out by using Quantachrome NOVA 4000e Surface Area and Pore Size Analyzer, and then the surface area, pore volume, and pore size were calculated by Brunauer–Emmett–Teller (BET) method and Barrett–Joyner–Halenda (BJH) model, respectively. The optical band gap was estimated from UV-vis diffuse reflectance spectroscopy (UV-Vis DRS) using a UV-2450 double beam spectrophotometer with BaSO4 as the reference. The photodegradation experiments were performed with a SGY-1 multifunctional photo reactor equipped with a 300 W high pressure mercury lamp, with a wavelengths of 365 nm.Electrochemical measurement
A standard three-electrode electrolytic cell was used for the electrochemical measurements with as-prepared samples as the working electrode, and a platinum electrode and a saturated calomel electrode as the counter and reference electrodes, respectively. All of the measurements were carried out in a 3 M KOH aqueous electrolyte solution on a CHI 660D electrochemical workstation. The cyclic voltammograms (CV) and galvanostatic charge–discharge (GCD) curves were obtained to determine the electrochemical properties. The electrochemical impedance spectroscopy (EIS) measurements were conducted by applying an AC voltage in the frequency range from 0.01 Hz to 100 kHz with an amplitude of 5 mV. The specific capacitance (Cm) of the electrode can be calculated from GCD curves according to the following equation:10
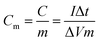 | (1) |
Photodegradation
MB is commonly adopted as a model organic pollutant to evaluate the photocatalytic performance of CuO.37,38 The experimental procedure was as follows, 0.1 g of the prepared CuO powders were dispersed into 1 L of MB aqueous solution with an initial concentration of 10 mg L−1, and the suspensions were ultrasonic stirred for 30 min for the sake of reaching the solubility equilibrium. The solutions were magnetically stirred in darkness for 20 min to achieve adsorption–desorption equilibrium between the CuO and the MB, after turning on the UV light, 6 mL of the suspension was periodically sampled every 10 min and centrifuged to remove the photocatalyst particles. The upper clear liquid was analyzed using a UV-vis spectrophotometer, and the characteristic absorption of MB at 662 nm was recorded to monitor the adsorption and degradation behavior. For comparison purpose, such as C-400, irradiating without photocatalysts were also performed under the same conditions. The percentage photodegradation was defined using the following equation:
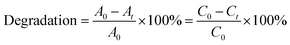 | (2) |
Results and discussions
XRD
Fig. 1 shows the XRD patterns of the CuO nanostructures with different AL doping mass and calcining temperature in air. The observed main peaks at 2θ values of 32.5°, 35.4°, 38.9°, 48.7°, 53.4°, 58.2°, 61.5°, 66.4°, 67.9° and 74.9° are indexed to the CuO crystal planes (110), (002), (111), (−202), (020), (202), (−113), (310), (220) and (004) respectively. All the diffraction peaks are fully matched with the nanocrystalline phase of CuO (JCPDS no. 5-0661) and no any other impurities such as Cu(OH)2 or Cu2O are observed during the measurement, indicating that the as-obtained CuO are extremely pure and well-crystalline. Typically, the crystallite size has been estimated from the XRD pattern using the Scherrer's equation:Where D is the average crystalline size, λ is the wavelength of Cu Kα radiation (λ = 0.15406 nm), β is the full width at half maximum of the diffraction peaks, and θ is the Bragg's angle. The average particle size of CuO was estimated to be around 25, 35, 18, 21, 27 and 24 nm for samples (corresponding to a, b, c, d, e and f, respectively).
By adding 0.5–3.0 g AL into CuO, the position of their diffraction peaks are still the same as that of pure CuO structure. Interestingly, with the increase of AL mass and calcination temperature, the diffraction peak intensity become more and more weak. Meanwhile, no remarkable shifts of any diffraction peaks are observed due to the variation in the CuO lattice parameters is negligible. The increase of lattice constant is due to phenolic hydroxyl and sulfonyl of AL are attached onto the surface of CuO nucleus. These diagrams exhibit that the diffraction peaks of the CuO nanoparticles depend on the calcination temperature, and increase with the increase of temperature.
