Organic corrosion inhibitors for aluminium and its alloys in acid solutions: a review†
Klodian Xhanari
ab and
Matjaž Finšgar
*a
aUniversity of Maribor, Faculty of Chemistry and Chemical Engineering, Smetanova ulica 17, 2000 Maribor, Slovenia. E-mail: matjaz.finsgar@um.si; Tel: +386 2 2294 447
bUniversity of Tirana, Faculty of Natural Sciences, Boulevard “Zogu I”, 1001 Tirana, Albania
First published on 22nd June 2016
Abstract
The aim of this review is to summarise the research work published in the last two decades on the use of organic compounds as corrosion inhibitors for aluminium and its alloys in acidic solutions. The focus is on HCl and H2SO4 solutions due to their extensive use in different applications, such as chemical and electrochemical etching, acid cleaning, anodising, and acid pickling of aluminium. Other acids are also reviewed. The inhibition effectiveness of numerous organic compounds and their possible inhibition type and mechanism are also discussed. Electrochemistry is mainly used to investigate inhibitor performance.
1. Introduction
Aluminium is usually considered to be the second most important metal, after iron. Aluminium is the third most common metal in the earth's crust and the fourth most common metal in general. The special mechanical properties of aluminium are related to its low density (2.7 g cm−3), which is slightly less than one third of the density of common steel. Therefore, aluminium and its alloys provide a high ratio of strength to weight. Moreover, they have high electrical and thermal conductivity and can be easily treated with a large number of metal machining techniques. In addition, recycling and reuse of aluminium and aluminium alloys is complete, which means that the recycled metal and products obtained are comparable in quality and characteristics to those before recycling.1 Finally, the price of aluminium and its alloys is relatively low. All these properties make aluminium and its alloys one of the most commonly used metals in a broad range of applications, including transport, building, packaging, electrical parts, the automotive industry, household appliances, etc.2,3Aluminium is highly corrosion resistant to the atmosphere and in aqueous solutions due to the formation of an oxide layer, which does not allow further oxidation of the metal. However, in certain environments, such as an acidic medium, aluminium becomes highly susceptible to corrosion. Different methods of mitigating corrosion are being sought. The use of corrosion inhibitors for closed system applications is one of the most effective and practical methods of corrosion protection.
The primary focus of this review is to summarise the research work presented in the past two decades related to the use of organic compounds as corrosion inhibitors for aluminium and its alloys in acidic solutions. Acidic solutions, HCl and H2SO4, in particular, are widely used in several processes, including acid cleaning, chemical or electrochemical etching, acid pickling, and anodising of aluminium.4–8 To the best of our knowledge, the last review article addressing organic corrosion inhibitors was presented by Jayalakshmi and Muralidharan9 in 1997. Therefore, in this review we only discuss research work published after 1998.
2. Aluminium alloys
Aluminium alloys are generally divided into two main groups, alloys that are non-heat treatable (1xxx, 3xxx, 4xxx, and 5xxx series) and alloys that are heat treatable (2xxx, 6xxx, and 7xxx series). The aluminium alloys that are non-heat treatable are generally considered to have a high resistance to general corrosion. Table 1 presents the composition and properties of aluminium alloys.2,10| Series | Structure | Tensile strength (MPa) | Melting temperature (°C) | Density (kg m−3) | Thermal conductivity at 20 °C (W m−1 K−1) | Electrical resistance at 20 °C (10−3 μΩ m) |
|---|---|---|---|---|---|---|
| 1xxx | Al | 70–175 | 645–660 | 2700–2720 | 225–243 | 26.7–33.9 |
| 2xxx | Al–Cu–Mg (1–2% Cu) | 170–310 | 502–645 | 2760–2840 | 113–193 | 34–62 |
| 2xxx | Al–Cu–Mg–Si (3–6% Cu) | 380–520 | ||||
| 3xxx | Al–Mn–Mg | 140–280 | 629–657 | 2710–2730 | 159–193 | 34–43 |
| 4xxx | Al–Si | 105–350 | 575–630 | 2680 | 163 | 40 |
| 5xxx | Al–Mg (1–2% Mg) | 140–280 | 574–657 | 2640–2710 | 117–201 | 32–59 |
| 5xxx | Al–Mg–Mn (3–6% Mg) | 280–380 | ||||
| 6xxx | Al–Mg–Si | 150–380 | 570–655 | 2690–2710 | 153–218 | 29–43 |
| 7xxx | Al–Zn–Mg | 380–520 | 477–645 | 2780–2830 | 137–180 | 36–52 |
| 7xxx | Al–Zn–Cu–Mg | 520–620 |
Alloys of the 1xxx series are technically pure and contain at least 99 wt% aluminium. They have lower strength compared to other aluminium alloys. Alloys of the 3xxx series (Al–Mn–Mg and Al–Mn alloys) have similar characteristics as the 1xxx series, but higher strength. Alloys of the 4xxx series (Al–Si alloys) have lower strength and are used for brazing and welding products and for cladding in architectural products. Silicon has a very small effect on the corrosion behaviour of these alloys. Alloys of the 5xxx series (Al–Mg) have the highest strength among the non-heat treatable aluminium alloys. These alloys are, in comparison with others, more resistant to general corrosion in weakly alkaline media. They are mainly used for decorative building elements.
Among the heat treatable alloys, the alloys of the 6xxx series (Al–Mg2Si) have moderate strength compared to other aluminium alloys, but they have general corrosion resistance similar to non-heat-treatable alloys. Alloys of the 6xxx series are very often used in products for the automotive industry. Alloys of the 7xxx series, which do not contain copper, are also resistant to general corrosion.
Other non-heat treatable alloys have a significantly lower resistance to general corrosion. This applies for alloys of the 2xxx series (Al–Cu, Al–Cu–Mg, Al–Cu–Si–Mg) and alloys of the 7xxx series (Al–Zn–Mg–Cu) that contain copper. The strength of these alloys is significantly higher due to the presence of copper, making them suitable for use in aerospace technology applications.
3. Corrosion of aluminium
The corrosion resistance of ultra-pure aluminium is higher than that of aluminium, which contains a certain degree of other elements. The high corrosion resistance of aluminium is due to the formation of an oxide layer on the aluminium surface. The oxide layer is stable in a pH interval of 5 to 8.5 (most stable at pH 5), as can be seen in the Pourbaix diagram in Fig. 1. The material's passivity can change with temperature, the previous passivation process of the metal, as well as the presence of substances, which can form aluminium-soluble complexes or insoluble salts. In acidic medium, the aluminium dissolves into Al3+, according to the following equation:| Al → Al3+ + 3e− | (1) |
| Al(OH)3(aq) + 3H+ → Al(aq)3+ + 3H2O | (2) |
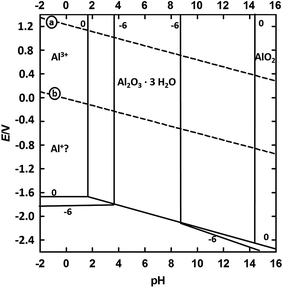 | ||
| Fig. 1 Pourbaix diagram for the system Al/H2O at 25 °C.2,3,11 | ||
The most important feature of aluminium corrosion susceptibility is the formation of an oxide layer that strongly binds on its surface. The thickness of the oxide layer formed in air at room temperature is about 5–10 nm. Fig. 2 shows how the natural oxide layer, which is composed of two layers, is structured when formed under atmospheric conditions. The very first layer is a compact amorphous protective layer of oxide, which acts as a barrier. This protective barrier is formed on the aluminium surface within milliseconds of exposure to air. A thicker porous layer is formed on top of this inner layer. The chemical properties of these oxides affect the corrosion properties of aluminium and its alloys.2,3,11
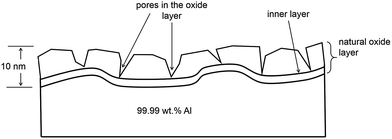 | ||
| Fig. 2 Natural oxide layer formed on the aluminium surface.2 | ||
Aluminium and its alloys often have a more negative corrosion potential than many metals and alloys. Therefore, the electrical contact of aluminium or aluminium alloys with other metals that have a significantly higher corrosion potential in a certain medium would result in galvanic corrosion.12 Another serious type of aluminium corrosion is intergranular corrosion. This type of corrosion spreads at grain boundaries. Crystal grains are not affected as they act as a cathode, while the grain boundaries of a corrodible material act as the anode.2,10 Exfoliation is a special form of intergranular corrosion. It can take place along grain boundaries. Corrosion products that form in the crystal grain boundaries can cause friction between the grain boundaries and, consequently, peeling, swelling, and the lifting of layers of material. Exfoliation occurs particularly in the products in which the metal is used in thin layers or plates. Another serious corrosion type of aluminium is pitting corrosion, which is especially dependent on the alloy type and corrosive medium. This type of corrosion will occur already after a few weeks of permanent contact of aluminium (or an alloy of aluminium) with a certain solution. This type of corrosion is especially frequent in chloride solutions.
4. Corrosion protection
There are several methods to protect aluminium and its alloys, among which the anodising process, conversion coatings, paints, organic coatings, and corrosion inhibitors are found to be the most effective.Anodising is an electrochemical process to increase the thickness of aluminium oxide, where aluminium metallic material is connected as an anode in the electrolytic cell. The anodised oxide layer is thus thicker (5–30 μm) than the natural oxide layer.2,3,10,13,14
Conversion coating consist of poorly soluble phosphate or chromium oxides, which are tightly bound to the surface of aluminium or its alloys.15–17 These coatings affect the appearance, corrosion potential, electrical resistance, and other surface properties. Conversion coatings are formed via a chemical oxidation–reduction process on the metal surface. However, the drawback of these coatings and the accompanying procedures is their negative environmental impact.2 Therefore, in recent years increasing interest has been shown in conversion coatings containing cerium18–23 and metallic ions24–28 or their combination.29,30
As mentioned above, another method of corrosion protection is to employ organic coatings. Before applying the organic coating, the aluminium surface should be cleaned and prepared. The process consists of degreasing the surface, followed by removal of the existing oxide layer and the application of the base layer and the primer. The surface cleaning and treatment influences the adhesion and anticorrosion properties of these coatings.31,32 The final layer is selected depending on the environmental conditions in which the product will be used.33–36 The use of organic–inorganic coatings in the protection of aluminium and its alloys has been also studied.37–39
4.1 Corrosion inhibitors
Corrosion inhibitors modify the metal surface either by forming insoluble compounds with aluminium or by forming a protective surface layer. Based on the electrochemical mechanism of action, the inhibitors can be classified as mixed-type inhibitors (inhibiting both cathodic and anodic reactions of the corrosion couple), anodic-type inhibitors (inhibiting the anodic reaction), or cathodic-type inhibitors (inhibiting the cathodic reaction). Inhibitors may bind on the surface of the metal by chemisorption or physisorption. They are mainly used to protect aluminium in closed systems, such as cooling systems in car engines, solar cells, etc. Of particular importance is the proper choice of an inhibitor according to the environment that the aluminium is exposed to.2,10 Chromates, nitrates, molybdates, tungstates, phosphates, and hydrogen phosphates are among the most tested inorganic inhibitors in the acidic corrosion of aluminium and its alloys.40–44 Organic compounds have found great use as corrosion inhibitors for metals in general, and of aluminium and its alloys in particular. These compounds adsorb on the metal surface through the heteroatoms N, S, O, and P, which serve as active centres for adsorption. The surface layer formed as a result of the adsorption protects the metal from further attack from the corrosive environment.5. Organic corrosion inhibitors
In the text that follows we discuss the research work performed in the last two decades on the inhibition of acidic corrosion of aluminium and its alloys by organic corrosion inhibitors. The use of the corrosion inhibitors is presented based on the acid type and within these sections we follow the trend of decreasing content of aluminium in the material. The summary of the findings, with a focus on the evaluation of inhibition effectiveness, are presented in Table S1 (in the ESI†). The minimum and maximum reported values of inhibition effectiveness are given. The aluminium or aluminium alloy type, corrosive medium (pH is given if reported), corrosion inhibitor and its concentration range, and corrosion testing methods are also given in Table S1.† As given below, weight loss (WL), linear sweep voltammetry (LSV) (including polarisation resistance (Rp), potentiodynamic (PDP), potentiostatic (PSP), and galvanostatic (GSP) polarisation), and electrochemical impedance spectroscopy (EIS) were among the most common methods used for evaluating corrosion inhibition effectiveness. Aluminium alloys with different chemical composition were investigated. In most of the cases, the authors do not designate the alloy type tested. In addition, a considerable change in the percentage of the elements can sometimes be observed for alloys with the same designation obtained from different manufacturers. Therefore, the chemical composition of the aluminium alloys tested, including their designation as given by the authors, is presented in Table 2.| Designation | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Ga | Ni | Zr | V | C | Ca | Pb | Ag | Li | Be | Na | Sr | In | Other | Al | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| — | ≤0.55 | ≤0.05 | ≤0.05 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.00 | 90 | |
| — | 0.0596 | 0.0260 | 0.0001 | 0.0002 | — | 0.0006 | 0.0124 | 0.0017 | — | — | — | — | — | — | — | — | 0.0004 | — | — | — | — | — | Balance | 64 |
| — | 0.25 | 0.35 | 0.05 | 0.03 | 0.03 | — | 0.05 | 0.03 | — | — | — | 0.05 | — | — | — | — | — | — | — | — | — | — | Balance | 84 and 114 |
| — | 0.03 | — | 0.02 | 0.02 | 0.03 | — | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.89 | 65, 128 and 130 |
| — | 0.03 | — | 6.00 | 0.02 | 0.03 | — | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 93.91 | 128 and 130 |
| — | 6.00 | — | 0.02 | 0.02 | 0.03 | — | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 93.91 | 128 and 130 |
| — | 0.60 | — | 0.25 | 0.15 | 1.00 | 0.25 | 0.25 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 97.50 | 96 |
| — | 0.50 | — | 4.50 | 0.60 | 1.50 | 0.10 | 0.25 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 92.25 | 96 |
| — | 0.4830 | 0.1799 | 0.0008 | 0.0083 | 0.4051 | 0.0040 | 0.0165 | 0.0145 | — | 0.0047 | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 160 and 162 |
| — | 0.05 | — | 0.05 | 0.05 | 0.05 | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.79 | 67 | |
| — | 0.02 | — | 0.01 | 0.002 | 0.02 | — | 0.005 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.95 | 60 |
| — | 0.50 | — | 0.10 | 0.10 | 0.10 | — | 0.10 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 88 and 91 | |
| — | — | 0.35 | — | — | — | — | — | — | — | — | — | — | 0.28 | 0.11 | — | — | — | — | — | — | — | — | 99.25 | 82 |
| — | 0.22851 | 0.578280 | 0.008090 | 0.27315 | 0.0731 | 0.005732 | 0.10291 | 0.01229 | — | 0.00548 | — | 0.01229 | — | — | 0.07663 | — | — | — | — | — | — | — | Balance | 61 |
| — | 0.07 | 0.31 | 0.0007 | 0.0021 | 0.0019 | 0.0003 | 0.0016 | 0.015 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.57 | 70–72 and 171 |
| — | 0.20 | 0.50 | 0.10 | — | — | — | 0.05 | 0.005 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 85, 86 and 89 |
| — | — | — | 2.5 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 152 |
| — | — | — | 7.0 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 152 |
| — | 0.634 | 0.099 | 4.320 | 0.223 | 0.210 | — | — | — | — | — | — | — | — | — | 0.072 | — | — | — | — | — | — | — | Balance | 143 |
| — | 0.2 | 0.5 | 0.1 | — | — | — | 0.005 | 0.005 | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 83 | |
| — | 0.15 | 0.19 | 0.02 | 0.005 | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.535 | 74 and 76–78 |
| — | 0.40 | 0.40 | 0.10 | 0.50 | 3.10 | 0.30 | 0.20 | 0.15 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 129 |
| — | 0.50 | 0.50 | 3.80 | 0.30 | 1.20 | 0.10 | 0.25 | 0.15 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 155 |
| — | 0.157 | 0.282 | 0.0025 | 0.024 | 0.51 | 0.023 | — | 0.006 | — | — | 0.002 | 0.0035 | — | 0.0011 | — | — | — | — | — | — | — | — | Balance | 140 |
| — | 0.80 | 0.70 | 0.15 | 0.15 | 1.20 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 97 |
| — | 0.100 | 0.250 | — | 0.047 | 0.007 | 0.008 | 0.003 | 0.007 | 0.012 | — | 0.001 | 0.007 | — | — | — | — | — | — | — | — | — | — | 99.555 | 73 |
| — | 0.1 | 0.05 | 0.01 | 0.006 | 0.005 | — | — | 0.002 | 0.005 | 0.01 | — | — | — | — | 0.03 | — | — | — | 0.001 | — | — | 0.007 | 99.8 | 144 |
| — | 10.502 | 0.250 | — | 0.047 | 0.007 | 0.008 | — | — | 0.012 | 0.002 | — | 0.007 | — | — | — | — | — | — | — | — | — | — | Balance | 131 |
| — | 0.3–0.7 | 0.6 | 0.1 | 0.3 | 0.4–0.9 | 0.2 | 0.2 | 0.1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 123 |
| — | 0.834 | — | 0.036 | — | 0.001 | — | 0.019 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.009 | 99.11 | 87 |
| — | 0.15 | 0.19 | 0.02 | 0.10 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.535 | 75 |
| — | 0.2885 | 0.5783 | 0.0809 | 0.2732 | 0.273 | 0.0057 | 0.1029 | 0.0125 | — | — | — | 0.0051 | — | — | 0.0766 | — | — | — | — | — | — | — | 98.60 | 106 |
| — | 0.62 | 0.51 | 3.83 | 0.59 | 0.53 | 0.12 | 0.09 | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 93.68 | 99 |
| — | — | 0.2 | 0.2 | — | — | — | 0.08 | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 98.5 | 141 |