As we all see, CA0.5-300 shows lower diffraction peaks and exhibits poorer crystallization than the other samples as a result of the residual of AL after calcination. Therefore, when the calcination temperature is higher, the intensity of the diffraction peak of the copper oxide becomes stronger, which indicates that the crystallization of the CuO is more complete and more comprehensive because of agglomeration.
The thermal behavior of CuO precursors was estimated by TGA and DTG measurements (shown in Fig. 2). As seen from Fig. 2A, the pure CuO precursor shows a distinct weight loss step (about 28.88%), which is attributed to removal of water from surface and dehydroxylation and decomposition of Cu(OH)2. For precursor of CA0.5 (shown in Fig. 2B), the result shows that the weight losses are about 6.83% and 19.06% between 90 and 310 °C respectively, which is due to the adsorbed water, interlayer water and partial decomposition of Cu(OH)2. The stage over 350–700 °C can be assigned to a continuous thermal depletion of deep-trapped hydroxyl groups and removal of carbon, and C–C bond cleavages between lignin units as well as aliphatic side chains of aromatic rings happen.39 Beyond 700 °C, the AL may be full carbonization.
SEM
Fig. 3 shows the SEM images of mesoporous CuO nanostructures obtained. It is clearly seen that nanoneedles are attached to sheet-like structure, and the size of CuO microsheet is in the range 110–200 nm from Fig. 3A. By comparing Fig. 3A, it is plain to see from Fig. 3B that the particle size of CuO sample decreases after AL is added. We could further find that the CuO has a large number of holes between the particles with about a diameter 10–30 nm. As shown in Fig. 3C, the particle sizes of CuO samples enlarge when dopant of AL increases. Meanwhile, the phenomenon of aggregation caused by the dopant of AL is more obvious and the quantity of interspace disappears rapidly. As discussed from the above, the concentration of AL is possible to have negative effects to the growth speed of CuO crystallites in different directions, and thus changes the morphology of the CuO nanostructures. With the increase of the calcination temperature, the average diameter largens and the crystallinity increases slightly (as shown in Fig. 3B and D), which can lead to loss of holes in a certain extent. The experimental SEM results show that AL doping could be conducive to making the CuO nanoparticles smaller because the remaining pores from the thermal decomposition of AL blocking the agglomeration of powders. | ||
| Fig. 3 SEM images of mesoporous CuO nanostructures obtained in the presence of AL with different mass: (A) 0 g at 400 °C (B) 0.5 g at 400 °C (C) 1.0 g at 400 °C (D) 0.5 g at 500 °C. | ||
BET
The porosity and textural parameters of the samples investigated by nitrogen adsorption and desorption experiments. The specific surface areas of the CA0.5-400 is 47.19 m2 g−1, higher than that of the C-400 (17.34 m2 g−1), as shown in Fig. 4A. According to the BDDT classification, all of the isotherms show type IV with H3 hysteresis loop, revealing that abundant mesoporous structures exist in the as-prepared CuO materials. The pore size distribution calculated from the desorption isotherm by using the BJH method displays that the CA0.5-400 has an average pore size of 17.43 nm. On the other side, for C-400, the average pore size is calculated to be about 3.84 nm, as shown in Fig. 4B. To clarify the influence of the recruitment of AL, the BET surface area of the other nanocomposites are found to be 25.28, 31.74, 21.75 and 27.73 m2 g−1 for CA0.5-300, CA1.0-400, CA2.0-400, and CA0.5-500, respectively. The results may attributed to the disaggregation of AL and appropriate calcination temperature. The mesoporous CuO nanocomposites can provide more binding site to accelerate diffusive transport of active species in photocatalytic process. | ||
| Fig. 4 (A) Nitrogen adsorption–desorption isotherms of C-400 (a) and CA0.5-400 (b). Inset: (B) BJH pore-size distribution plot of C-400 (a) and CA0.5-400 (b). | ||
UV-vis
The optical properties of the samples are investigated by the UV-vis absorption spectra analysis, as displayed in Fig. 5. It can be clearly observed that the broad absorption peek at 300 nm, 360 nm and 550 nm respectively, revealing strong light absorption in the UV region because transitions of electrons in an excited electronic state from the valence band to the conduction band occur under UV irradiation. The more the weight percentage of AL in the samples is, the weaker the light absorption capacity of the CuO is. This absorbance band can be attributed to the plasmon resonance absorption of CuO nanostructures.40 It is noticeable that the appropriate AL adding into CuO lead to a red shift in the optical absorption edge.For crystalline semiconductors, a classical approach is employed to calculate the optical band gap energy (Eg) following the equation:41
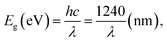 | (3) |
FT-IR
FT-IR spectra of the CuO samples are shown in Fig. 6 (seen in Fig. S3 and S4†). We can see that the organic groups exist in large quantity before calcination. The weak absorption band appeared at 3435 cm−1 is assigned to the stretching vibration of the O–H bond, which can be attributed to the stretching vibration of the physisorbed water and undecomposed AL. The peak at 1630 cm−1 is due to –OH bending vibration, and the peak at 1380 cm−1 may be caused by the Cu–OH in-plane bending vibration, the presence of strong peak at 530 cm−1, which is corresponded to monoclinic phase of CuO. No characteristic peaks for impurity are observed from C-500, indicating that all the organic molecules could be removed completely from the AL/CuO mixture after calcination at 500 °C, and the well-crystallized CuO structure is obtained at this temperature.Photocatalytic activity test
Photocatalytic performance is effective for the catalytic degradation of organic dyes, which typically require the assistance of excellent semiconductor photocatalyst, such as TiO2, ZnO, SnO2 and so forth. And the dispersion case of CuO, C-500, CA0.5 and CA0.5-500 in the aqueous medium is shown in Fig. S2.† In order to understand the photocatalytic effect in details, we schematically represent schematic illustration of the probable electron transfer process in the CuO system when illuminated and it is shown in Fig. 7. As we can see from the picture, UV-light from mercury lamp generates electron–hole pair in CuO and the holes in the VB of CuO can react with water adhering to the surface to convert the OH− into hydroxyl radical ˙OH, which could be changed into CO2 and H2O by the MB or MO molecules. Whilst the photoexcited electrons in the CB of CuO are captured by dissolved O2 only to yield highly oxidative ˙O2− and which in turn produces ˙OH.42,43 The ˙OH and ˙O2− radicals can cause the rupture of the cyclic structures of MB or MO molecules and subsequent total mineralization.44Fig. 8A represents the time dependent photodegradation rate of MB in the presence of the CuO catalysts, respectively. It is evident that the photodegradation of MB in the presence of AL-doped CuO was significantly quicker than that in the presence of CuO undoped, and the catalytic efficiency of CuO decrease gradually with the increasing of AL-doped. Fig. 8C shows the photodegradation ratio of MB in the presence of CuO-doped with 0.5 g AL at different calcination temperatures. The CuO which is calcined at 400 °C exhibited higher photodegradation rate than what is calcined at others temperature, moreover, the difference is very evident. This result can be explained that lower temperature cannot sustain the complete decomposition of AL and higher temperature induces the agglomeration of particle. Thus, the grain-size is smaller and the specific surface area is larger at 400 °C, which may be the reason of high activity for the removal of the dyes in water. In summary, the suitable calcination temperature and AL dopant both play a crucial role in the formation of the CuO and improve the catalytic properties of CuO catalyst effectively.