| — | — | 0.2 | — | — | — | — | 0.08 | 0.08 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 98.5% | 164 |
| — | 0.03 | — | 4.5 | 0.02 | 0.20 | 0.001 | 0.022 | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | 0.005 | 0.005 | 95.212 | 163 |
| — | 0.03 | — | 7.5 | 0.02 | 0.20 | 0.001 | 0.022 | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | 0.005 | 0.005 | 92.212 | 163 |
| — | 0.20 | 0.20 | 0.02 | — | — | — | 0.08 | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 161 |
| — | 0.48 | 0.50 | 0.048 | 0.012 | — | — | — | 0.005 | — | 0.013 | — | 0.016 | — | 0.01 | — | — | — | — | — | — | 0.10 | 0.071 | 98.70 | 93 |
| — | 0.09 | 0.35 | — | — | — | — | 0.02 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 99.6 | 68 |
| — | — | 0.2 | 0.2 | — | — | — | 0.08 | 0.03 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 98.5 | 95 |
| — | — | — | — | — | — | — | — | — | — | — | — | — | 5.06 | — | — | — | — | — | — | — | — | — | 94.94 | 98 |
| AA1050 | 0.25 | 0.40 | 0.05 | 0.05 | 0.05 | 0.07 | 0.03 | — | — | — | — | — | — | — | — | — | 0.03 | — | — | — | 0.002 | Balance | 145 | |
| AA1060 | 0.25 | 0.35 | 0.05 | 0.03 | 0.03 | — | 0.05 | 0.03 | — | — | — | 0.05 | — | — | — | — | — | — | — | — | — | — | Balance | 116 |
| AA1060 | 0.12 | 0.02 | — | 0.04 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 113 |
| AA3102 | 0.07 | 0.42 | — | 0.21 | — | — | — | 0.01 | — | — | — | 0.01 | — | — | — | — | — | — | — | — | — | — | Balance | 121 |
| AA2024 | 0.50 | 0.50 | 3.80 | 0.30 | 1.20 | 0.10 | 0.25 | 0.15 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 119 and 150 |
| AA6061 | 0.40–0.80 | 0.70 | 0.15–0.40 | 0.15 | 0.80–1.20 | 0.04–0.35 | 0.25 | 0.15 | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.15 | Balance | 122, 124, 151 and 153 |
| AA6063 | 0.20–0.60 | 0.35 | 0.10 | 0.10 | 0.45–0.90 | 0.10 | 0.10 | 0.10 | — | — | — | — | — | — | — | — | — | — | — | — | — | 0.15 | Balance | 124 and 153 |
| Al–8% Si–3% Cu | 8.2828 | 0.7246 | 3.1938 | 0.0999 | 0.1448 | 0.0148 | 0.3594 | 0.0142 | — | 0.0236 | — | — | — | — | 0.1148 | — | — | — | — | — | — | 0.0249 | Balance | 127 |
| Al–4% Cu | 0.8098 | 0.4400 | 4.0219 | 0.6413 | 0.4218 | 0.0331 | 0.1199 | 0.0255 | — | 0.0089 | — | — | — | — | 0.0220 | — | — | — | — | — | — | 0.0063 | Balance | 127 |
| Al–12% Cu | 0.0086 | 0.1240 | 12.25 | <0.0010 | <0.0005 | <0.0020 | 0.0026 | <0.0005 | — | <0.0020 | 0.0075 | 0.0095 | Balance | 127 | ||||||||||
| Al–22% Cu–4% Fe | 0.17 | 3.93 | 21.85 | — | — | — | 0.10 | — | — | — | — | — | — | — | 0.77 | — | — | — | — | — | — | — | Balance | 127 |
| E-110 | 5.20 | 0.47 | 3.10 | 0.35 | 0.29 | 0.008 | 0.19 | 0.03 | — | 0.03 | — | — | — | — | 0.04 | — | — | — | — | — | — | 0.001 | Balance | 125 and 126 |
| E-140 | 12.00 | 0.64 | 0.85 | 0.38 | 0.20 | 0.01 | 0.48 | 0.03 | — | 0.09 | — | — | — | — | 0.06 | — | — | — | — | — | — | 0.001 | Balance | 125 and 126 |
| E-150 | 12.30 | 0.82 | 2.35 | 0.26 | 0.23 | 0.01 | 0.58 | 0.03 | — | 0.08 | — | — | — | — | 0.04 | — | — | — | — | — | — | 0.02 | Balance | 125 and 126 |
| E-160 | 8.60 | 0.88 | 3.40 | 0.21 | 0.22 | 0.01 | 0.75 | 0.03 | — | 0.09 | — | — | — | — | 0.09 | — | — | — | — | — | — | 0.02 | Balance | 125 and 126 |
| E-171 | 10.00 | 0.44 | 0.24 | 0.38 | 0.48 | 0.01 | 0.23 | 0.02 | — | 0.008 | — | — | — | — | 0.09 | — | — | — | — | — | — | 0.005 | Balance | 125 and 126 |
| E-195 | 18.23 | 0.23 | 0.94 | 0.011 | 1.02 | 0.002 | 0.004 | 0.01 | — | 0.97 | — | — | — | — | — | — | — | — | — | — | — | — | Balance | 125 and 126 |
5.1 Organic corrosion inhibitors in HCl solution
As given below and in Table S1,† most of the organic compounds used as corrosion inhibitors were tested in HCl solution, which causes significant corrosion and therefore needs to be inhibited. A quite broad concentration range of HCl was tested, varying between 0.1 and 5 M.Dibetsoe et al.45 investigated seven macrocyclic compounds comprising four phthalocyanines (Pcs), i.e. 1,4,8,11,15,18,22,25-octabutoxy-29H,31H-phthalocyanine (Pc1), 2,3,9,10,16,17,23,24-octakis(octyloxy)-29H,31H-phthalocyanine (Pc2), 2,9,16,23-tetra-tert-butyl-29H,31H-phthalocyanine (Pc3), and 29H,31H-phthalocyanine (Pc4), and three naphthalocyanines, i.e. 5,9,14,18,23,27,32,36-octabutoxy-2,3-naphthalocyanine (nPc1), 2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine (nPc2), and 2,3-naphthalocyanine (nPc3), as corrosion inhibitors for 100% aluminium in 1 M HCl solution, using the WL and PDP techniques. The inhibition effectiveness generally increased with increasing concentration of the compounds added. Pc1 and nPc3 gave the highest inhibition effectiveness at the concentrations studied. There is a non-uniform trend of the inhibition effectiveness at higher concentrations, at all temperatures, but especially at higher temperatures. The authors attributed this to the varying aggregative interactions of the inhibitor molecules, which at higher concentrations and temperatures favour the unsubstituted Pc4 more than the substituted Pc2 and Pc3 compounds. In addition, the lower inhibition effectiveness of Pc4 is due to the absence of an electron-donating alkyl or alkoxy substituents. The inhibition effectiveness in general decreased with an increase in temperature. The inhibition effectiveness was found to further increase with the addition of KI as a result of the synergistic effect. The authors reported that these compounds have a mixed-type (both physisorption and chemisorption) adsorption mechanism. They discussed the ability of these compounds to protect aluminium in terms of their ability to donate/accept electrons to/from the appropriate p- and/or d-orbitals of the metal.
Lashgari and Malek46 studied the ability of phenol to inhibit the corrosion of pure aluminium in 0.5 M HCl and 0.5 M NaOH solutions at 25–45 °C. The authors reported substantially higher corrosion rates for aluminium in the alkaline media compared to the acidic one. Moreover, they claimed that phenol physisorbs on the aluminium surface in an acidic environment, while suggesting chemisorption in alkaline media. PDP measurements showed that phenol is a mixed-type inhibitor and that inhibition effectiveness decreased with increasing temperature.
Zohreh et al.47 investigated the inhibition effectiveness of benzene-1,2,4,5-tetracarboxylic dianhydride (PMDH) as a corrosion inhibitor for 99.999% aluminium in 1 M HCl solution at 25–55 °C, using the polarisation and EIS techniques. The inhibition effectiveness of this compound increased with increasing compound concentration at all fixed temperatures. The authors reported that the inhibition effectiveness decreased with increasing temperature and they attributed that to the higher desorption of the inhibitor from the aluminium surface. Polarisation measurements showed that PMDH acted as a mixed-type inhibitor. Based on the thermodynamic data, the authors suggested physisorption as the possible adsorption mechanism for PMDH.
Khaled and Amin48 tested the inhibition effectiveness of imidazole (IM) and two IM derivatives, i.e. 2-amino-4,5-imidazoledicarbonitril (AID) and 5-amino-4-imidazolecarboxamide (AIC) in the corrosion of 99.999% aluminium in 1 M HCl solution at 25 °C, using the WL, Tafel extrapolation, and EIS techniques. The inhibition effectiveness of all three compounds increased with increasing compound concentration and followed the order AID > AIC > IM. Polarisation curves showed that these compounds acted mainly as cathodic-type inhibitors. The authors concluded that the inhibition action of these compounds is due to the fact that they form a film on the aluminium surface by physisorption followed by chemisorption, further enhanced via hydrogen bond formation.
Özdemir et al.49 synthesised two phthalocyanines, i.e. 1(4)-tetrakis[(2-mercapto)pyridine]phthalocyanine and 2,3-octakis[(2-mercapto)pyridine]phthalocyanine, and tested them as corrosion inhibitors for 99.998% aluminium in 0.1 M HCl solution at 15–35 °C, using the PDP and EIS techniques. The authors concluded that the inhibition effectiveness of these compounds increased with increasing concentration, reaching the maximum value at 5 × 10−4 M for the first compound and at 1 × 10−5 M for the second compound. The inhibition effectiveness decreased with increasing temperature. The authors suggested that these phthalocyanines physisorbed on the aluminium surface. The first compound was more effective compared to the second one. PDP measurements showed that these phthalocyanines predominately acted as cathodic-type corrosion inhibitors.
Aytaç50 synthesised two bromide and ethoxy substituted Schiff bases, i.e. N-4-bromobenzilidene-2-aminophenol (APh-Br) and N-4-ethoxybenzilidene-2-aminophenol (APh-OCH2CH3) and their corresponding Cu(II)-, Ni(II)- and Co(II)-complexes, i.e. [Cu(APh–Br)] and [Cu(APh–OCH2CH3)], [Ni(APh–Br)], and [Ni(APh–OCH2CH3)], [Co(APh–Br)], and [Co(APh–OCH2CH3)]. These compounds were tested as corrosion inhibitors for 99.998% aluminium in 0.1 M HCl solution at 25 °C, using the EIS, PDP, Rp, and HE techniques. The values of the inhibition effectiveness obtained with different techniques showed a good correlation, apart from the Rp, values, which were too low for all tested compounds. The inhibition effectiveness changes according to the order [Ni(APh–OCH2CH3)] > [Cu(APh–OCH2CH3)] > [Cu(APh–Br)] > [Ni(APh–Br)] > [(APh–Br)] > [Co(APh–Br)] > [Co(APh–OCH2CH3)] > [(APh–OCH2CH3)]. PDP measurements showed that these compounds suppress both anodic and cathodic corrosion processes. SEM investigation revealed the formation of a uniform film on the aluminium surface as a result of the adsorption of these compounds.
Zapata-Loría and Pech-Canul51 studied the inhibition effectiveness of glutamic acid as a corrosion inhibitor for 99.99% aluminium in 0.1 M HCl solution at 25 °C, using the Rp, PDP, and EIS techniques. The inhibition effectiveness of glutamic acid increased with increasing concentration. Based on the PDP measurements, the authors reported that glutamic acid acted as a mixed-type inhibitor. The authors confirmed the adsorption of the compound on the aluminium surface with a decrease in the double-layer capacitance. Based on the X-ray photoelectron spectroscopy (XPS) analysis, the authors reported the presence of amino (ionized –NH3+ and neutral –NH2) and carboxylic groups in the adsorbed layer. They concluded that glutamic acid chemisorbed, forming a stable chelate on the aluminium surface.
El-Haddad and Fouda52 reported the use of imidazole and 2-methyl imidazole as corrosion inhibitors for 99.99% aluminium in 0.5 M HCl solution at 30 °C, using the PDP, EIS, and EFM techniques. PDP curves showed that imidazole derivatives are mixed-type inhibitors. The inhibition effectiveness increased with increasing inhibitor concentration, methyl imidazole being the most effective. The inhibition effect of these compounds is due to the formation of a film on the aluminium surface that inhibited metal dissolution. The authors showed through quantum chemical calculations that imidazole derivatives can adsorb on the aluminium surface through the nitrogen atoms as well as the π-electrons in the imidazole ring.
Hazazi and Abdallah53 investigated the influence of three prazole compounds, i.e. sodium salt of 2-((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methylsulfinyl)-1H-benzimidazole (Omeprazole), sodium salt of 1-(2-((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H-benzimidazol-6-yl)-2,2-difluoroethanone (Pantoprazole sodium sesquihydrate), and 5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl)-1H-benzimidazole (Rabeprazole sodium), in the corrosion of 99.99% aluminium in 1 M HCl solution at 30–60 °C. The inhibition effectiveness increased with increasing inhibitor concentration and with decreasing temperature. The order of inhibition effectiveness is Rabeprazole sodium > Pantoprazole sodium sesquihydrate > Omeprazole. The polarisation measurements showed that these compounds act as mixed-type inhibitors. EIS indicated that the corrosion reaction is controlled by a charge transfer process. The authors reported that the inhibition action of prazole compounds is due to the formation of an insoluble complex adsorbed on the aluminium surface.
Abd El Haleem et al.54 investigated the corrosion behaviour of 99.99% aluminium in 2 M HCl solution in the presence of five nitrogen and/or sulphur-containing compounds, i.e. phenylhydrazine, urea, thiourea, N-allylthiourea, and thiosemicarbazide, using the WL, HE, thermometry, and GSP techniques. The authors attributed the inhibition effect of these compounds to the adsorption on the metal surface and/or the precipitation of their corresponding chloro-complexes on the metal surface. Thermodynamic data showed that these compounds physisorb on the aluminium surface. GSP measurements showed that these organic additives acted as mixed-type inhibitors. The corrosion rate decreased with increasing inhibitor concentration and with increasing molecular weights. The inhibition effectiveness decreased in the order thiosemicarbazide > N-allylthiourea > thiourea > urea > phenylhydrazine.
El-Etre55 studied the performance of vanillin, i.e. 4-hydroxy-3-methoxy benzaldehyde, as an inhibitor in the corrosion of 99.99% aluminium in 5 M HCl solution at 30 °C, using the WL, thermometry, HE, and PSP techniques. Vanillin was first dissolved in a 20/80 ethyl alcohol–water mixture and then added to the acid. The inhibition effectiveness increased with increasing concentration of vanillin. PSP measurements showed that vanillin affects both anodic and cathodic reactions, therefore acting as a mixed-type inhibitor. The authors concluded that this compound adsorbed on the aluminium surface through the active centres (carbonyl, methoxy and hydroxyl groups) contained in its structure.
Maayta and Al-Rawashdeh56 studied the corrosion of 99.95% aluminium in 0.2 and 0.5 M HCl solutions in the presence of sulfonic acid (SA), sodium cumene sulfonate (SCS), and sodium alkyl sulphate (SAS), using the WL and PSP techniques. The inhibition effectiveness of all three compounds increased with increasing inhibitor concentration and decreasing acid concentration and temperature. The ability of these compounds to inhibit the corrosion of aluminium in these conditions decreased in the order SA > SCS > SAS. The authors attributed this order to the differences in molecular size, geometry, and mode of adsorption. The decrease in inhibition effectiveness and surface coverage with increasing temperature indicated a weak interaction between the inhibitor molecules and the aluminium surface. The large increase in the cathodic Tafel slope revealed that the inhibitors slow down the rate of the cathodic reaction.
Al-Rawashdeh and Maayta57 investigated the inhibition performance of the cationic surfactant cetyl trimethylammonium chloride (CTAC) in the corrosion of 99.95% aluminium in 0.2 M and 0.5 M HCl solutions at 30–60 °C, using the WL and PDP techniques. The inhibition effectiveness increased with increasing surfactant concentration and decreased with increasing acid concentration. The increase in temperature resulted in a decrease in the inhibition effectiveness and the authors attributed that to the increase in aluminium dissolution at higher temperatures. Moreover, this behaviour combined with the decrease in surface coverage with increasing temperature, according to the authors, suggested a weak interaction between the surfactant molecules and the aluminium surface. The formation of a multilayer of CTAC on the aluminium surface was also proposed. On the basis of the measured Tafel slope values obtained, the authors concluded that CTAC controls the cathodic reaction.
Abdallah et al.58 reported on the use of carboxy methyl chitosan and carboxy methyl cellulose as corrosion inhibitors for 99.95% aluminium in 0.5 M HCl solution, using the WL, GSP, and PSP techniques at 30–60 °C. The inhibition effectiveness increased with increasing inhibitor concentration, but decreased with temperature. Carboxy methyl cellulose is the most effective inhibitor. PSP measurements showed that these compounds acted as mixed-type inhibitors. The authors reported that these compounds protect the aluminium surface from pitting corrosion by shifting the pitting corrosion potential to a more noble direction.
Abdallah et al.59 tested three polyethylene glycol compounds (MW = 600, 2000, and 6000 g mol−1) as corrosion inhibitors for 99.95% aluminium in 0.5 M HCl solution, using the WL, PDP, and GSP techniques at 30 °C. The inhibition effectiveness of these compounds increased with increasing concentration, but decreased with increasing temperature. The authors reported that the inhibition action of these compounds depends on their chemical structure, increasing with increasing molecular weight. Based on PDP measurements the authors concluded that the polyethylene glycol compounds acted as mixed-type inhibitors.
Zhang and Hua60 tested three newly synthesised alkylimidazolium ionic liquids, i.e. 1-butyl-3-methylimidazolium chloride (BMIC), 1-hexyl-3-methylimidazolium chloride (HMIC), and 1-octyl-3-methylimidazolium chloride (OMIC), as corrosion inhibitors for 99.95% aluminium in 1 M HCl solution at 30 °C, using the WL, PDP and EIS techniques. The authors reported that the inhibitor effectiveness followed the order OMIC > HMIC > BMIC. The inhibition effectiveness of these compounds increased with increasing inhibitor concentration, but decreased with increasing temperature. PDP measurements showed that all of the inhibitors acted as mixed-type inhibitors. The authors concluded that the inhibition effect of these compounds is mainly due to the fact that they physisorb on the aluminium surface.
Umoren and Solomon61 reported that polyacrylamide was an efficient corrosion inhibitor for 99.91% aluminium in 0.5 M HCl solution at 30 and 60 °C, using the WL, HE, and thermometric techniques. The inhibition effectiveness increased with increasing polyacrylamide concentration, but decreased with increasing temperature. A synergistic effect was observed when KBr and KI were added, KI being the most effective. The authors proposed physisorption as the possible mechanism for the adsorption of polyacrylamide.