Through the comparison of Fig. 8 and 9, the degradation efficiency for MB is apparently higher than that for MO. The highest degradation rate was observed for CA0.5-400, and the efficiency values were 97.83% and 66.76% respectively for MB and MO. For comparative purpose, an identical experiment under UV light illumination was conducted without photocatalyst. However, MB and MO degradation are relatively slow, indicating that MB and MO are also stable molecular and the photolysis can be ignored. Based on the above experimental results and probable photocatalysis mechanism, we can conclude that CuO photocatalyst could efficiently improve the photocatalytic degradation of MB and MO, which is obviously attributed to the these photogenerated hole–electron pairs react with water and adsorbed oxygen to form and O2− with powerful oxidizability,45,46 and react with the dyes adsorbed on the surface of CuO.
In addition, the linear plots in Fig. 8B and D and 9B and D display that the photodegradation of MB and MO by CuO under UV irradiation followed pseudo first-order kinetic reaction. The rate constants of the samples in removal of MB and MO can be well described by Langmuir equation:
| −ln(Ct/C0) = kt | (4) |
| Sample | k/min−1 (MB) | R2 (MB) | k/min−1 (MO) | R2 (MO) |
|---|---|---|---|---|
| C-400 | 0.01300 | 0.99691 | 0.00415 | 0.99243 |
| CA0.5-400 | 0.03667 | 0.97980 | 0.01291 | 0.98552 |
| CA1.0-400 | 0.02941 | 0.98772 | 0.00944 | 0.99251 |
| CA2.0-400 | 0.02007 | 0.99547 | 0.00702 | 0.99640 |
| CA3.0-400 | 0.01416 | 0.99192 | 0.00585 | 0.99464 |
| CA0.5-300 | 0.02355 | 0.99586 | 0.00649 | 0.97764 |
| CA0.5-500 | 0.01955 | 0.97055 | 0.00867 | 0.98436 |
To evaluate the electrochemical properties of the as-prepared samples, cyclic voltammetry and galvanostatic charge–discharge were conducted in 3 M KOH electrolyte. As shown in Fig. 10A, all CV curves show typical pseudocapacitive characteristics with distinct redox peaks in the potential window of 0–0.5 V at a scan rate of 5 mV s−1, which could be attributed to the redox (Cu2+ to Cu+ and Cu+ to Cu2+) process.47 It is can be noticed that the dopant of AL is increased, the shape of the CV changes, and the potentials of the anodic and cathodic peaks shift towards more positive and negative directions, respectively. Especially for the CA3.0-400 sample, the anodic peak appears at about 4.5 V when the dopant of AL increases to 3.0 g and the specific capacitance significantly improves. This may be ascribed to carbon particles from the carbonization of residual AL in high temperature.36,37 Chen et al.48 have reported the self-assembly of NiO nanoparticles embedded in lignin-derived mesoporous carbon (MPC) frameworks for supercapacitor applications, demonstrating a high specific capacitance (880.2 F g−1 at a current density of 1.0 A g−1) with enhanced rate capability and cycle stability. The galvanostatic charge–discharge curves of the CuO samples at a current density of 1 A g−1 are shown in Fig. 10B. The specific capacitances of C-400, CA0.5-400, CA1.0-400, CA2.0-400 and CA3.0-400 are 230, 330.4, 436.6, 510.4 and 644.8 F g−1 using the eqn (1), respectively. It can be seen that the AL-doped CuO samples exhibit remarkably enhanced specific capacitance compared to that of pure CuO, which is in good agreement with the CV measurement. Furthermore, the specific capacitances of the CuO samples are further changed by varying the mass of AL to starch in the range of 0 to 3.0, corresponding to Fig. 11A. Cycling stability of the CA samples is further investigated by charging–discharging the electrode between 0 and 0.5 V at a current density of 1 A g−1 as shown in Fig. 11B. From these data plots, it is seen that for all CuO samples the supercapacitances suddenly decrease and remain 57.4, 88.4, 81.3, 76.6 and 69.3% after 1000 cycles, indicating that electrochemical stabilities are not straight increased by addition of AL and can be seen from Fig. 11B. The superior capacitance retention of the CA0.5-400 is attributed to big active specific surface areas and appropriate pore volumes, and then accelerates the diffusion of OH ions. The CA3.0-400 electrode presents inferior cyclic voltammetry performance, which may cause by the instability and easy collapse of the structure. The carbon desultorily existed into the CuO nanoparticles can overcome the poor conductivity of metal oxide, but cannot strengthen the stability of the structure.
 | ||
| Fig. 10 (A) Cyclic voltammetry curves, (B) galvanostatic charge–discharge curves of the CA-400 electrodes in 3 M KOH electrolyte. | ||
 | ||
| Fig. 11 (A) Specific capacitance, (B) cycling performance of the CA-400 electrodes at current density of 1 A g−1, and (C) Nyquist plots for the CA-400 electrodes at open circuit potential. | ||
The electrochemical impedance spectroscopy further commendably supports the above results, the Nyquist plots are shown in Fig. 11C. All electrodes show a semicircle at high frequencies followed by a linear slope at the medium or low frequency region. The depressed semicircle in the high frequency region represents the kinetic resistance to the ion transfer at the electrode–electrolyte interface, which can be attributed to the Cu2+/Cu+ redox reaction. The steeper line at low frequency region demonstrates the capacitive nature of the electrode.49,50 And compared with any other samples, the CA0.5-400 electrode exhibits a better charge transfer resistance and favorable internal resistance, suggesting a higher electrical conductivity, and a larger electro-active surface area. The above results, the CA0.5-400 of choice for ideal supercapacitor electrode.
Conclusions
A simple and eco-friendly solid-phase method for synthesizing mesoporous CuO with AL as a template was put forward in this study. The AL-doped amount and calcination temperature may be responsible for the sizes, morphologies, microstructures, electrochemical performance and photocatalytic performances of the resulting CuO catalysts. The addition of template AL is advantageous to preparation of CuO with a small particle size in the solid state reaction system. The as-prepared CuO photocatalysts showed excellent catalytic and electrochemical properties because of smaller particle size, large specific surface area. Because the solid phase method is simple and cheap, as well as the low price of the AL derived from industrial waste. Summarized by the research above, the CA nanoparticles with excellent catalytic and electrochemical performances are promising to be used as a commercial catalyst for dye wastewater and advanced electrode materials for supercapacitors.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (61571245), the Open Project Program of State Key Laboratory of Analytical Chemistry for Life Science (Nanjing University) (KLACLS1010), the Program of Department of Education of Jiangsu Province (12KJD610003), the Natural Science Foundation of Jiangsu Province (BK20131249), and the Senior Personnel Scientific Research Foundation of Jiangsu University (15JDG084) for financial support of this research.References
- K. Mageshwari and S. S. Mali, Powder Technol., 2013, 249, 456 CrossRef CAS.
- R. Saravanan, S. Karthikeyan and V. K. Gupta, Mater. Sci. Eng., C, 2013, 33, 91 CrossRef CAS PubMed.
- A. Sadollahkhani, Z. H. Ibupoto, S. Elhag, O. Nur and M. Willander, Ceram. Int., 2014, 40, 11311 CrossRef CAS.
- S. Q. Wei, Y. Y. Chen, Y. Y. Ma and Z. C. Shao, J. Mol. Catal. A: Chem., 2010, 331, 112 CrossRef CAS.
- Y. C. Hong, G. B. Tian, B. J. Jiang, A. P. Wu, Q. Zhang, G. H. Tian and H. G. Fu, J. Mater. Chem. A, 2013, 1, 5700 CAS.
- G. H. A. Bagheri, M. Sabbaghan and Z. Mirgani, Spectrochim. Acta, Part A, 2015, 137, 1286 CrossRef PubMed.