Meena et al.62 tested gelatin and the surfactant Triton X-100 as corrosion inhibitors for 99.9% aluminium in 0.1 N HCl solution, using the Tafel extrapolation and WL techniques. The inhibition effectiveness increased with increasing concentrations of both compounds. However, there was not a good correlation between the inhibition effectiveness values obtained by the two methods, especially at the lowest concentration values and even more so for Triton X-100. Triton X-100 was found to be a better inhibitor than gelatin under the studied conditions.
Awad et al.63 studied the performance of three polyethylene glycols (MW = 300, 400, and 600 g mol−1) as corrosion inhibitors for 99.9% aluminium in 1 M HCl solution, using the WL and polarisation techniques. The inhibition effectiveness increased with an increase in both the molecular weight and the concentration of the compounds added, but decreased with temperature. This is in agreement with the work of Abdallah et al.,59 who studied the inhibition effectiveness of polyethylene glycols (MW = 600, 2000, and 6000 g mol−1) in the corrosion of 99.95% aluminium in 0.5 M HCl solution. Polarisation measurements showed that polyethylene glycol acted as a mixed-type inhibitor in accordance with what was reported previously by Abdallah et al.59 The authors reported that these polymers physisorbed on the aluminium surface.
Dadgarinezhad et al.64 synthesised bis-(2-hydroxy-3-methoxy)-1,6-diaminohexane salicylaldimine and tested it as a corrosion inhibitor for 99.899% aluminium in 0.5–2.0 M HCl solutions at 25 °C, using the WL, PDP, and EIS techniques. The authors reported that at all acid concentrations tested, the inhibition effectiveness of this compound increased with increasing compound concentration up to 100 ppm. A further increase in the compound concentration resulted in a decrease in inhibition effectiveness. No influence of the HCl concentration on the inhibition effectiveness was reported when 100 ppm of the inhibitor was used. PDP measurements showed that bis-(2-hydroxy-3-methoxy)-1,6-diaminohexane salicylaldimine acted as a mixed-type inhibitor.
Foad El-Sherbini et al.65 investigated the inhibition effectiveness of three ethoxylated fatty acids, i.e. polyoxyethylene 20 mono oleate [OL(EO)20], polyoxyethylene 40 mono oleate [OL(EO)40], and polyoxyethylene 80 mono oleate [OL(EO)80], in the corrosion of 99.89% aluminium in 1 M HCl solution at 25 °C, using the WL and PDP techniques. The inhibition effectiveness increased with increasing compound concentration and with increasing chain length of the fatty acid, in the order [OL(EO)20] < [OL(EO)40] < [OL(EO)80]. PDP measurements showed that these compounds acted as anodic-type inhibitors. The authors concluded that the adsorption of these compounds on the aluminium surface fits the kinetic-thermodynamic model well.
Zor et al.66 synthesised 4-phenyl-3-thiosemicarbazide and tested it as a corrosion inhibitor for 99.8% aluminium in aerated and stirred 0.1 M HCl solutions at 25–70 °C, using the PDP and Rp techniques. The inhibition effectiveness of this compound increased with increasing compound concentration, but decreased with increasing temperature. Based on the thermodynamic calculations, the authors suggested physisorption as the possible adsorption mechanism for 4-phenyl-3-thiosemicarbazide.
Abd El Rehim et al.67 reported that 1,1(lauryl amido)propyl ammonium chloride is an effective inhibitor in the corrosion of 99.79% aluminium in 1 M HCl solution at 10–60 °C, using the WL PDP and EIS techniques. The inhibition effectiveness increased with increasing inhibitor concentration, but decreased with increasing temperature. Maximum inhibition effectiveness was achieved around the surfactant's CMC (≈2 × 10−3 M at 30 °C). PDP measurements showed that this surfactant acted predominately as an anodic-type inhibitor, adsorbing on the aluminium surface and blocking its active sites.
Grubač et al.68 studied the corrosion behaviour of 99.6% aluminium in 0.17 M HCl solution at 20–60 °C in the presence of two N-arylpyrroles, i.e. 1-(2-fluorophenyl)-2,5-dimethylpyrrole and 1-(2-fluorophenyl)-2,5-dimethylpyrrole-3-carbaldehyde, using the PDP and EIS techniques. The inhibition effectiveness increased with increasing inhibition effectiveness and temperature. 1-(2-Fluorophenyl)-2,5-dimethylpyrrole-3-carbaldehyde gave the highest inhibition effectiveness. Based on the thermodynamic calculations, the authors suggested physisorption as the possible adsorption mechanism for these compounds.
Metikoš-Huković et al.69 investigated the inhibition effectiveness of five substituted N-arylpyrroles, i.e. 1-(2-methylphenyl)-2,5-dimethylpyrrole, 1-(2-fluorophenyl)-2,5-dimethylpyrrole, 1-(2-chlorophenyl)-2,5-dimethylpyrrole, 1-(2-iodophenyl)-2,5-dimethylpyrrole, and 1-(2-fluorophenyl)-2,5-dimethylpyrrole-3-carbaldehyde, as corrosion inhibitors for 99.6% aluminium in 0.5 M HCl solution, using the PDP, Rp, and EIS techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration. The highest inhibition effectiveness was observed for 1-(2-fluorophenyl)-2,5-dimethylpyrrole-3-carbaldehyde and the authors attributed that to the increased electron density of this molecule due to the carbaldehyde group, leading to stronger adsorption on the aluminium surface. PDP measurements showed that these compounds acted as cathodic-type inhibitors.
El-Deeb and Mohamed70 synthesised 3-(10-sodium sulfonate decyloxy)aniline monomeric surfactant and the analogue polymeric surfactant poly[3-(decyloxy sulfonic acid)aniline] (PC10) and tested them as corrosion inhibitors for 99.57% aluminium in 0.5 M HCl solution at 30–60 °C, using the WL and PDP techniques. The inhibition effectiveness of both surfactants increased with increasing inhibitor concentration up to 5 ppm concentration for both compounds and then slightly decreased. The corrosion rate increased with increasing temperature. PDP measurements showed that these compounds acted as mixed-type inhibitors, with the predominant effect on the anodic reaction of the corrosion couple. Thermodynamic data suggested that the adsorption was exothermic, and occurred mainly through a physisorption mechanism.
El-Deeb et al.71 reported that 3-(12-sodiumsulfonate dodecyloxy) aniline monomer (MC12) and poly-3-(dodecyloxy sulfonic acid) aniline (PC12SO3H) inhibited the corrosion of 99.57% aluminium in 0.5 M HCl solution at 30–60 °C, using the WL and PDP techniques. For both compounds the inhibition effectiveness increased with increasing inhibitor concentration, but decreased with increasing temperature. The inhibition effectiveness of polymeric surfactant is higher than that of the monomeric surfactant. PDP measurements showed that these compounds acted as mixed-type inhibitors with the predominant effect on the anodic reaction of the corrosion couple.
Sayyah et al.72 studied the influence of the terminal side chain of a polymeric surfactant on inhibition effectiveness in the corrosion of 99.57% aluminium in 0.5 M HCl solution. Poly-3-dodecyloxy aniline polymeric surfactant (PC12H) was tested by the authors at 30–60 °C, using the PDP technique, and the data were compared with those of PC12SO3H published by El-Deeb et al.71 The inhibition effectiveness of PC12H increased with increasing concentration, but decreased with temperature. PC12H showed higher inhibition effectiveness compared to PC12SO3H under the same conditions. In order to explain this order, the authors performed quantum chemical calculations. They concluded that PC12H has higher electron-donating behaviour and electron-accepting capability compared to PC12SO3H. However, PC12H polarises more easily than PC12SO3H and hence adsorbed better on the aluminium surface. PDP measurements showed that PC12H acted as an anodic-type inhibitor. Physisorption was the suggested adsorption mechanism for both polymeric surfactants.
Shalabi et al.73 synthesised three sulfonylacetophenoneazo derivatives, i.e. 1-phenyl-2-(2-phenyl hydrazono)-2-(phenylsulfonyl)ethanone (compound 1), 2-(2-(40-methylbiphenyl-4-yl)hydrazono)-1-phenyl-2-(phenyl sulfonyl)ethanone (compound 2), and 2-(2-(40-methoxybiphenyl-4-yl)hydrazono)-1-phenyl-2-(phenylsulfonyl)ethanone (compound 3), and tested them as corrosion inhibitors for 99.555% aluminium in 0.5 M HCl solution at 25–55 °C, using the PDP, EIS, and EFM techniques. The inhibition effectiveness increased with increasing compound concentration, but decreased with increasing temperature, following the order (compound 3) > (compound 2) > (compound 1). The authors attributed the highest inhibition effectiveness of the third compound to the presence of the p-OCH3 group, which is an electron-repelling group that increases the electron charge density. Based on PDP measurements, they showed that all three sulfonylacetophenoneazo derivatives acted as mixed-type inhibitors, with the predominant effect on the cathodic reaction. EIS measurements showed a decrease in the double layer capacitance with respect to the blank solution when the compounds are added.
Okafor et al.74 tested three thiosemicarbazone derivatives, i.e. 2-acetyl pyridine-(4-phenylthiosemicarbazone), 2-acetyl pyridine-(4-phenyl-iso-methylthiosemicarbazone), and 2-acetyl pyridine-(4-phenyl-iso-ethylthiosemicarbazone), as corrosion inhibitors for 99.535% aluminium in 0.1 M HCl solution at 30 and 50 °C, using the WL and HE techniques. The authors report that they tested the inhibitors in the concentration range 0.1 × 10−4 to 5 × 10−4 M. They concluded that the inhibition effectiveness increased with inhibitor concentration and decreased with temperature. However, the data showed that this is true only for higher concentrations (1 × 10−4 and 5 × 10−4 M). At low inhibitor concentrations, these compounds do not protect aluminium, but accelerate corrosion. Moreover, apart from the high concentration values mentioned before, there is no correlation between the data obtained by the two techniques (WL and HE) used to determine the corrosion rates. The authors proposed physisorption as the possible adsorption mechanism.
Mohamed et al.75 investigated the inhibitive action of three quaternary ammonium salts, i.e. hexadecyltrimethyl ammonium chloride, dodecyltrimethyl ammonium chloride, and dimethyl dioctadecyl ammonium bromide, in the corrosion of 99.535% aluminium in 1 M HCl solution, using the thermometric, WL, and polarisation techniques at 30 °C. The authors reported that inhibitor effectiveness increased with increasing size of the alkyl chain. They attributed this behaviour to the van der Waal's attraction forces between the organic cations electrostatically adsorbed on the aluminium surface covered with primary adsorbed halide ions. The inhibition effectiveness of dodecyltrimethyl ammonium chloride increased with increasing concentration. The authors reported that the inhibition effectiveness followed the order hexadecyltrimethyl ammonium chloride > dimethyl dioctadecyl ammonium bromide > dodecyltrimethyl ammonium chloride. Based on the polarisation curves, the authors concluded that these compounds acted as mixed-type inhibitors.
Fouda et al.76 tested the inhibition effectiveness of four benzaldehyde, 2-hydroxybenzol hydrazone derivatives, i.e. 3-methyl benzaldehyde, 2-hydroxy benzoyl-hydrazone (mixture 1), 3-hydrozybenzaldehyde, 2-hydroxy benzol-hydrazone (mixture 2), 4-bromo benzaldehyde, 2-hydroxy benzol-hydrazone and benzaldehyde (mixture 3), and 2-hydroxy benzoyl-hydrazone (compound 4), as corrosion inhibitors for 99.535% aluminium in 2 N HCl solution at 30–55 °C, using the thermometric and polarisation techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration, but rapidly decreased with increasing temperature in the studied range. The authors reported that the inhibition effectiveness followed the order mixture 2 > mixture 3 > mixture 1 > compound 4. They attributed this to an increase in the electron-donor character. Polarisation measurements showed that these compounds acted as mixed-type inhibitors with the predominant inhibition effect on the cathodic reaction.
Elewady and Mostafa77 synthesised four ketonic secondary Mannich bases, i.e. N,3-diphenylpropanamide (DPPA), 3-(4-chlorophenyl)-N-phenylpropanamide (CPPA), N-phenyl-3-(p-tolyl)propanamide (PTPA), and 3-(4-methoxyphenyl)-N-phenylpropanamide (MPPA), and tested them as corrosion inhibitors for 99.53% aluminium in 2 M HCl solution at 25–40 °C, using the WL and GSP techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration, but decreased with increasing temperature. The authors reported that the inhibition effectiveness followed the order MPPA > PTPA > CPPA > DPPA. They explained this order with the polar effect of the p-substituent on the aromatic ring (electron-donor characteristics). GSP measurements suggested that these compounds acted as mixed-type inhibitors with the predominant effect on the cathodic reaction.
Fouda et al.78 synthesised four tertiary ketonic Mannich bases, i.e. β-(piperidino)-p-methoxy-propiophenone hydrochloride (compound 1), β-(piperidino)-p-methyl-propiophenone hydrochloride (compound 2), β-(piperidino)-p-hydroxy-propiophenone hydrochloride (compound 3), and β-(piperidino)-propiophenone hydrochloride (compound 4), and tested them as corrosion inhibitors for 99.535% aluminium in 2 M HCl solution at 25 °C, using the WL and GSP techniques. The inhibition effectiveness increased with increasing inhibitor concentration, but decreased with increasing temperature. The authors reported that the inhibition effectiveness followed the order compound 1 > compound 2 > compound 3 > compound 4. They explained the highest inhibition effectiveness of compound 1 with its higher electron realising effect due to the p-OCH3 group, which may act as an additional centre of adsorption. GSP measurements showed that these compounds acted as mixed-type inhibitors, with the predominant effect on the cathodic reaction of the corrosion couple.
Elewady et al.79 studied the corrosion of 99.53% aluminium in 1 M HCl solution at 30–50 °C, in the presence of ethyl trimethyl ammonium bromide (ETMAB). The inhibition effectiveness increased with the increase in the ETMAB concentration, but decreased with the increase of temperature. It is worth pointing out that the authors reported a significant drop in the inhibition effectiveness for the same immersion time when increasing the temperature with only 5 °C (from 30 to 35 °C the η changes from 52.50% to 10.9% for the lowest concentration of ETMAB used). Meanwhile, for the same concentration of ETMAB further increase in temperature of 5 °C shows only an insignificant change in the IE values (from 10.4% to 9.0%). The authors report that ETMAB acted as a mixed-type inhibitor and that it physisorbed on the aluminium surface. The inhibition effectiveness increased with the addition of I−, SCN−, and Br−. The addition of the iodide ions gave the highest inhibition effectiveness, while SCN− and Br− demonstrated a similar effect in the inhibition effectiveness of ETMAB. In the presence of these ions, the protective film on the aluminium surface is assumed to be an Al–anion–ETMAB complex.
Zor et al.80 tested the effect of temperature (30–60 °C) on the inhibition effectiveness of sodium dodecyl benzene sulfonate (SDBS) as a corrosion inhibitor for 99.5% aluminium in 0.1 M HCl solution, using the WL and PSP techniques. The authors reported that the inhibition of SDBS increased with increasing inhibitor concentration, but decreased with increasing temperature. PSP measurements showed that SDBS acted as a mixed-type inhibitor. The authors also tested the inhibition of SDBS in the corrosion of 99.5% iron in the same corrosive environment and concluded that SDBS protected aluminium better than iron.
Elewady et al.81 investigated the inhibition effectiveness of five anionic surfactants, i.e. octyl sulphate sodium salt, decyl sulphate sodium salt, dodecyl sulphate sodium salt, hexadecyl sulphate sodium salt, and dodecyl benzene sulfonate, in the corrosion of 99.39% aluminium in 1 M HCl solution at 30 °C by the WL and GSP techniques. The inhibition effectiveness of all anionic surfactants tested increased with increasing concentration and decreased with increasing temperature. Dodecyl benzene sulfonate gave the best protection among the tested compounds. The authors attributed this to the higher molecular size and the presence of the benzene ring, which donates π-electrons to the adsorption centres. The order of decreasing inhibition effectiveness is octyl sulphate sodium salt < decyl sulphate sodium salt < dodecyl sulphate sodium salt < hexadecyl sulphate sodium salt < dodecyl benzene sulfonate. GSP measurements showed that these compounds acted as mixed-type inhibitors. The authors tested the protection ability of these surfactants in the presence of 0.01 M KI. The data showed that the inhibition effectiveness increased when KI was added.
El-Dahan et al.82 tested hexamethylenetetramine (HMTA) as an inhibitor in the corrosion of aluminium in 2 M HCl solution, as well as in combination with KI or CaCl2. It should be noted that by adding CaCl2 as a possible synergist, the authors introduced into the corrosion environment chloride ions, which generally promote corrosion. The corrosion inhibition effectiveness depended on both the type and concentration of the additives. The highest inhibition effectiveness of HMTA was achieved for the combinations 600 ppm HMTA + 20 ppm KI and 300 ppm HMTA + 500 ppm CaCl2. The authors suggested that there is a competitive adsorption on the metal surface between the aggressive Cl− ion and the inhibitor molecules.
Şafak et al.83 synthesised three Schiff bases, i.e. 1,5-bis-[2-(2-hydroxybenzylideneamino)phenoxy]-3-oxopentane (compound 1), 1,5-bis-[2-(5-chloro-2-hydroxybenzylideneamino)-phenoxy]-3-oxopentane (compound 2), and 1,5-bis-[2-(5-bromo-2-hydroxybenzylideneamino)-phenoxy]-3-oxopentane (compound 3), and tested them as corrosion inhibitors for 99.19% aluminium in 0.1 M HCl solution by electrochemical techniques at 25 °C. The adsorption of inhibitors and inhibition effectiveness increased with increasing inhibitor concentration. The inhibition effectiveness followed the order compound 3 > compound 2 > compound 1. The authors attributed the difference in the inhibition effectiveness of the three compounds to their structure. The electronegative bromine and chlorine atoms facilitate the adsorption of the molecule on the aluminium surface. The polarisation curves showed that all three Schiff bases acted as cathodic-type inhibitors. The thermodynamic parameters suggested a strong interaction of the inhibitors with the aluminium surface and chemisorption as the possible adsorption mechanism.