- S. G. Kumar and K. S. R. K. Rao, RSC Adv., 2015, 5, 3306 RSC.
- L. G. Devi and R. Kavitha, RSC Adv., 2014, 4, 28265 RSC.
- S. Zhu, M. Wu, M. H. Ge, H. Zhang, S. K. Li and C. H. Li, J. Power Sources, 2016, 306, 593 CrossRef CAS.
- F. L. Lai, Y. E. Miao, Y. P. Huang, T. S. Chung and T. X. Liu, J. Phys. Chem. C, 2015, 119, 13442 CAS.
- W. Z. Wang, Y. Zhuang and L. Li, Mater. Lett., 2008, 62, 1724 CrossRef CAS.
- Y. Q. Wang, T. T. Jiang, D. W. Meng, Y. Jun, Y. C. Li, Q. Ma and J. Han, Appl. Surf. Sci., 2014, 317, 414 CrossRef CAS.
- X. Q. Liu, Z. Li, Q. Zhang, F. Li and T. Kong, Mater. Lett., 2012, 72, 49 CrossRef CAS.
- A. Nezamzadeh-Ejhieh and M. Amiri, Powder Technol., 2013, 235, 279 CrossRef CAS.
- M. M. Rashad, S. Soltan, A. A. Ramadan, M. F. Bekheet and D. A. Rayan, Ceram. Int., 2015, 41, 12237 CrossRef CAS.
- M. Rabbanin, R. Rahimi and M. Bozorgpour, Mater. Lett., 2014, 119, 39 CrossRef.
- L. J. Wang, Q. Zhou and Y. J. Liang, Appl. Surf. Sci., 2013, 271, 136 CrossRef CAS.
- T. Arbuzova, B. Gizhevskii, S. Naumov, A. Korolev, V. Arbuzov, K. Shalnov and A. Druzhkov, J. Magn. Magn. Mater., 2003, 258/259, 342 CrossRef.
- H. Jiang, H. Endo, H. Natori, M. Nagai and K. Kobayashi, Mater. Res. Bull., 2009, 44, 700 CrossRef CAS.
- Y. Lu, X. M. Liu, K. W. Qiu, J. B. Cheng, W. X. Wang, H. L. Yan, C. C. Tang, J. K. Kim and Y. S. Luo, ACS Appl. Mater. Interfaces, 2015, 7, 9682 CAS.
- D. D. Li, J. Hu, R. Q. Wu and J. G. Lu, Nanotechnology, 2010, 21, 485 Search PubMed.
- H. X. Zhang and M. L. Zhang, Mater. Chem. Phys., 2008, 108, 184 CrossRef CAS.
- A. Jegatha Christy, L. C. Nehru and M. Umadevi, Powder Technol., 2013, 235, 783 CrossRef.
- Z. F. Liu, Z. G. Jin, W. Li and J. J. Qiu, Mater. Lett., 2005, 59, 3620 CrossRef CAS.
- M. Wu, D. H. Zhao, J. H. Pang, X. M. Zhang, M. F. Li, F. Xu and R. C. Sun, Ind. Crops Prod., 2015, 66, 123 CrossRef CAS.
- W. L. Xiong, D. J. Yang, R. S. Zhong, Y. Li, H. F. Zhou and X. Q. Qiu, Ind. Crops Prod., 2015, 74, 285 CrossRef CAS.
- Z. P. Yan, J. H. Li, S. Chang, T. Cui, Y. Jiang, M. H. Yu, L. Zhang, G. Zhao, P. L. Qi and S. Z. Li, Fuel, 2015, 158, 152 CrossRef CAS.
- Y. Z. Wang, D. Sudipta and N. Yan, Chem. Commun., 2016, 52, 6210 RSC.
- Y. R. Guo, F. D. Yu, G. Z. Fang and Q. J. Pan, J. Alloys Compd., 2013, 552, 70 CrossRef CAS.