Li et al.84 investigated the corrosion of 99.16% aluminium in a 1 M HCl solution in the presence of tetradecylpyridinium bromide (TDPB), using the PDP and EIS techniques. The inhibition effectiveness of TDPB increased with inhibitor concentration, but decreased with increasing temperature. The authors concluded that TDPB acted as a cathodic-type inhibitor and adsorbed on the metal surface through electrostatic attraction between AlClads− and [TDP]+, sharing electrons between the N atoms and the vacant p-orbitals of aluminium.
Yurt and Aykın85 tested two synthesised diphenolic Schiff bases, i.e. 1,8-bis-[2-(5-chloro-2-hydroxybenzylideneamino)-phenoxy]-3,6-dioxooctane (TC) and 1,8-bis-[2-(5-bromo-2-hydroxybenzylideneamino)-phenoxy]-3,6-dioxooctane (TB), in the corrosion of 99.15% aluminium in 0.1 M HCl solution, using the PDP and electrochemical quartz crystal microbalance (EQCM) techniques. The inhibition effectiveness increased with the increase in inhibitor concentration. The authors concluded that the inhibition action of these compounds is mainly due to their adsorption on the aluminium surface. According to them the adsorption is by a chemical mechanism, involving sharing electrons between the N atoms and the vacant p-orbitals of aluminium.
Yurt et al.86 synthesised three Schiff bases, i.e. 2-[2-aza-2-(5-methyl(2-pyridyl))vinyl]phenol, 2-[2-aza-2-(5-methyl(2-pyridyl))vinyl]-4-bromophenol, and 2-[2-aza-2-(5-methyl(2-pyridyl))vinyl]-4-chlorophenol, and tested them as corrosion inhibitors for 99.15% aluminium in 0.1 M HCl solution, using the PDP, EIS, and Rp techniques. The inhibition effectiveness increased with increasing compound concentration. However, there is a poor correlation between the inhibition effectiveness values obtained with different techniques (especially between PDP and Rp). PDP measurements showed that the four Schiff bases acted as mixed-type inhibitors. According to the authors the variation in the inhibition effectiveness depended on the functional groups substituted on the benzene ring. The presence of bromine and chlorine atoms facilitated the adsorption of the molecules on the aluminium surface.
Rethinnagiri et al.87 reported on the inhibition effectiveness of 3-amino-1,2,4-triazole (ATA) as a corrosion inhibitor for 99.11% aluminium in 1 M HCl solution at 30–60 °C, using the WL and PDP techniques. The authors reported that the inhibition effectiveness of ATA increased with increasing concentration and decreased with increasing temperature. Based on PDP measurements, they concluded that ATA acted as a mixed-type inhibitor. SEM images showed the formation of a protective layer on the aluminium surface. Physisorption was the adsorption mechanism proposed by the authors.
Gomma and Wahdan88 synthesised and tested four Schiff bases, i.e. aniline, N-benzylidene, ethylenediamine, N,N′-dibenzylidene, aniline, N-(p-methoxybenzylidene), and ethylenediamine + N,N′-di(p-methoxybenzylidene), as corrosion inhibitors for 99.1% aluminium in 2 N HCl solution, using the polarisation technique. The inhibition effectiveness increased with increasing concentration of the Schiff bases and with increasing temperature. Aniline, N-(p-methoxybenzylidene) gave the highest inhibition effectiveness. Polarisation measurements showed that the inhibitors are of a cathodic-type.
Bereket et al.89 synthesised seven benzimidazole-2-tione and benzoxazole-2-tione derivatives, i.e. benzoxazole-2-tione (BOZ), benzimidazole-2-tione (BNZ), 5-methyl benzoxazole-2-tione (CH3-BOZ), 5-methyl-benzimidazole-2-tione (CH3-BNZ), 5-chloro benzoxazole-2-tione (Cl-BOZ), 5-chloro-benzimidazole-2-tione (Cl-BNZ), and 5-nitro benzoxazole-2-tione (NO2-BOZ), and tested them as corrosion inhibitors for 99% aluminium in 0.1 M HCl solution at 20–60 °C, using the PSP technique. The inhibition effectiveness of these compounds increased with increasing compound concentration, but decreased with temperature. The authors reported that the inhibition effectiveness of the benzimidazole-2-tione derivatives decreased in the order BNZ > CH3-BNZ > Cl-BNZ, while for the benzoxazole-2-tione derivatives, in the order CH3-BOZ > BOZ > Cl-BOZ > NO2-BOZ. Based on the PSP measurements, the authors concluded that BOZ, CH3-BOZ, BNZ, CH3-BNZ, and Cl-BNZ acted as cathodic-type inhibitors, Cl-BOZ as a mixed-type inhibitor, while NO2-BOZ acted as an anodic-type inhibitor.
Zhang et al.90 synthesised four gemini surfactants, i.e. hexanediyl-1,6-bis-(diethyl alkyl ammonium bromide) CmC6Cm(Et)·2Br (m = 10, 12, 14, 16), and concluded that they are effective inhibitors for aluminium corrosion in 1 M HCl solution at 25 °C, using WL and HE measurements. The inhibition effectiveness of these surfactants increased with increasing surfactant concentration. At the same concentration of surfactant, the inhibition effectiveness increased with increasing surfactant chain length (m). These data, combined with the fact that for surfactant concentrations near the critical micelle concentration (CMC) the inhibitor effectiveness scarcely changes, revealed that the inhibition action of these surfactants is mainly due to adsorption on aluminium. The authors suggested that the adsorption of these compounds on the aluminium/solution interface can be considered to be cooperative adsorption by the synergistic effect of bromide ions.
Ashassi-Sorkhabi et al.91 synthesised four Schiff bases, i.e. benzylidene-2-methoxy-phenyl-amine, 2-methoxy-phenyl-4-methyl-benzylidene-amine, 4-chloro-benzylidene-2-methoxy-phenyl-amine, and 4-nitro-benzylidene-2-methoxy-phenyl-amine, and tested them as corrosion inhibitors for 99% aluminium in 1 M HCl solution. The tests were performed at 25 °C, using WL, PDP and EIS techniques. The inhibition effectiveness of all compounds increased with increasing inhibitor concentration and decreasing temperature. The comparison of the data showed that the inhibition effectiveness follows the order (benzylidene-2-methoxy-phenyl-amine) > (2-methoxy-phenyl-4-methyl-benzylidene-amine) > (4-chloro-benzylidene-2-methoxy-phenyl-amine) > (4-nitro-benzylidene-2-methoxy-phenyl-amine). The authors concluded that inhibitor performance strongly depends on the type of functional groups substituted on the benzene ring. Polarisation measurements revealed that these compounds acted as mixed-type inhibitors. The authors concluded that the adsorption of these Schiff bases on the aluminium surface is exothermic, and occured through physisorption.
Fouda et al.92 synthesised two chalcone derivatives, i.e. 3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one and 3-(4-hydroxyphenyl)-1-(4-nitrophenyl)prop-2-en-1-one, and tested their inhibition effectiveness in the corrosion of pure (purity higher than 98.8%) aluminium in 0.5 M HCl solution by WL and electrochemical techniques (PDP, EIS, and EFM) at 25 °C. The inhibition effectiveness increased with increasing compound concentration, but decreased with increasing temperature. 3-(4-Hydroxyphenyl)-1-(4-nitrophenyl)prop-2-en-1-one gave the highest inhibition effectiveness under the studied conditions. According to the authors these chalcone derivatives changed the mechanism of hydrogen evolution and metal dissolution, acting as mixed-type inhibitors. EIS measurements showed that the addition of these compounds decreased the double layer capacitance with respect to blank solution, indicating their adsorption on the aluminium surface.
Muhammad et al.93 studied the inhibition effectiveness of glutamic acid as a corrosion inhibitor for 98.70% aluminium in 0.20–1.00 M HCl solutions at 30–50 °C, using the WL, HE, and thermometric techniques. The authors reported that the inhibition effectiveness of glutamic acid increased when its concentration increased, but decreased with increasing temperature, the period of immersion, and the concentration of the HCl solution. It has to be noted that there is a poor correlation between the inhibition effectiveness calculated from the different techniques (with thermometric giving the highest η values). Based on the thermodynamic calculations, it was reported that glutamic acid physisorbs on the aluminium surface.
Umoren et al.94 tested polyvinyl pyrrolidone, polyacrylamide, and their mixtures as corrosion inhibitors for 98.5% aluminium in 0.1 M and 2.0 M HCl solutions at 30–60 °C, using the WL, HE, and thermometric techniques. It was shown that polyvinyl pyrrolidone is a better inhibitor than polyacrylamide. The inhibition effectiveness was found to increase with increasing compound concentration, but decreased with increasing temperature. Upon mixing the two compounds, it was found that inhibition effectiveness improved, increasing with increasing amount of PVP in the mixture. The optimum blending ratio (polyvinyl pyrrolidone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polyacrylamide) of 3
polyacrylamide) of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) gave the maximum inhibition effectiveness. Based on the thermodynamic data, the authors suggested that these compounds physisorb on the metal surface.
1 (v/v) gave the maximum inhibition effectiveness. Based on the thermodynamic data, the authors suggested that these compounds physisorb on the metal surface.
Bansiwal et al.95 investigated the inhibition effectiveness of four Schiff bases, i.e. 2-anisalidine-pyridine, 2-anisalidine-pyrimidine, 2-salicylidine-pyridine, and 2-salicylidine-pyrimidine, as corrosion inhibitors for 98.5% aluminium in 1–5 M HCl solutions at 25 °C, using the WL and thermometric techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration and acid concentration. The authors concluded that there is a good correlation between the inhibition effectiveness values obtained with the two techniques. However, the data clearly show that this is not true especially at lower concentrations of the inhibitors (for example, for 0.2 wt% of 2-anisalidine-pyrimidine added, the η values are 41.5% with the thermometric technique and 92.8% with WL). The highest inhibition effectiveness was obtained for 2-salicylidine-pyridine. The authors attributed the inhibition action of these Schiff bases to their adsorption on the metal surface via the unshared electrons in the nitrogen atoms, as well as through interactions between the organic molecules adsorbed on anodic and cathodic sites.
Osman and Rehim96 evaluated the corrosion inhibition effectiveness of four ethoxylated fatty acids, i.e. polyoxyethylene (80) monostearate and polyoxyethylene (20, 40, 80) monooleate, in the corrosion of two types of aluminium (containing 97.5% and 92.25% aluminium, respectively) in 1 M HCl solution, using the WL technique. The inhibition effectiveness increased with increasing concentration of the additives, with increasing ethylene oxide units, and the presence of a π-bond in their molecular structure. An increase in temperature enhances the corrosion process.
Muniandy et al.97 synthesised three Schiff bases, i.e. N,N′-bis-(2-hydroxybenzylidene)-1,3-diaminobenzene (SB1), N,N′-bis-(4-bromobenzylidene)-1,3-diaminobenzene (SB2), and N,N′-bis-(2-hydroxy-5-bromobenzylidene)-1,3-diaminobenzene (SB3), and tested them as corrosion inhibitors for 97% aluminium in 0.5 M HCl solution, using the WL and PDP techniques. The inhibition effectiveness of all three compounds increased with increasing compound concentration and followed the order SB3 > SB2 > SB1. PDP measurements revealed that all three Schiff bases act as mixed-type inhibitors, with a predominately cathodic action. The authors concluded that these compounds adsorbed on the aluminium surface, forming protective layers.
Tamilarasan and Sreekanth98 synthesised tris-(5-methyl-2-thioxo-1,3,4-thiadiazole)borate and tested it as a corrosion inhibitor for 94.94% aluminium in 1 M HCl solution at 25 °C, using the Tafel extrapolation and EIS techniques. The authors reported that the inhibition effectiveness of this compound increased with increasing compound concentration. Based on the Tafel extrapolation, they concluded that tris-(5-methyl-2-thioxo-1,3,4-thiadiazole)borate acted as a mixed-type inhibitor with the predominant effect on the cathodic reaction. The authors reported a mixed adsorption mechanism (chemisorption and physisorption) based on the thermodynamic calculations.
Noor143 studied the inhibition action of 1-methyl-4-[4′(-H)-styryl]pyridinium iodide, 1-methyl-4-[4′(-Cl)-styryl]pyridinium iodide and 1-methyl-4-[4′(-H)-styryl]pyridinium iodide in the corrosion of 94.44% aluminium in 0.5 M HCl solution at 30–70 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness increased with increasing inhibitor concentration and after a certain concentration it does not change appreciably with inhibitor concentration. An increase in temperature slightly decreased the inhibition effectiveness, showing good protective properties even at 70 °C (η values for the three tested compounds varied from 82.76 to 94.02%). PDP measurements revealed that these compounds acted as cathodic-type inhibitors without changing the cathodic reaction mechanism. Based on thermodynamic calculations the authors concluded that these inhibitors physisorbed on the metal surface.
Mehdaoui et al.99 distilled Algerian crude oil and the four petroleum fractions obtained, i.e. gasoil sulfonate, kerosene sulfonate, heavy solvent sulfonate, and total gasoline sulfonate, were tested as corrosion inhibitors for 93.68% aluminium in 1 M HCl solution at 20 °C, using the WL, polarisation, and EIS techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration and followed the order gasoil sulfonate > kerosene sulfonate > heavy solvent sulfonate > total gasoline sulfonate. Polarisation measurements showed that these compounds acted as predominantly cathodic-type inhibitors. The authors studied the performance of gasoil sulfonate at 20–50 °C and reported that its inhibition effectiveness decreased with increasing temperature. Based on the thermodynamic calculations, they concluded that these compounds physisorb on the aluminium surface and the adsorption process is exothermic.
Bereket and Pinarbaşi100 synthesised three benzotriazole derivatives, i.e. 5-methyl benzotriazole (CH3-BTA), 5-chloro benzotriazole (Cl-BTA), and 5-nitro benzotriazole (NO2-BTA), and tested them together with benzotriazole (BTA) as corrosion inhibitors for pure aluminium in 0.1 M HCl solution at 20 °C, using the PSP technique. It was reported that the inhibition effectiveness of these compounds increased with increasing compound concentration, following the order NO2-BTA > CH3-BTA > Cl-BTA > BTA. Based on the PSP measurements, the authors concluded that these compounds acted as predominantly cathodic-type inhibitors.
Branzoi et al.101 performed a comparative study on the inhibition role of three surfactants, i.e. Tween 20, Tween 81, and hexadecylpyridinium bromide, in the corrosion of pure aluminium in 0.5 M HCl solution, using the PSP and PDP techniques. The highest inhibition effectiveness was observed for concentration values close to the CMC and then the effectiveness remained stable or decreased slightly when the concentration was increased. The commercial surfactant Tween 81 was found to be more effective as a corrosion inhibitor in the selected corrosion environment, with the inhibitor effectiveness following the order Tween 81 > hexadecylpyrinidium bromide > Tween 20. These surfactants strongly adsorb on the aluminium surface forming a protective film that isolated the metal from the aggressive anions.
Patel et al.102 tested two newly synthesised Schiff bases, i.e. N-benzylidene benzylamine and benzenemethanamine-α-methyl-N-(phenylmethylene), as corrosion inhibitors for pure aluminium in 1 M HCl solution at 35–65 °C, using the GSP and EIS techniques. The inhibition effectiveness increased with increasing compound concentration, but decreased with increasing temperature. The second compound is a better inhibitor under the studied conditions. GSP measurements showed that both compounds act as mixed-type inhibitors. These Schiff bases physisorbed on the aluminium surface and the thermodynamic data showed that the adsorption was exothermic.
Patel et al.103 synthesised a Schiff base, i.e. o-chloroaniline-N-benzylidene, and tested it as a corrosion inhibitor for pure aluminium in 1 M HCl solution at 35–65 °C, using the WL, polarisation, and EIS techniques. The inhibition effectiveness increased with increasing concentration of the compound, but decreased with increasing temperature. However, according to the data, there is only a slight change in the inhibition effectiveness with increasing temperature (for example, at 35 °C the η = 99.4%, while at 65 °C the η = 96.6%). The Schiff base acted as a mixed-type inhibitor, with the predominant effect on the cathodic reaction. Based on the thermodynamic parameters, the authors proposed physisorption as the adsorption mechanism for this Schiff base. The EIS measurements showed that with the addition of the inhibitor there was an increase in the Rct value, while the Cdl value decreased, indicating the formation of a surface film.
Naik and Shah104 synthesised two Schiff bases of benzaldehyde, i.e. o- and p-anisidine-N-benzylidene, and used them to investigate the influence of the position of the substituted group on the inhibition effectiveness of these compounds in the corrosion of pure aluminium in 1 M HCl solution at 35 °C, using the WL, polarisation, and EIS techniques. The inhibition effectiveness of both Schiff bases increased when their concentration increased. The authors reported that p-anisidine-N-benzylidene is a better inhibitor than o-anisidine-N-benzylidene. Polarisation measurements showed that both compounds acted as mixed-type inhibitors. According to the authors, the ability of these compounds to inhibit corrosion is attributed to the presence of imine and methoxy groups, which increased the electron density on the nitrogen atom of the imine group. As a result, the molecule will adsorb strongly on the aluminium surface. The authors reported that both chemisorption and physisorption simultaneously influenced the inhibition effectiveness of these Schiff bases.
Desai and Kapopara105 studied the inhibition effectiveness of xylenol orange in the corrosion of 2S aluminium alloy in 0.4–0.6 M HCl solutions at 28–60 °C, using the WL and PDP techniques. The inhibition effectiveness increased with increasing compound concentration, but decreased with increasing temperature. PDP measurements showed that xylenol orange acted as a mixed-type inhibitor with a predominantly cathodic effect. Based on the higher values of the activation energy, the authors proposed physisorption as the possible adsorption mechanism.
Umoren et al.106 tested polyethylene glycol (PEG) and PVP as well as their mixtures as corrosion inhibitors for 3SR (98.60% aluminium) aluminium alloy in 0.1 M HCl solution at 30–60 °C, using the WL and HE techniques. The inhibition effectiveness of the single compounds increased with increasing compound concentration, but decreased with increasing temperature. The authors reported that PEG–PVP in different v/v ratios synergistically increased the inhibition effectiveness compared to the use of single compounds. The highest inhibition effectiveness was obtained for PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVP in the mixture ratio 1
PVP in the mixture ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v. Physical adsorption was the proposed mechanism of adsorption for these compounds and their mixtures.
3 v/v. Physical adsorption was the proposed mechanism of adsorption for these compounds and their mixtures.
Eddy et al.107 concluded that adenine, guanine, and hypoxanthine are excellent inhibitors for the corrosion of AA1060 aluminium alloy in 0.1 M HCl solution at 30 and 60 °C, using the WL, PDP, and EIS techniques. The effectiveness of all three compounds increased with increasing inhibitor concentration and decreased with increasing temperature. Guanine gave the highest inhibition effectiveness under the studied conditions, with the others following the order guanine > hypoxanthine > adenine. The compounds physisorbed on the aluminium/HCl interface and the adsorption was found to be exothermic. PDP measurements showed that all three compounds inhibited the cathodic reaction to a greater extent than the anodic reaction of the corrosion couple.
Oguzie108 studied the inhibition effectiveness of Crystal violet dye as a corrosion inhibitor for AA1060 aluminium alloy (98.8% aluminium) in aerated 1 M HCl solution at 30–60 °C, using the WL technique. The inhibition effectiveness of Crystal violet dye increased with increasing inhibitor concentration, but decreased with temperature. Based on the thermodynamic calculations, the authors suggested physisorption as the possible adsorption mechanism for this inhibitor. The authors studied the inhibition effectiveness of Crystal violet dye for the same alloy in 0.5 M KOH solution and found that this compound protects the alloy better in HCl solution.
Oguzie et al.109 reported that Malachite green dye, especially at high concentrations, is an effective corrosion inhibitor for AA1060 aluminium alloy (98.8% aluminium) in aerated 1 M HCl solution at 30–60 °C, using the WL technique. The inhibition effectiveness increased with increasing Malachite green dye concentration, but decreased with increasing temperature. A synergistic effect was observed when KI was added to the solutions containing low concentrations of Malachite green dye. The same compound was also tested for the corrosion of the same alloy in 0.5 M KOH solution. It was reported that Malachite green dye is a better inhibitor in HCl than in KOH solution under the studied conditions. Based on molecular dynamics stimulations, the authors concluded that Malachite green dye adsorbed on the aluminium surface via the N atom of the aminic group.
Obot et al.110 investigated the inhibition effectiveness of 2,3-diaminonaphthalene (2,3-DAN) as a corrosion inhibitor for AA1060 aluminium alloy in 1 M HCl solution at 30 and 40 °C, using the HE technique. The authors reported that the inhibition effectiveness increased with increasing 2,3-DAN concentration, but decreased with increasing temperature. A synergistic effect was also reported when KI was added to the solutions containing 2,3-DAN. Based on quantum chemical calculations, the authors concluded that 2,3-DAN adsorbed on the aluminium surface mainly through the nitrogen atoms. Physisorption was the proposed adsorption mechanism of this compound. The same conclusions were previously published by the same group.111
Arukalam112 tested hydroxypropyl methylcellulose (HPMC) as a corrosion inhibitor for AA1060 (98% aluminium) in 1 M HCl solution at 28 °C, using the WL, PDP and EIS technique. The inhibition effectiveness increased with increasing compound concentration. PDP measurements revealed that HPMC acted as a mixed-type inhibitor. Based on the EIS measurements, the authors concluded that HPMC adsorbed on the aluminium surface via a physisorption mechanism.
Arukalam and Obidiegwu113 studied the corrosion of AA1060 aluminium alloy in 1.0 and 1.5 M HCl solution in the presence of hydroxyethyl cellulose, using the WL technique under atmospheric exposure. The corrosion rate increased with increasing acid concentration, but decreased with increasing inhibitor concentration.
Li et al.114 investigated the potential of three oxime compounds, i.e. acetone oxime (AO), 2-butanone oxime (BO), and cyclohexanone oxime (CO), as corrosion inhibitors for AA1060 aluminium alloy in 1 M HCl solution at 20 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness increased with an increase in the concentration of all compounds, with decreasing temperature, and followed the order CO > BO > AO. The data showed an increase in inhibition effectiveness when increasing immersion time up to 2 h and then gradually decreased from 2 to 6 h. PDP measurements showed that these compounds acted as cathodic-type inhibitors. The authors showed that polarisation and charge-transfer resistance values increased with inhibitor concentration while the capacitance values decreased. Molecular dynamic stimulations revealed that the oxime molecules adsorb on the aluminium surface in a nearly flat manner.
Korde et al.115 synthesised 5-ethyl-4-(4-methoxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate and tested it as a corrosion inhibitor for AA1060 aluminium alloy in 1 M HCl solution at 35 °C, using the WL and PDP techniques. The inhibition effectiveness increased with increasing compound concentration. PDP measurements revealed that the compound acted as a mixed-type inhibitor. The compound spontaneously adsorbed on the aluminium surface. The authors conclude that the adsorption of this compound on the aluminium surface is due to three various modes of interaction, electrostatic, interaction by π-electrons, and interaction by non-bonding electrons.
Li et al.116 found that o-phenanthroline acts as an inhibitor in the corrosion of AA1060 aluminium alloy in 1 M HCl solution, using the WL, PDP, EIS, and SEM techniques. The inhibition effectiveness increased with increasing inhibitor concentration, and decreased with increasing temperature. The authors reported that the inhibition effectiveness first increased with immersion time (from 0.5 to 2 h) and then decreased gradually with immersion time. The inhibition effectiveness increased with increasing acid concentration in the range 0.5–1.0 M, and then decreased significantly with acid concentration in 1.0–3.0 M solutions. PDP measurements showed that o-phenanthroline acted as a cathodic-type inhibitor.
Oguzie et al.117 tested methylene blue dye as an inhibitor in the corrosion of AA1060 aluminium alloy in 2 M HCl solution at 30 and 60 °C, using the WL technique. The inhibition effectiveness increased with increasing methylene blue concentration, but decreased with increasing temperature. However, the highest η obtained was 59.46%. The addition of halides synergistically increased the inhibition effectiveness in the order KCl < KBr < KI. The highest η obtained in this case was 79.74%. Thermodynamic calculations indicated that the inhibitor molecules physisorbed on the aluminium surface.
Oguzie and Ebenso118 investigated the inhibition effectiveness of Congo Red dye as a corrosion inhibitor for AA1060 aluminium alloy in 2 M HCl solution at 30 and 60 °C, using the WL technique. The inhibition effectiveness of this dye increased when its concentration increased, but decreased with increasing temperature. The addition of KCl, KBr, and KI to the solutions containing Congo Red dye further increased the inhibition effectiveness, following the order KCl < KBr < KI.
Musa et al.119 tested 1-(2H)-phthalazinone (PTO) as a corrosion inhibitor for AA2024 aluminium alloy in 1.0 M HCl solution at 30 °C, using the PDP and EIS techniques. The inhibition effectiveness increased with increasing PTO concentration, even more so with the addition of KI. PDP measurements revealed that PTO acted as a mixed-type inhibitor. The authors reported that the synergistic effect of iodide ions decreased with increasing PTO concentration and that a competitive inhibition mechanism exists between the iodide anion and PTO cation. They concluded that PTO physisorbed in the absence of KI and chemisorbed when iodide was added.
Li et al.120 studied the effectiveness of tryptophan as an inhibitor in the corrosion of AA2024 aluminium alloy in 1 M HCl solution, using the WL, polarisation, and EIS techniques. The inhibition effectiveness increased with increasing tryptophan concentration. The compound acted as a cathodic-type inhibitor. Quantum chemical calculations indicated that the interactions between the metal surface and tryptophan occur mainly over the indole ring plane.
Aytaç et al.121 reported on the inhibition ability of four newly synthesised Schiff bases, i.e. 2-hydroxyacetophenone-etansulphonylhydrazone, salicylaldehyde-etansulphonylhydrazone, 5-bromosalicylaldehyde-etansulphonylhydrazone, and 5-chlorosalicylaldehyde-etansulphonylaldehyde, in the corrosion of AA3102 aluminium alloy in 0.1 M HCl solution using the HE and EIS techniques. The inhibition effectiveness reached a maximum at a low concentration of these added Schiff bases, decreasing at higher concentrations. However, there is no clear trend in the change in inhibition effectiveness with the concentration of the compounds. The results showed that the most effective inhibitors are 5-bromosalicylaldehyde-etansulphonylhydrazone and 5-chlorosalicylaldehyde-etansulphonylaldehyde. These compounds have electronegative atoms (chlorine and bromine) as para substituents on the phenol ring. In the case of these halogen-containing Schiff bases, the dispersion forces (which are higher for the bromine-containing compound due to its molecular weight) assisted adsorption in addition to other adsorption forces. The methyl group in 2-hydroxyacetophenone-etansulphonylhydrazone resulted in steric hindrance to the adsorption of these molecules on the aluminium surface. The authors concluded that the inhibition effectiveness decreased in the order (2-hydroxyacetophenone-etansulphonylhydrazone) < (salicylaldehyde-etansulphonylhydrazone) < (5-chlorosalicylaldehyde-etansulphonylaldehyde) < (5-bromosalicylaldehyde-etansulphonylhydrazone).
Fakrudeen and Bheema122 synthesised two Schiff bases, i.e. N,N′-bis-(salicylidene)-1,4-diaminobutane (SDB) and N,N′-bis-(3-methoxy salicylidene)-1,4-diaminobutane (MSDB), and tested them as corrosion inhibitors for AA6061 aluminium alloy in 1 M HCl solution at 30 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness of both compounds increased with increasing compound concentration, but decreased with increasing temperature and immersion time. The data confirmed that MSDB protects aluminium better than SDB under the tested conditions. PDP measurements showed that these compounds acted as mixed-type inhibitors, but more effectively inhibit the cathodic reaction. The authors also reported that the charge transfer resistance increased, while double layer capacitance decreased with increasing inhibitor concentration. They concluded that these Schiff bases protect AA6061 aluminium alloy by the formation of a protective barrier film on its surface.
Bhat and Alva123 reported on the use of nicotinic acid hydrazide as a corrosion inhibitor for A63400 aluminium in 1 M HCl solution at 30 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness increased with increasing inhibitor concentration, but decreased with increasing temperature, which, according to the authors, indicates physisorption as the possible adsorption mechanism. PDP measurements showed that nicotinic acid hydrazide acted as a mixed-type inhibitor.
Fakrudeen et al.124 synthesised two Schiff bases, i.e. N,N′-bis-(salicylidene)-1,4-diaminophenelyne (SDP) and N,N′-bis-(3-methoxy salicylidene)-1,4-diaminophenelyne (MSDP), and tested them as corrosion inhibitors for AA6061 and AA6063 aluminium alloy in 1 M HCl solution at 30–60 °C, using the WL technique. The inhibition effectiveness increased with increasing Schiff base concentration, but decreased with increasing temperature and immersion time for both alloys. MSDP is a better inhibitor than SDP for both alloys under the studied conditions. According to the authors, the adsorption of the studied Schiff bases occurs due to electrostatic interaction.
Buyuksagis and Aksut125 investigated the inhibition effectiveness of six alcohols, i.e. 1-buten-3-ol, 2-methyl-3-butyn-2-ol, 3-methyl-2-buten-1-ol, 3-methyl-3-buten-1-ol, 3-methyl-1-pentyn-3-ol, and 5-hexen-1-ol, as corrosion inhibitors for pure aluminium and six aluminium alloys with aluminium content varying from 78.53% to 90.21% in 1 N HCl solution, using the PDP technique. The authors reported that the inhibition effectiveness of the studied alcohols increased with increasing inhibitor concentration. PDP measurements showed that these compounds acted as cathodic-type inhibitors. These alcohols were previously studied by the authors as corrosion inhibitors for the same alloys, but in 1 N H2SO4 solution.126 These alcohols protect aluminium alloys in 1 N H2SO4 much better than in 1 N HCl solution, at the same concentrations.
Önal and Aksüt127 studied the inhibition effectiveness of tolyltriazole (TTA) as a corrosion inhibitor for four aluminium alloys, i.e. Al–8% Si–3% Cu, Al–4% Cu, Al–12% Cu, and Al–22% Cu–4% Fe, in 1 M HCl solution (pH = 0.5) at 15–35 °C, using the PDP and Rp techniques. The inhibition effectiveness of TTA increased with increasing inhibitor concentration, but decreased with increasing temperature and pH. The authors tested TTA also in 1 M NaCl solutions (pH = 6 and 11) and showed that TTA is a more effective inhibitor at pH = 0.5 compared to the other solutions. PDP measurements showed that TTA acted as a mixed-type inhibitor. The authors concluded that the inhibition effect of TTA is due to its adsorption on Cu particles and the formation of a Cu(I)–TTA film. They suggested that this film is formed from the interaction of CuCl2− complex with TTA (TTAH2+, TTAH, or TTA− form).
Abd El Rehim et al.128 synthesised the non-ionic surfactant dodecyl phenol ethoxidate and then tested it as an inhibitor in the corrosion of pure aluminium and two aluminium alloys (Al + 6% Cu and Al + 6% Si) in 1 M HCl solution at 10–60 °C. The corrosion resistance of the three samples in 1 M HCl solution decreased in the order (Al + 6% Si) > (Al + 6% Cu) > Al. The same order was also found for the inhibition effectiveness of the tested non-ionic surfactant. The inhibition effectiveness increased with increasing inhibitor concentration, but decreased with increasing temperature. The non-ionic surfactant acted mainly as an anodic-type inhibitor and it adsorbed on the surface of the aluminium samples.
Halambek et al.129 tested two newly prepared compounds, i.e. 4-(methoxymethyl)-1,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (compound 1) and 4-amino-3,5-bis-[6-(methoxymethyl)-3,4-dimethyl-2-oxo-1,2-dihydropyridine-1-yl]-1,2,4-triazole-2(H) (compound 2), as corrosion inhibitors for Al–3Mg alloy (94.85% aluminium) in 0.5 M and 1 M HCl solution, using the WL and PDP techniques. The inhibition effectiveness increased with increasing concentration of both compounds. However, for compound 2 the inhibition effectiveness was found to decrease for concentration values higher than 10−4 M in both acid concentrations. Compound 2 is a better inhibitor than compound 1. The authors attribute this behaviour to the higher molecular size and the presence of more nitrogen and oxygen atoms and probably better protonation in acid media. PDP measurements showed that both compounds act as mixed-type inhibitors. Thermodynamic calculations and FTIR analysis revealed that both compounds adsorb on the aluminium surface based on two types of interactions, physisorption and chemisorption. FTIR showed that compound 1 can be adsorbed on the basis of donor–acceptor interactions between the lone pair of electrons of oxygen from the carbonyl group and/or the nitrogen atom carbonitrile group and the vacant p-orbital of the aluminium atom on the surface. In the case of compound 2, based on the FTIR results, the authors suggested binding through the oxygen atom of the carbonyl group and nitrogen atoms from the 1,2,4-triazole ring.
Abd El Rehim et al.130 studied the corrosion inhibition of 99.89% aluminium and aluminium alloys with 6% Cu and 6% Si, respectively, in 1 M HCl solution, using sodium benzene sulphonate (SBS) as an inhibitor. The inhibition effectiveness increased with increasing inhibitor concentration and decreased with increasing temperature. A comparison of the experimental data showed that the inhibition effectiveness for the three aluminium samples decreased in the order (Al + 6% Si) > (Al + 6% Cu) > Al. PDP measurements showed that SBS acted as a cathodic-type inhibitor, adsorbing on the surface of the three aluminium samples and blocking their active sites.
Fouda and Abdallah131 investigated the inhibition effectiveness of 4-amino-1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (aminophenazone) and 1,5-dimethyl-2-phenyl-1H-pyrazol-3(2H)-one (phenazone) in ethanol in the corrosion of Al–Si alloy in 1 M HCl solution, using the PDP and EIS techniques. The inhibition effectiveness increased with increasing inhibitor concentration and decreased with increasing temperature. Aminophenazone is a better inhibitor under the studied conditions. The authors attributed this to the fact that this compound has three active centres (one O-atom and two N-atoms) in addition to one more active centre (the N atom at NH2). This compound lies flat on the alloy surface, covering more surface area, hence increasing the inhibition effectiveness. PDP measurements showed that both compounds acted as mixed-type inhibitors. Thermodynamic calculations indicated that the compounds adsorbed on the metal surface through physisorption.
El-Mahdy and Mahmoud132 synthesised 5-benzylidine-1-methyl-2-methylthio-imidazole-4-one and tested it as an inhibitor in the corrosion of pure aluminium in 0.5 M HCl solution at 30 °C, using the WL, Rp, and HE techniques. The inhibition effectiveness increased with increasing compound concentration and with increasing temperature. The corrosion rate of aluminium increased with increasing temperature in the absence and presence of inhibitor due to the increase in aluminium dissolution at higher temperatures. Based on the decrease in activation energy and the increase in inhibition effectiveness in the presence of inhibitor with increasing temperature, the authors suggested chemisorption as the adsorption mechanism.
Abiola et al.133 tested the inhibition effectiveness of diphenylthiocarbazone (DPTC) and diphenyl carbazone (DPC) in the corrosion of 2S aluminium alloy in 0.5 M HCl solution at 30 °C, using the WL technique. The inhibition effectiveness increased with increasing compound concentration and followed the order DPTC > DPC. The authors explained the difference in inhibition effectiveness on the basis of the difference in the electron-donor ability of the sulphur and oxygen atoms.
Khaled and Al-Qahtani134 investigated the inhibition effectiveness of four tetrazole derivatives, i.e. 1-phenyl-1H-tetrazole-5-thiol, 1-phenyl-1H-tetrazole, 1H-tetrazole-5-amine, and 1H-tetrazole, in the corrosion of aluminium in 1 M HCl solution at 25 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness increased with increasing tetrazole concentration. 1-Phenyl-1H-tetrazole-5-thiol was found to give the highest inhibition effectiveness; the others followed the order (1-phenyl-1H-tetrazole-5-thiol) > (1-phenyl-1H-tetrazole) > (1H-tetrazol-5-amine) > (1H-tetrazole). The corrosion process is inhibited by the adsorption of these molecules on the aluminium surface. Double layer capacitances decreased with respect to the blank solution when tetrazole derivatives were added, as a result of their adsorption on the aluminium surface. The authors proposed a mixed mode of adsorption (physisorption and chemisorption) as the corrosion inhibition mechanism.