- X. H. Wang, Y. K. Zhang, C. Hao, F. Feng, H. B. Yin and N. C. Si, Ind. Eng. Chem. Res., 2014, 53, 6585 CrossRef CAS.
- F. Feng, C. Hao, H. L. Zhang, W. J. Xie, X. H. Wang and Y. T. Zhao, J. Mater. Sci.: Mater. Electron., 2015, 26, 6704 CrossRef CAS.
- M. Umetsu, X. Man, K. Okuda, M. Tahereh, S. Ohara and J. Zhang, Chem. Lett., 2006, 35, 732 CrossRef CAS.
- W. L. Zhang, J. Yin, Z. Q. Lin, H. B. Lin, H. Y. Lu, Y. Wang and W. M. Huang, Electrochim. Acta, 2015, 176, 1136 CrossRef CAS.
- S. X. Hu, S. L. Zhang, N. Pan and Y. L. Hsieh, J. Power Sources, 2014, 270, 106 CrossRef CAS.
- H. Li, D. Yuan, C. H. Tang, S. X. Wang, J. T. Sun, Z. B. Li, T. Tang, F. K. Wang, H. Gong and C. B. He, Carbon, 2016, 100, 151 CrossRef CAS.
- C. L. Lai, Z. P. Zhou, L. F. Zhang, X. X. Wang, Q. X. Zhou, Y. Zhao, Y. C. Wang, X. F. Wu, Z. T. Zhu and H. Fong, J. Power Sources, 2014, 247, 134 CrossRef CAS.
- J. Liang, H. Tan, C. H. Xiao, G. J. Zhou, S. W. Guo and S. J. Ding, J. Power Sources, 2015, 285, 210 CrossRef CAS.
- T. T. Jiang, Y. Q. Wang, D. W. Meng and M. H. Yu, Superlattices Microstruct., 2015, 85, 1 CrossRef CAS.
- Y. Q. Wang, T. T. Jiang and D. W. Meng, Appl. Surf. Sci., 2015, 355, 191 CrossRef CAS.
- M. Umadevi and A. J. Christy, Spectrochim. Acta, Part A, 2013, 109, 133 CrossRef CAS PubMed.
- J. C. Sin, S. M. Lam, K. T. Lee and A. R. Mohamed, J. Mol. Catal. A: Chem., 2015, 409, 1 CrossRef CAS.
- P. Wilhelm and D. Stephan, J. Photochem. Photobiol., A, 2007, 185, 19 CrossRef CAS.
- M. W. Xiao, L. S. Wang, Y. D. Wu, X. J. Huang and Z. Dang, Nanotechnology, 2008, 19, 015706 CrossRef PubMed.
- S. P. Meshram, P. V. Adhyapak, U. P. Mulik and D. P. Amalnerkar, Chem. Eng. J., 2012, 204, 158 CrossRef.
- X. D. Lou, J. Han, W. F. Chu, X. Jia, J. W. Wang, Y. Y. Liu and W. H. Tang, Mater. Sci. Eng., B, 2007, 137, 268 CrossRef CAS.
- S. G. Kumar and L. G. Devi, J. Phys. Chem. A, 2011, 115, 13211 CrossRef CAS PubMed.
- S. K. Shinde, D. P. Dubal, G. S. Ghodake and V. J. Fulari, RSC Adv., 2015, 5, 4443 RSC.
- F. Chen, W. J. Zhou, H. F. Yao, P. Fan, J. T. Yang, Z. D. Fei and M. Q. Zhong, Green Chem., 2013, 15, 3057 RSC.
- Y. Fan, P. F. Liu and Z. J. Yang, Ionics, 2015, 21, 185 CrossRef CAS.
- S. E. Moosavifard, M. F. El-Kady, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, ACS Appl. Mater. Interfaces, 2015, 7, 4851 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11911e |
| This journal is © The Royal Society of Chemistry 2016 |