Umoren et al.135 concluded that PVP acted as an inhibitor for the corrosion of aluminium (composition not given) in 2 M HCl solution at 30–60 °C, using the HE technique. Inhibition effectiveness increased with increasing compound concentration and decreased with increasing temperature. The addition of KI to the acid solution containing PVP increased the inhibition effectiveness through the synergistic effect. The authors proposed physisorption as the possible mechanism. These conclusions are in accordance with what was reported previously by Umoren et al.94 on the use of PVP as a corrosion inhibitor for 98.5% aluminium in 0.1 and 2 M HCl solutions at 30–60 °C.
Quraishi et al.136 synthesised four imidazoline derivatives, i.e. 2-pentadecyl-1,3-imidazoline (PDI), 2-undecyl-1,3-imidazoline (UDI), 2-heptadecyl-1,3-imidazoline (HDI), and 2-nonyl-1,3-imidazoline (NI), and tested them as corrosion inhibitors for cold rolled aluminium in 1 M HCl solution at 30 °C, using the PDP and EIS techniques. The inhibition effectiveness followed the order UDI > NI > PDI > HDI. The authors reported that for UDI the inhibition effectiveness increased with increasing inhibitor concentration. PDP measurements showed that these compounds acted as mixed-type inhibitors. Based on SEM analysis, the authors concluded that these imidazoline derivatives inhibited corrosion due to the formation of film-like deposits on the inhibited aluminium surfaces.
5.2 Organic corrosion inhibitors in H2SO4 solution
Umoren et al.137 reported on the use of polyacrylic acid (PAA) as a corrosion inhibitor for 99.99% aluminium in 0.5 M H2SO4 solutions at 30 °C, using the PDP and EIS techniques. The inhibition effectiveness of PAA increased with increasing inhibitor concentration and was further enhanced by the addition of KI. Based on the PDP measurements, the authors suggested that PAA acted as a mixed-type inhibitor. According to the authors, Fourier transform infrared spectroscopy (FTIR) analyses showed that the synergistic effect of iodide ions and PAA was due to their co-adsorption, which is of a cooperative nature. Based on the thermodynamic calculations, they concluded that PAA physisorbed on the aluminium surface. The inhibition mechanism of PAA in the corrosion of 99.99% aluminium in 0.5 M H2SO4 was further studied by the authors138 using in situ atomic force microscopy (AFM). The in situ AFM morphology of the surface film suggested that PAA in the presence of iodide was adsorbed on the aluminium surface after taking a more orderly arrangement, providing in this way more uniform coverage at potentials below and above the potential of zero charge.Foad El-Sherbini et al.65 investigated the inhibition effectiveness of three ethoxylated fatty acids, i.e. [OL(EO)20], [OL(EO)40], and [OL(EO)80], in the corrosion of 99.89% aluminium in 1 M H2SO4 solution at 25 °C, using the WL and PDP techniques. The inhibition effectiveness increased with increasing compound concentration and with increasing chain length of the fatty acid, in the order OL[EO]20 < OL[EO]40 < OL[EO]80. These compounds acted as anodic-type inhibitors. The inhibition effectiveness of these ethoxylated fatty acids is higher in 1 M HCl solution for the same material and temperature, compared to 1 M H2SO4 solution, as reported in the same publication.
Ravari and Dadgareenezhad139 concluded that propargyl alcohol is an effective inhibitor in the corrosion of 99.3% aluminium in 0.5 M H2SO4 solution at room temperature, using the PDP and EIS techniques. The inhibition effectiveness of PA increased with increasing inhibitor concentration. The authors also reported on the synergistic effects between PA and zinc sulphate. They attribute this to the stabilisation of PA adsorption in the presence of the zinc salt. The thermodynamic calculations showed that both PA and the mixture PA + Zn2+ are physisorbed on the aluminium surface.
Sanni et al.140 tested zinc gluconate as an inhibitor in the corrosion of 98.99% aluminium in 0.5 M H2SO4 solution at 28 °C, using the WL, PDP, and Rp techniques. PDP measurements showed that zinc gluconate acted as a mixed-type inhibitor.
Sethi et al.141 synthesised three Schiff bases, i.e. N-(4-N,N-dimethylaminobenzal)-p-anisidine, N-(4-N,N-dimethylaminobenzal)-p-toluidine, and N-(4-N,N-dimethylaminobenzal)-2,4-dinitroaniline, and studied their effectiveness as corrosion inhibitors for 98.5% aluminium in 0.1–2.0 N H2SO4 solutions, using the WL technique. The authors reported that the inhibition effectiveness of these compounds increased not only with increasing compound concentration, but with increasing acid concentration as well. It was also reported that the addition of Na2SO4 further increased the inhibition effectiveness of these Schiff bases. The inhibition effectiveness decreased in the order N-(4-N,N-dimethylaminobenzal)-p-anisidine > N-(4-N,N-dimethylaminobenzal)-p-toluidine > N-(4-N,N-dimethylaminobenzal)-2,4-dinitroaniline, at all acid concentrations tested.
Echem and James142 synthesised 2-[(3-nitrobenzylidene)amino] (SBNAP) and tested it as a corrosion inhibitor for 3SR (98.7076% aluminium) aluminium alloy in 2 M H2SO4 solution at 30–50 °C, using the WL and HE techniques. The inhibition effectiveness of SBNAP increased with increasing inhibitor concentration, but decreased with temperature. Based on thermodynamic calculations, the authors concluded that SBNAP physisorbed on the aluminium surface and the adsorption reaction is endothermic.
Noor143 studied the inhibition action of 1-methyl-4-[4′(-H)-styryl]pyridinium iodide, 1-methyl-4-[4′(-Cl)-styryl]pyridinium iodide and 1-methyl-4-[4′(-H)-styryl]pyridinium iodide in the corrosion of 94.44% aluminium in 0.5 M H2SO4 solution at 30 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness increased with increasing inhibitor concentration and after a certain concentration it does not change appreciably with inhibitor concentration. PDP measurements revealed that these compounds act as cathodic-type inhibitors without changing the cathodic reaction mechanism. Thermodynamic data indicated that the inhibitor physisorbed on the metal surface. The author compared the inhibition effectiveness of these compounds in two acids (HCl and H2SO4) and found that for the concentration studied (10−4 M) the compounds protect aluminium better in HCl than in H2SO4, emphasising the predominance of the physisorption mechanism.
Ehsani et al.144 synthesised 3,3′-(1,4-phenylene)-bis-(2-imino-2,3-dihydrobenzo[d]oxazole-5,3-iyl)-bis-(4-thylbenzenesulfonate) (1,4-Ph(OX)2(Ts)2) and tested it as a corrosion inhibitor for AA1005 aluminium alloy in 0.5 M H2SO4 solution at 25–45 °C, using the WL, PDP, and EIS techniques. The inhibition effectiveness increased with increasing compound concentration. The data showed no clear trend in the change of inhibition effectiveness with increasing temperature. The anodic Tafel slope decreased upon the addition of 1,4-Ph(OX)2(Ts)2, while the cathodic Tafel slope values showed only small changes. The compound inhibited the corrosion of aluminium under the studied conditions by the adsorption on the metal surface. Quantum chemical calculations showed that the compound adsorbs through adsorption sites located mainly around the nitrogen atoms. Thermodynamic calculations showed that the adsorption process occurs through electrostatic interactions between the positively charged nitrogen atoms of 1,4-Ph(OX)2(Ts)2 and the negatively charged metal surface.
Mercier et al.145 reported on the inhibition effectiveness of 1,2-diaminoethane (DAE) in the corrosion of AA1050 aluminium alloy in 0.1 mM H2SO4 solution at pH = 3. DAE inhibited both uniform and localised corrosion. According to the authors the compound adsorbed on the oxide film surface either by direct adsorption of the protonated molecule on the Al–OH site (in competition with protons) or via a ligand exchange mechanism with the proton of the Al–OH2+ site. The adsorption process led to the formation of monodentate hydrogen-bond surface complexes that block reactive sites.
Ebenso146 found that 2-acetylphenothiazine (2APTZ) acts as an inhibitor for AA1060 aluminium alloy corrosion in 0.5 M H2SO4 solution at 30–60 °C. A synergistic effect was observed when halides are added to the 2APTZ, at very low concentrations. The highest inhibition effectiveness was obtained for the addition of KI and decreased in the order I− > Br− > Cl−. According to the author this indicated that the radii and the electronegativity of the halides play an important role in the adsorption process. The author suggested that 2APTZ chemisorbed on the aluminium surface, blocking its active sites.
Innocent et al.147 investigated hydroxypropyl methylcellulose (HPMC) as a corrosion inhibitor for AA1060 aluminium alloy in 0.5 M H2SO4 solution, using the WL and electrochemical techniques. The compound inhibited the corrosion process by adsorption at the metal/solution interphase. The inhibition effectiveness increased with inhibitor concentration. The addition of KI further increased the inhibition effectiveness, restricted to the cathodic sites on the aluminium surface. The anodic sites were covered by a stable passive oxide layer that hindered inhibitor adsorption.
Arukalam et al.148 investigated the use of hydroxyethyl cellulose as a corrosion inhibitor for AA1060 aluminium alloy in 0.5 M H2SO4 solution, using the WL and electrochemical techniques. The inhibition effectiveness increased with increasing inhibitor concentration. The addition of KI in the solution containing inhibitor resulted in lower inhibitor effectiveness. According to the authors the fact that the inhibition effectiveness increased with increasing temperature, while the activation energy decreased in the presence of the inhibited systems suggests a chemisorption mechanism for hydroxyethyl cellulose. Polarisation measurements showed that hydroxyethyl cellulose is a cathodic-type inhibitor.
Solomon and Umoren149 evaluated the inhibition effectiveness of poly(methacrylic acid) (PMAA) in the corrosion of AA1060 aluminium alloy in 0.5 M H2SO4 solution, using the WL and electrochemical techniques. They concluded that PMAA is a modest inhibitor (with the highest η = 48.7%) for aluminium under the studied conditions. The inhibition effectiveness increased with increasing compound concentration and decreased with increasing temperature. However, the addition of iodide ions to PMAA increased the inhibition effectiveness through the synergistic effect. PDP measurements showed that both PMAA alone and PMAA in combination with the iodide ions acted as mixed-type corrosion inhibitors. The authors reported that these compounds physisorbed on the aluminium surface.
Arukalam112 tested HPMC as a corrosion inhibitor for AA1060 (98% aluminium) in 0.5 M H2SO4 solution at 28 °C, using the WL technique. The inhibition effectiveness increased with increasing compound concentration. The inhibition effectiveness of the same compound in the corrosion of AA1060 (98% aluminium) was also reported for 1 M HCl solution in the same publication. The data showed that HPMC was more effective in hydrochloric acid than in sulphuric acid. The authors explained this behaviour based on the cooperative mechanism of inhibition. In the presence of strongly adsorbable chloride ions, the surface charge is changed to negative, resulting in the joint adsorption of the anion with the cation, leading to a positive synergistic effect. Polarisation studies revealed that HPMC acted as a cathodic-type inhibitor in sulphuric acid solutions, but mixed-type inhibitor behaviour was reported regarding hydrochloric solutions.
Mohamad et al.150 synthesised N,N′-((2E,2′E)-2,2′-(1,4-phenylenebis(methanylylidene))-bis-(hydrazinecarbonothioyl))-bis-(2-oxo-2H-chromene-3-carboxamide) (PBBC) and tested it as a corrosion inhibitor for AA2024 aluminium alloy in 1 M H2SO4 solution at 30–60 °C, using the EIS and PDP techniques. The inhibition effectiveness increased with increasing concentration of PBBC, but decreased with increasing temperature. PDP measurements showed that the compound acted as a mixed-type inhibitor. The inhibition effectiveness of the synthesised compound increased further upon the addition of KI. The mixture of the compound and KI was reported to act as a cathodic-type inhibitor. The authors reported that chemisorption is the mechanism at lower temperatures, while physisorption is favoured at higher temperature. Quantum calculations revealed that the high charge density on oxygen, sulphur, nitrogen, and some carbon atoms could act as a centre of adsorption with the aluminium surface.
Arellanes-Lozada et al.151 synthesised three poly(ionic liquids) derived from imidazole, i.e. poly(1-vinyl-3-dodecylimidazolium hexafluorophosphate) (PImC12), poly(1-vinyl-3-octylimidazolium hexafluorophosphate) (PImC8), and poly(1-vinyl-3-butylimidazolium hexafluorophosphate) (PImC4), and tested them as corrosion inhibitors for AA6061 aluminium alloy in 0.1–1.0 M H2SO4 solution, using the WL and Rp techniques and by measuring the concentration of the dissolved species with ICP-OES. The inhibition effectiveness increased with increasing compound concentration. The data show no clear trend as regards how the inhibition effectiveness changes with time. These compounds inhibit corrosion by the formation of a film on the alloy. Physisorption was the mechanism proposed by the authors. The inhibition effectiveness decreased in the order PImC12 > PImC8 > PImC4. The slight improvement in effectiveness was attributed to the higher molecular weight and the longer alkyl chain through steric hindrance and the interactions of poly ionic liquids.
Buyuksagis and Aksut126 investigated the inhibition effectiveness of six alcohols, i.e. 1-buten-3-ol, 2-methyl-3-butyn-2-ol, 3-methyl-2-buten-1-ol, 3-methyl-3-buten-1-ol, 3-methyl-1-pentyn-3-ol, and 5-hexen-1-ol as corrosion inhibitors for pure aluminium and six aluminium alloys with aluminium content varying from 78.53% to 90.21% in 1 N H2SO4 solution using the PDP technique. The authors reported that the inhibition effectiveness of the studied alcohols increased with increasing concentration of the alcohols. PDP measurements showed that these compounds acted as cathodic-type inhibitors.
Abd El Rehim et al.152 tested SDBS as a corrosion inhibitor for 99.98% aluminium and two aluminium–copper alloys (Al + 2.5% Cu and Al + 7.0% Cu) in 1 M H2SO4 solution, using electrochemical techniques. The inhibition effectiveness increased with increasing compound concentration and immersion time, but decreased with increasing temperature. The authors reported that the maximum inhibition effectiveness was observed at concentrations around the CMC (1.2 × 10−3 M). Physisorption was proposed as the mechanism of corrosion inhibition. According to the authors the compound formed a film on the metal surface that retards the reduction of dissolved oxygen and inhibits the growth of aluminium oxide in acid solution (a mixed-type inhibitor).
Rosliza et al.153 studied the corrosion inhibition of AA6061 and AA6063 aluminium alloys in 0.1 M H2SO4 solution in the presence of sodium benzoate (NaBz), using the WL and electrochemical techniques (PDP, Rp, and EIS) for different immersion times (5–30 days). The inhibition effectiveness of the AA6061 alloy decreased with increasing immersion time, while for the AA6063 alloy the measurements first showed a decrease in inhibition effectiveness with increasing immersion time (up to 15 days of immersion) and then an increase in inhibition effectiveness from 25 days of immersion. The highest inhibition effectiveness was observed after 30 days of immersion. PDP measurements showed that NaBz acted as a cathodic-type inhibitor. Based on the EIS measurements, the authors reported that the corrosion process was mainly kinetically controlled and no change in the corrosion mechanism was observed due to either the immersion time or the addition of inhibitor. According to the authors NaBz adsorbed as a monolayer on the aluminium surface and physisorption was the proposed mechanism.
Umoren et al.154 concluded that PEG and polyvinyl alcohol (PVA) acted as inhibitors for aluminium corrosion in 0.1 M H2SO4 solution, using the WL, HE, and thermometric techniques at 30 °C. The inhibition effectiveness of both compounds increased with increasing concentration, but decreased with increasing temperature. PEG was found to be a better inhibitor than PVA. Thermodynamic data suggested that inhibitors were physisorbed on the aluminium surface.
Quraishi et al.136 synthesised four imidazoline derivatives, i.e. 2-pentadecyl-1,3-imidazoline (PDI), 2-undecyl-1,3-imidazoline (UDI), 2-heptadecyl-1,3-imidazoline (HDI), and 2-nonyl-1,3-imidazoline (NI), and tested them as corrosion inhibitors for cold rolled aluminium in 0.5 M H2SO4 solution at 30 °C, using the PDP and EIS techniques. This article also reported the use of these compounds for the same material, but in 1 M HCl solution. The same results are valid in the case of 0.5 M H2SO4 solution as for 1 M HCl solution. The imidazoline derivatives showed higher inhibition effectiveness in 1 M HCl solutions compared to the 0.5 M H2SO4 solution, at the same concentration and temperature.
5.3 Organic corrosion inhibitors in HNO3 solution
Musa et al.155 tested 4,4-dimethyloxazolidine-2-thione (DMT) as a corrosion inhibitor for AA2024 aluminium alloy in 0.5 M HNO3 solution at 30 °C, using the EIS and PDP techniques. The inhibition effectiveness increased with increasing compound concentration. PDP measurements showed that DMT acted as a cathodic-type inhibitor. DMT adsorbed on the aluminium surface, forming a smooth, dense protective layer. The thermodynamic data showed that the adsorption mechanism is spontaneous and of a mixed type (physisorption and chemisorption).John and Joseph156 studied the inhibition effectiveness of 4-amino-6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5-(2H)-one (compound 1) and 4-amino-6-benzyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5-(2H)-one (compound 2) in the corrosion of 100% aluminium in 1 N HNO3 solution at 25 °C, using the EIS technique. The inhibition effectiveness increased with increasing compound concentration. Compound 1 was found to be a better inhibitor due to the increase in the electron density around the inhibitor molecule as a result of the substitution. EIS measurements showed that the charge transfer resistance values increased while the double layer capacitance decreased with increasing inhibitor concentration. The authors attribute this behaviour to the formation of a protective layer on the aluminium surface. The authors concluded that the inhibitors electrostatically adsorb on the electrode surface based on the increase in the thickness of the protective layer and the decrease in the double layer capacitance with increasing inhibitor concentration.
John et al.157 synthesised 4-amino-4H-1,2,4-triazole-3,5-dimethanol (ATD), (4-(benzylideneamino)-4H-1,2,4-triazole-3,5-diyl)dimethanol (BATD), and (4-(4-(dimethylamino)benzylideneamino)-4H-1,2,4-triazole-3,5-diyl)dimethanol (DBATD) and tested them as corrosion inhibitors for 100% aluminium in 1 N HNO3 solution at 25 °C, using the Tafel extrapolation and EIS techniques. The inhibition effectiveness increased with increasing compound concentration, and decreased with longer immersion times, following the order DBATD > BATD > ATD. The authors attributed this order to the increase in the electron density around the inhibitor molecule due to the substitution. There is no good correlation between the data obtained with the two methods for high compound concentration. Polarisation measurements showed that all three compounds acted as mixed-type inhibitors.
Khaled158 evaluated the protection effectiveness of three thiosemicarbazone derivatives, i.e. 3-pyridinecarboxaldehyde thiosemicarbazone, isonicotinaldehyde thiosemicarbazone, and 2-pyridinecarboxaldehyde thiosemicarbazone, as corrosion inhibitors for 99.999% aluminium in 1 M HNO3 solution, using the WL and EIS techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration. PDP curves demonstrated that the thiosemicarbazone derivatives acted as mixed-type inhibitors. The EIS indicated that the addition of inhibitors increased the charge-transfer resistance of the corrosion process, and hence the inhibition performance. Thiosemicarbazone derivatives can adsorb on the surface through the sulphur and nitrogen atoms as well as π-electrons in the pyridyl structure. The author proposed physisorption followed by chemisorption as the mechanism of the inhibition process, which is enhanced via the formation of hydrogen bonding.
5.4 Organic corrosion inhibitors in H3PO4 solution
Amin et al.159 tested purine as an inhibitor in the corrosion of high-purity aluminium in 1 M deaerated stirred H3PO4 solution. They concluded that purine moderately reduced the acid corrosion of aluminium. The inhibition effectiveness increased with increasing purine concentration and immersion time. A significant increase in the inhibition effectiveness was obtained due to the synergistic effect of the addition of KI. PDP measurements showed that both purine and the mixture purine and KI acted as mixed-type inhibitors. The authors proposed physisorption followed by chemisorption as the mechanism of corrosion inhibition. A surface film of the inhibitor is formed on the electrode surface via the electrostatic adsorption of protonated purine molecules on the positively charged aluminium surface covered with a chemisorbed layer of negatively charged iodide ions.Fouda et al.160 investigated the inhibition effect of three chalcone derivatives, i.e. benzalacetophenone, dibenzalacetone, and dibenzal-1,4-diacetylbenzene, in the corrosion of 99.52% aluminium in 1 M H3PO4 solution, using the WL and GSP techniques. The effectiveness of these compounds increased with increasing compound concentration, but decreased with increasing temperature. The order of the inhibition effectiveness is compound III > compound II > compound I. The authors explained this order on the basis of the chemical structure and adsorption active centres of the compounds. The GSP measurements showed that these chalcone derivatives acted as mixed-type inhibitors. The inhibition effectiveness of these compounds increased further (by more than 30%) with the addition of 10−2 M potassium halides (KI, KBr, and KCl) due to the synergistic effect.
Fouda et al.161 synthesised five p-thiazolidinone derivatives, i.e. 5(-4-nitrophenylazo)-3-phenylamino-2-thioxo-4-thiazolidinone (compound 1), 5(-4-chlorophenylazo)-3-phenylamino-2-thioxo-4-thiazolidinone (compound 2), 5(-4-hydrophenylazo)-3-phenylamino-2-thioxo-4-thiazolidinone (compound 3), 5(-4-methylphenylazo)-3-phenylamino-2-thioxo-4-thiazolidinone (compound 4), and 5(-4-methoxyphenylazo)-3-phenylamino-2-thioxo-4-thiazolidinone (compound 5), and tested them as corrosion inhibitors for 99.47% aluminium in 3 M H3PO4 solution at 30 °C, using the WL and GSP techniques. The inhibition effectiveness of these compounds increased with increasing compound concentration, but decreased with increasing temperature, following the order compound 5 > compound 4 > compound 3 > compound 2 > compound 1. A further increase in the inhibition effectiveness was reported when small amounts of KI, KBr, and KSCN were added to the solutions containing the studied derivatives, following the order KI > KSCN > KBr. GSP measurements showed that these compounds acted as mixed-type inhibitors.
Fouda et al.162 studied the corrosion of aluminium in 1 M H3PO4 solution in the presence of ethanolamines (ethanol-, diethanol-, and triethanol-amine), using the WL, GSP, and quantum chemical calculation methods. The inhibition effectiveness increased with increasing compound concentration and increasing number of ethanol groups (inhibition effectiveness increased in the order triethanol amine > diethanolamine > ethanolamine). The ethanolamines influence both cathodic and anodic processes. The authors also investigated the effect of the addition of halide ions to various concentrations of ethanolamines. The inhibition effectiveness increased in the presence of 10−2 M solutions of KI, KBr, and KCl due to the synergistic effect.
Amin163 investigated the inhibition effectiveness of the anionic surfactant sodium oleate as a corrosion inhibitor for 99.99% aluminium and two aluminium alloys containing 4.5% Cu (Al–4.5% Cu) and 7.5% Cu (Al–7.5% Cu) in deaerated stirred 1 M H3PO4 solution at 25 °C, using the polarisation technique. The inhibition effectiveness of sodium oleate increased with increasing concentration and with increasing copper content in the Al samples. The authors attributed this behaviour to the enhancement of the electrostatic adsorption of the oleate ions on the aluminium surface that results from the presence of copper. They reported that maximum inhibition effectiveness was observed at concentrations around the CMC of sodium oleate (1.5 × 10−3 M). Polarisation measurements showed that sodium oleate acted as a mixed-type inhibitor for 99% aluminium, while it was found to act mainly as a cathodic-type inhibitor for the two alloys tested.
5.5 Organic corrosion inhibitors in chloroacetic acids solutions
Devender et al.164 investigated the inhibition effectiveness of three newly synthesised Schiff bases, i.e. N-(4-N,N-dimethylamino) benzylidene-4-methoxyaniline (compound 1), N-(4-N,N-dimethylamino) benzylidene-2-methylaniline (compound 2), and N-(4-N,N-dimethylamino)benzylidene-3-methylaniline (compound 3), in the corrosion of 98.5% aluminium in 0.1–2.0 N trichloroacetic acid solutions at 25 °C, using the WL and thermometric techniques. The authors reported that the inhibition effectiveness of these Schiff bases increased with increasing Schiff base and trichloroacetic acid concentration. The inhibition effectiveness followed the order compound 1 > compound 2 > compound 3, for all trichloroacetic acid concentrations tested.Ebenso et al.165 reported on the inhibition of AA1060 aluminium alloy (98% purity) in 0.1–0.5 M monochloroacetic, dichlochloroacetic, and trichloroacetic acids at 30 and 40 °C, in the presence of 2-acetylphenothiazine (2APTZ), using the WL and HE techniques. The inhibition effectiveness of 2APTZ increased with increasing inhibitor concentration. 2APTZ was more effective at 30 than at 40 °C. The authors reported that for the same concentration of inhibitor used, the inhibition effectiveness decreased with an increase in the chlorine atoms in the acid (the more chlorine atoms in the molecule, the less effective is the corrosion inhibitor). They suggested physisorption as the possible adsorption mechanism.
Desai and Vashi166 studied sulphathiazole as a corrosion inhibitor for 2S aluminium alloy in 0.01–0.10 M trichloroacetic acid solution at 40–60 °C, using the WL and GSP techniques. The inhibition effectiveness decreased with increasing acid concentration and with increasing temperature. GSP measurements suggested that sulphathiazole acted as a mixed-type inhibitor. Thermodynamic calculations suggested that sulphathiazole physisorbed on the aluminium surface.
Desai and Vashi167 studied the inhibition effectiveness of methylthymolblue complexon as a corrosion inhibitor for 2S aluminium alloy (no composition given) in 0.01–0.10 M trichloroacetic acid at 28–60 °C, using the WL and GSP techniques. The authors reported that the inhibition of methylthymolblue complexon increased with increasing inhibitor concentration, but decreased with increasing acid concentration and temperature. GSP measurements showed that PTU mainly acted as a cathodic-type inhibitor.
Desai and Vashi168 tested phenylthiourea (PTU) as a corrosion inhibitor for 2S aluminium alloy (no composition given) in 0.01–0.10 M trichloroacetic acid at 28–60 °C, using the WL and GSP techniques. The authors reported that the inhibition effectiveness of PTU increased with increasing inhibitor concentration, but decreased with increasing acid concentration and temperature. GSP measurements showed that PTU mainly acted as a cathodic-type inhibitor. The authors attributed the inhibition action of PTU to the presence of extensively delocalised electrons on the phenyl rings. The planarity of the molecule and the presence of lone pair electrons on N and S atoms further increased the adsorption of PTU on the aluminium surface.
5.6 Organic corrosion inhibitors in other acidic solutions
Nithya and Rajendran169 reported on the corrosion of 95% aluminium in an aqueous solution at pH = 3 (the composition of the solution was not given) in the presence of diethylene triamine pentamethylene phosphonic acid (DTPMP) as a corrosion inhibitor, using the WL and PDP techniques. The inhibition effectiveness of DTPMP increased with increasing its concentration. A further increase was reported upon the addition of Zn2+. The highest inhibition effectiveness (η = 98%) was obtained for the combination 200 ppm DTPMP and 10 ppm of Zn2+. No change in the inhibition effectiveness was observed for higher concentrations of DTPMP and Zn2+. PDP measurements showed that DTPMP acted as an anodic-type inhibitor. FTIR analyses showed the formation of a protective film on the aluminium surface.Ashassi-Sorkhabi et al.170 studied six amino acids, i.e. alanine, leucine, methionine, valine, proline, and tryptophan, as corrosion inhibitors for 99.99% aluminium in a mixture of 1 M HCl and 1 M H2SO4. The inhibition effectiveness increased with increasing amino acid concentration and with decreasing temperature. All amino acids acted as mixed-type inhibitors, with a significant increase in inhibition effectiveness in the case of an aromatic ring and hetero atoms, such as sulphur and nitrogen on the amino acid structure. The inhibition effectiveness followed the order tryptophan > methionine > proline > leucine > valine > alanine.
Rosliza et al.153 studied the corrosion inhibition of AA6061 and AA6063 aluminium alloys in 0.5 M CH3COOH solution in the presence of sodium benzoate (NaBz), using the WL and electrochemical techniques (PDP, Rp, and EIS) for different immersion times (5–30 days). The inhibition effectiveness of NaBz in the corrosion of AA6061 alloy in acetic acid increased with increasing immersion time, while the AA6063 alloy data showed the same behaviour as for the sulphuric acid (with a change in the trend at 15 days of immersion) investigated in the same paper. The measurements first showed a decrease in the inhibition effectiveness with immersion time (up to 20 days of immersion) and then an increase from 25 days of immersion was observed. The inhibition effectiveness values showed that NaBz protects the two alloys in 0.5 M CH3COOH solution better compared to the 0.1 M H2SO4 solution. The same conclusions mentioned above for the corrosion of these alloys in sulphuric acid are also valid for corrosion in acetic acid.
6. Conclusions
Due to their specific mechanical properties, aluminium and its alloys are among the most used materials in the world. The exposure of aluminium and its alloys to various acidic environments leads to corrosion, which results in material damage and consequent economic losses. The use of organic corrosion inhibitors is one of the most widely employed methods of corrosion protection. This review focuses on the use of organic corrosion inhibitors in acidic media, with an emphasis on HCl and H2SO4 solutions. These acids are widely used in acid cleaning, chemical or electrochemical etching, acid pickling, and anodising of aluminium. The main conclusions of this review are:• The selection of the proper organic compound is highly dependent on the corrosive environment and aluminium alloy type.
• It is shown that pure aluminium, and 1xxx, 2xxx, and 7xxx series were the most tested materials.
• As presented herein, over the past two decades, amines, N-heterocyclic compounds, azole, imidazole, and thiazole derivatives, different kinds of polymers, organic dyes and Schiff bases have been the most tested organic compounds as corrosion inhibitors for aluminium and it alloys in acidic solutions.
• The inhibition effectiveness of these compounds frequently decreases with increasing temperature and increases with increasing corrosion inhibitor concentration.
• The corrosion inhibition effectiveness of these compounds was tested at room temperature and up to 70 °C.
• The methods used to test corrosion inhibition effectiveness are also discussed in this review, with electrochemical testing being the predominant method.
Abbreviations
| AFM | Atomic force microscopy |
| CMC | Critical micelle concentration |
| EFM | Electrochemical frequency modulation |
| EIS | Electrochemical impedance spectroscopy |
| EQCM | Electrochemical quartz crystal microbalance |
| FTIR | Fourier transform infrared spectroscopy |
| GSP | Galvanostatic polarisation |
| HE | Hydrogen evolution |
| LSV | Linear sweep voltammetry |
| MW | Molecular weight |
| PDP | Potentiodynamic polarisation |
| PSP | Potentiostatic polarisation |
| Rp | Polarisation resistance |
| SEM | Scanning electron microscope |
| WL | Weight loss |
| XPS | X-ray photoelectron spectroscopy |
| η | Inhibition effectiveness |
References
- J. G. Kaufman, Introduction to aluminium alloys and tempers, ASM International, Ohio, 2000 Search PubMed
.
- J. R. Davis, Corrosion of aluminium and aluminium alloys, ASM International, Ohio, 1999 Search PubMed
.
- R. W. Revie, Uhlig's corrosion handbook, John Willey & Sons Inc, New York, 3rd edn, 2011 Search PubMed
.
- S. Ono and H. Habazaki, Corros. Sci., 2009, 51, 2364–2370 CrossRef CAS
.
- R.-g. Xiao, K.-p. Yan, J.-x. Yan and J.-z. Wang, Corros. Sci., 2008, 50, 1576–1583 CrossRef CAS
.
- H.-J. Oh, J.-H. Lee, H.-J. Ahn, Y. Jeong, N.-J. Park, S.-S. Kim and C.-S. Chi, Materials Science and Engineering: A, 2007, 449–451, 348–351 CrossRef
.
- K. Mizuno, A. Nylund and I. Olefjord, Corros. Sci., 2001, 43, 381–396 CrossRef CAS
.
- D. Elabar, A. Němcová, T. Hashimoto, P. Skeldon and G. E. Thompson, Corros. Sci., 2015, 100, 377–385 CrossRef CAS
.
- M. Jayalakshmi and V. S. Muralidharan, Corros. Rev., 1997, 15, 315–340 CAS
.
- C. Vargel, Corrosion of aluminium, Elsevier, Oxford, 2004 Search PubMed
.
- M. Stratmann and G. S. Frankel, Corrosion and oxide films, Wiley-VCH, Weinheim, 2003 Search PubMed
.
- M. Finšgar, Corros. Sci., 2013, 68, 51–56 CrossRef
.
- J. S. Zhang, X. H. Zhao, Y. Zuo and J. P. Xiong, Surf. Coat. Technol., 2008, 202, 3149–3156 CrossRef CAS
.
- L. Domingues, J. C. S. Fernandes, M. D. Belo, M. G. S. Ferreira and L. Guerra-Rosa, Corros. Sci., 2003, 45, 149–160 CrossRef
.
- J. Qi, T. Hashimoto, J. Walton, X. Zhou, P. Skeldon and G. E. Thompson, J. Electrochem. Soc., 2016, 163, C25–C35 CrossRef CAS
.
- L. L. Li and G. M. Swain, Corrosion, 2013, 69, 1205–1216 CrossRef CAS
.
- Y. Liu, P. Skeldon, G. E. Thompson, H. Habazaki and K. Shimizu, Corros. Sci., 2005, 47, 341–354 CrossRef CAS
.
- B. Valdez, S. Kiyota, M. Stoytcheva, R. Zlatev and J. M. Bastidas, Corros. Sci., 2014, 87, 141–149 CrossRef CAS
.
- B. F. Rivera, B. Y. Johnson, M. J. O'Keefe and W. G. Fahrenholtz, Surf. Coat. Technol., 2004, 176, 349–356 CrossRef CAS
.
- W. G. Fahrenholtz, M. J. O'Keefe, H. Zhou and J. T. Grant, Surf. Coat. Technol., 2002, 155, 208–213 CrossRef CAS
.
- A. De Nicolò, L. Paussa, A. Gobessi, A. Lanzutti, C. Cepek, F. Andreatta and L. Fedrizzi, Surf. Coat. Technol., 2016, 287, 33–43 CrossRef
.
- J. Gulicovski, J. Bajat, B. Jokic, V. Panic, V. Miskovic-Stankovic and S. Milonjic, J. Solid State Electrochem., 2016, 20, 293–303 CrossRef CAS
.
- T. G. Harvey, Corros. Eng., Sci. Technol., 2013, 48, 248–269 CrossRef CAS
.
- X. Zuo, W. Li, S. Mu, J. Du, Y. Yang and P. Tang, Prog. Org. Coat., 2015, 87, 61–68 CrossRef CAS
.
- S. S. Golru, M. M. Attar and B. Ramezanzadeh, J. Ind. Eng. Chem., 2015, 24, 233–244 CrossRef CAS
.
- P. Santa Coloma, U. Izagirre, Y. Belaustegi, J. B. Jorcin, F. J. Cano and N. Lapeña, Appl. Surf. Sci., 2015, 345, 24–35 CrossRef CAS
.
- S.-x. Yu, R.-j. Zhang, Y.-f. Tang, Y.-l. Ma and W.-c. Du, J. Nanomater., 2013, 2013, 594273, DOI:10.1155/2013/594273
.
- F. O. George, P. Skeldon and G. E. Thompson, Corros. Sci., 2012, 65, 231–237 CrossRef CAS
.
- M. Bahrami, G. H. Borhani, S. R. Bakhshi and A. Ghasemi, J. Sol-Gel Sci. Technol., 2015, 76, 552–561 CrossRef CAS
.
- C. C. Chang, C. C. Wang, C. W. Wu, S. C. Liu and F. D. Mai, Appl. Surf. Sci., 2008, 255, 1531–1533 CrossRef CAS
.
- M. Niknahad, S. Moradian and S. M. Mirabedini, Corros. Sci., 2010, 52, 1948–1957 CrossRef CAS
.
- S. S. Golru, M. M. Attar and B. Ramezanzadeh, Prog. Org. Coat., 2015, 87, 52–60 CrossRef CAS
.
- B. D. Mert, Corros. Sci., 2016, 103, 88–94 CrossRef
.
- M.-Y. Jiang, L.-K. Wu, J.-M. Hu and J.-Q. Zhang, Corros. Sci., 2015, 92, 118–126 CrossRef
.
- J. Carneiro, J. Tedim and M. G. S. Ferreira, Prog. Org. Coat., 2015, 89, 348–356 CrossRef CAS
.
- E. Gonzalez, J. Pavez, I. Azocar, J. H. Zagal, X. Zhou, F. Melo, G. E. Thompson and M. A. Páez, Electrochim. Acta, 2011, 56, 7586–7595 CrossRef CAS
.
- Y. Liu, D. Sun, H. You and J. S. Chung, Appl. Surf. Sci., 2005, 246, 82–89 CrossRef CAS
.
- Y.-H. Han, A. Taylor and K. M. Knowles, Surf. Coat. Technol., 2008, 202, 1859–1868 CrossRef CAS
.
- F. Girardi, F. Graziola, P. Aldighieri, L. Fedrizzi, S. Gross and R. Di Maggio, Prog. Org. Coat., 2008, 62, 376–381 CrossRef CAS
.
- P. Kwolek, A. Kamiński, K. Dychtoń, M. Drajewicz and J. Sieniawski, Corros. Sci., 2016, 106, 208–216 CrossRef CAS
.
- E. E. Abd El Aal, S. Abd El Wanees, A. Farouk and S. M. Abd El Haleem, Corros. Sci., 2013, 68, 14–24 CrossRef CAS
.
- K.-H. Na and S.-I. Pyun, Corros. Sci., 2007, 49, 2663–2675 CrossRef CAS
.
- K.-H. Na and S.-I. Pyun, J. Electroanal. Chem., 2006, 596, 7–12 CrossRef CAS
.
- M. A. Ameer, A. A. Ghoneim and A. M. Fekry, Int. J. Electrochem. Sci., 2012, 7, 4418–4431 CAS
.
- M. Dibetsoe, L. Olasunkanmi, O. Fayemi, S. Yesudass, B. Ramaganthan, I. Bahadur, A. Adekunle, M. Kabanda and E. Ebenso, Molecules, 2015, 20, 15701 CrossRef CAS PubMed
.
- M. Lashgari and A. M. Malek, Electrochim. Acta, 2010, 55, 5253–5257 CrossRef CAS
.
- S. Zohreh, B. Wan Jeffrey and Z. Sharifuddin Mohd, Anti-Corros. Methods Mater., 2010, 57, 21–27 CrossRef
.
- K. F. Khaled and M. A. Amin, J. Appl. Electrochem., 2009, 39, 2553–2568 CrossRef CAS
.
- O. K. Özdemir, A. Aytaç, D. Atilla and M. Durmuş, J. Mater. Sci., 2011, 46, 752–758 CrossRef
.
- A. Aytaç, J. Mater. Sci., 2010, 45, 6812–6818 CrossRef
.
- A. D. Zapata-Loría and M. A. Pech-Canul, Chem. Eng. Commun., 2014, 201, 855–869 CrossRef
.
- M. N. El-Haddad and A. S. Fouda, J. Mol. Liq., 2015, 209, 480–486 CrossRef CAS
.
- O. A. Hazazi and M. Abdallah, Int. J. Electrochem. Sci., 2013, 8, 8138–8152 CAS
.
- S. M. Abd El Haleem, S. Abd El Wanees, E. E. Abd El Aal and A. Farouk, Corros. Sci., 2013, 68, 1–13 CrossRef CAS
.
- A. Y. El-Etre, Corros. Sci., 2001, 43, 1031–1039 CrossRef CAS
.
- A. K. Maayta and N. A. F. Al-Rawashdeh, Corros. Sci., 2004, 46, 1129–1140 CrossRef CAS
.
- N. A. F. Al-Rawashdeh and A. K. Maayta, Anti-Corros. Methods Mater., 2005, 52, 160–166 CrossRef CAS
.
- M. Abdallah, I. Zaafarany, A. Fawzy, M. A. Radwan and E. Abdfattah, Am. J. Sci., 2013, 9, 580–586 Search PubMed
.
- M. Abdallah, H. E. Megahed, M. A. Radwan and E. Abdfattah, Am. J. Sci., 2012, 8, 49–55 Search PubMed
.
- Q. Zhang and Y. Hua, Mater. Chem. Phys., 2010, 119, 57–64 CrossRef CAS
.
- S. A. Umoren and M. M. Solomon, Arabian J. Sci. Eng., 2010, 35, 115–129 CAS
.
- S. L. Meena and P. S. Verma, Indian J. Chem. Technol., 2014, 21, 220–223 CAS
.
- M. K. Awad, M. S. Metwally, S. A. Soliman, A. A. El-Zomrawy and M. A. Bedair, J. Ind. Eng. Chem., 2014, 20, 796–808 CrossRef CAS
.
- A. Dadgarinezhad, F. B. Ravari and I. Sheikhshoaie, Asian J. Chem., 2009, 21, 3763–3771 CAS
.
- E. E. Foad El-Sherbini, S. M. Abd-El-Wahab and M. A. Deyab, Mater. Chem. Phys., 2003, 82, 631–637 CrossRef CAS
.
- S. Zor, H. Ozkazanc, T. Arslan and F. Kandemirli, Corrosion, 2010, 66, 045006 CrossRef
.
- S. S. Abd El Rehim, H. H. Hassan and M. A. Amin, Mater. Chem. Phys., 2001, 70, 64–72 CrossRef CAS
.
- Z. Grubač, R. Babić and M. Metikoš-Huković, J. Appl. Electrochem., 2002, 32, 431–438 CrossRef
.
- M. Metikoš-Huković, R. Babić and Z. Grubač, J. Appl. Electrochem., 2002, 32, 35–41 CrossRef
.
- M. M. El-Deeb and S. M. Mohamed, J. Appl. Polym. Sci., 2011, 122, 3030–3037 CrossRef CAS
.
- M. M. El-Deeb, S. M. Sayyah, S. S. Abd El-Rehim and S. M. Mohamed, Arabian J. Chem., 2015, 8, 527–537 CrossRef CAS
.
- S. M. Sayyah, M. M. El-Deeb, S. S. Abd El-Rehim, R. A. Ghanem and S. M. Mohamed, Port. Electrochim. Acta, 2014, 32, 417–429 CrossRef CAS
.
- K. Shalabi, Y. M. Abdallah and A. S. Fouda, Res. Chem. Intermed., 2015, 41, 4687–4711 CrossRef CAS
.
- P. C. Okafor, E. E. Ebenso and U. J. Ekpe, Bull. Chem. Soc. Ethiop., 2004, 18, 181–192 CrossRef CAS
.
- A. M. K. Mohamed, A. Al-Nadjm and A. A. S. Fouda, J. Chim. Phys. Phys.-Chim. Biol., 1998, 95, 2061–2069 CrossRef CAS
.
- A. S. Fouda, M. M. Gouda and S. I. Abd El-Rahman, Chem. Pharm. Bull., 2000, 48, 636–640 CrossRef CAS PubMed
.
- G. Y. Elewady and H. A. Mostafa, Desalination, 2009, 247, 573–582 CrossRef CAS
.
- A. S. Fouda, G. Y. El Ewady, H. A. Mostafa and Y. M. El-Toukhee, Chem. Eng. Commun., 2009, 197, 366–376 CrossRef
.
- G. Y. Elewady, I. A. El-Said and A. S. Fouda, Int. J. Electrochem. Sci., 2008, 3, 644–655 CAS
.
- S. Zor, P. Dogan and B. Yazici, Corros. Rev., 2005, 23, 217–232 CAS
.
- G. Y. Elewady, I. A. El-Said and A. S. Fouda, Int. J. Electrochem. Sci., 2008, 3, 177–190 CAS
.
- H. A. El-Dahan, T. Y. Soror and R. M. El-Sherif, Mater. Chem. Phys., 2005, 89, 260–267 CrossRef CAS
.
- S. Şafak, B. Duran, A. Yurt and G. Türkoğlu, Corros. Sci., 2012, 54, 251–259 CrossRef
.
- X. Li, S. Deng and H. Fu, Corros. Sci., 2011, 53, 1529–1536 CrossRef CAS
.
- A. Yurt and Ö. Aykın, Corros. Sci., 2011, 53, 3725–3732 CrossRef CAS
.
- A. Yurt, S. Ulutas and H. Dal, Appl. Surf. Sci., 2006, 253, 919–925 CrossRef CAS
.
- V. Rethinnagiri, P. Jeyaprakash, M. Arunkumar, V. Maheswaran and A. Madhiyalagan, Adv. Appl. Sci. Res., 2012, 3, 1718–1726 CAS
.
- G. K. Gomma and M. H. Wahdan, Mater. Chem. Phys., 1995, 39, 209–213 CrossRef CAS
.
- G. Bereket, A. Pınarbaşı and C. Öğretir, Anti-Corros. Methods Mater., 2004, 51, 282–293 CrossRef CAS
.
- Q. Zhang, Z. Gao, F. Xu and X. Zou, Colloids Surf., A, 2011, 380, 191–200 CrossRef CAS
.
- H. Ashassi-Sorkhabi, B. Shabani, B. Aligholipour and D. Seifzadeh, Appl. Surf. Sci., 2006, 252, 4039–4047 CrossRef CAS
.
- A. S. Fouda, K. Shalabi and N. H. Mohamed, International Journal of Innovative Research in Science Engineering and Technology, 2014, 3, 9861–9875 Search PubMed
.
- A. B. Muhammad, A. Uzairu, J. F. Iyun and H. Abba, IOSR J. Appl. Chem., 2014, 7, 50–62 CrossRef CAS
.
- S. A. Umoren and E. E. Ebenso, Indian J. Chem. Technol., 2008, 15, 355–363 CAS
.
- A. Bansiwal, P. Anthony and S. P. Mathur, Br. Corros. J., 2000, 35, 301–303 CrossRef CAS
.
- M. M. Osman and S. S. A. E. Rehim, Mater. Chem. Phys., 1998, 53, 34–40 CrossRef CAS
.
- M. T. Muniandy, A. A. Rahim, H. Osman, A. M. Shah, S. Yahya and P. B. Raja, Surf. Rev. Lett., 2011, 18, 127–133 CrossRef CAS
.
- R. Tamilarasan and A. Sreekanth, RSC Adv., 2013, 3, 23681–23691 RSC
.
- R. Mehdaoui, A. Khelifa and O. Aaboubi, Res. Chem. Intermed., 2015, 41, 705–720 CrossRef CAS
.
- G. Bereket and A. Pinarbaşi, Corros. Eng., Sci. Technol., 2004, 39, 308–312 CrossRef CAS
.
- V. Branzoi, F. Golgovici and F. Branzoi, Mater. Chem. Phys., 2003, 78, 122–131 CrossRef
.
- A. S. Patel, V. A. Panchal and N. K. Shah, Bull. Mater. Sci., 2012, 35, 283–290 CrossRef CAS
.
- A. S. Patel, V. A. Panchal, G. V. Mudaliar and N. K. Shah, J. Saudi Chem. Soc., 2013, 17, 53–59 CrossRef CAS
.
- U. J. Naik and N. K. Shah, International Journal of Innovative Research in Science Engineering and Technology, 2014, 3, 10422–10431 CrossRef
.
- P. S. Desai and S. M. Kapopara, Indian J. Chem. Technol., 2014, 21, 139–145 CAS
.
- S. A. Umoren, U. M. Eduok and M. M. Solomon, Pigm. Resin Technol., 2014, 43, 299–313 CrossRef CAS
.
- N. O. Eddy, H. Momoh-Yahaya and E. E. Oguzie, J. Adv. Res., 2015, 6, 203–217 CrossRef CAS PubMed
.
- E. E. Oguzie, Chem. Eng. Commun., 2009, 196, 591–601 CrossRef CAS
.
- E. E. Oguzie, C. O. Akalezi, C. K. Enenebeaku and J. N. Aneke, Chem. Eng. Commun., 2010, 198, 46–60 CrossRef
.
- I. B. Obot, N. O. Obi-Egbedi and S. A. Umoren, Corros. Sci., 2009, 51, 276–282 CrossRef CAS
.
- I. B. Obot and N. O. Obi-Egbedi, Surf. Rev. Lett., 2008, 15, 903–910 CrossRef CAS
.
- I. O. Arukalam, I. C. Madufor, O. Ogbobe and E. E. Oguzie, Pigm. Resin Technol., 2014, 43, 151–158 CrossRef CAS
.
- I. O. Arukalam and M. U. Obidiegwu, Acad. Res. Int., 2011, 1, 484–491 Search PubMed
.
- X. H. Li, S. D. Deng and X. G. Xie, Corros. Sci., 2014, 81, 162–175 CrossRef CAS
.
- R. Korde, C. B. Verma, E. E. Ebenso and M. A. Quraishi, Int. J. Electrochem. Sci., 2015, 10, 1081–1093 Search PubMed
.
- X. Li, S. Deng and X. Xie, J. Taiwan Inst. Chem. Eng., 2014, 45, 1865–1875 CrossRef CAS
.
- E. E. Oguzie, B. N. Okolue, E. E. Ebenso, G. N. Onuoha and A. I. Onuchukwu, Mater. Chem. Phys., 2004, 87, 394–401 CrossRef CAS
.
- E. E. Oguzie and E. E. Ebenso, Pigm. Resin Technol., 2006, 35, 30–35 CrossRef CAS
.
- A. Y. Musa, A. A. H. Kadhum, A. B. Mohamad, M. S. Takriff and E. P. Chee, Curr. Appl. Phys., 2012, 12, 325–330 CrossRef
.
- X. Li, B. Xiang, X.-l. Zuo, Q. Wang and Z.-d. Wei, J. Mater. Eng. Perform., 2011, 20, 265–270 CrossRef CAS
.
- A. Aytaç, Ü. Özmen and M. Kabasakaloğlu, Mater. Chem. Phys., 2005, 89, 176–181 CrossRef
.
- S. P. Fakrudeen and R. V. Bheema, J. Mater. Environ. Sci., 2013, 4, 326–337 CAS
.
- J. I. Bhat and V. D. P. Alva, J. Korean Chem. Soc., 2014, 58, 85–91 CrossRef CAS
.
- S. P. Fakrudeen, H. C. A. Murthy and V. B. Raju, J. Chil. Chem. Soc., 2012, 57, 1364–1371 CrossRef CAS
.
- A. Buyuksagis and A. A. Aksut, Prot. Met., 2008, 44, 514–520 CrossRef CAS
.
- A. Buyuksagis and A. A. Aksut, Prot. Met., 2008, 44, 280–288 CrossRef CAS
.
- A. N. Önal and A. A. Aksüt, Anti-Corros. Methods Mater., 2000, 47, 339–349 CrossRef
.
- S. S. Abd El Rehim, H. H. Hassan and M. A. Amin, Corros. Sci., 2004, 46, 5–25 CrossRef CAS
.
- J. Halambek, M. Jukic, K. Berkovic and J. Vorkapic-Furac, Int. J. Electrochem. Sci., 2012, 7, 1580–1601 CAS
.
- S. S. Abd El Rehim, H. H. Hassan and M. A. Amin, Mater. Chem. Phys., 2003, 78, 337–348 CrossRef CAS
.
- A. S. Fouda and Y. M. Abdallah, Arabian J. Sci. Eng., 2014, 39, 5363–5371 CrossRef CAS
.
- G. A. El-Mahdy and S. S. Mahmoud, Corrosion, 1995, 51, 436–440 CrossRef CAS
.
- O. K. Abiola, N. C. Oforka, A. O. Ifelebuegu, T. M. Fasina and A. I. Babatunde, J. Mar. Sci.: Res. Dev., 2008/2009, 11, 1–8 Search PubMed
.
- K. F. Khaled and M. M. Al-Qahtani, Mater. Chem. Phys., 2009, 113, 150–158 CrossRef CAS
.
- S. A. Umoren, I. B. Obot and I. O. Igwe, Open Corros. J., 2009, 2, 1–7 CrossRef CAS
.
- M. A. Quraishi, M. Z. A. Rafiquee, S. Khan and N. Saxena, J. Appl. Electrochem., 2007, 37, 1153–1162 CrossRef CAS
.
- S. A. Umoren, Y. Li and F. H. Wang, J. Solid State Electrochem., 2010, 14, 2293–2305 CrossRef CAS
.
- S. A. Umoren, C. Pan, Y. Li and F. H. Wang, J. Adhes. Sci. Technol., 2013, 28, 31–37 CrossRef
.
- F. B. Ravari and A. Dadgareenezhad, J. Chil. Chem. Soc., 2013, 58, 1853–1857 CrossRef CAS
.
- O. Sanni, C. A. Loto and A. P. I. Popola, Pol. J. Chem. Technol., 2013, 15, 60–64 CAS
.
- T. Sethi, A. Chaturvedi, R. K. Upadhyaya and S. P. Mathur, Prot. Met. Phys. Chem. Surf., 2009, 45, 466–471 CrossRef CAS
.
- O. G. Echem and A. O. James, Int. J. Appl. Res., 2015, 1, 353–359 Search PubMed
.
- E. A. Noor, Mater. Chem. Phys., 2009, 114, 533–541 CrossRef CAS
.
- A. Ehsani, M. Nasrollahzadeh, M. G. Mahjani, R. Moshrefi and H. Mostaanzadeh, J. Ind. Eng. Chem., 2014, 20, 4363–4370 CrossRef CAS
.
- D. Mercier, M. Herinx and M. G. Barthés-Labrousse, Corros. Sci., 2010, 52, 3405–3412 CrossRef CAS
.
- E. E. Ebenso, Mater. Chem. Phys., 2003, 79, 58–70 CrossRef CAS
.
- A. Innocent Okechi, M. Innocent Chimezie, O. Okoro and E. O. Emeka, Pigm. Resin Technol., 2014, 43, 151–158 CrossRef
.
- I. O. Arukalam, I. C. Madufor, O. Ogbobe and E. Oguzie, Open Corros. J., 2014, 6, 1–10 CrossRef
.
- M. M. Solomon and S. A. Umoren, Measurement, 2015, 76, 104–116 CrossRef
.
- A. Mohamad, A. Kadhum, A. Al-Amiery, L. Ying and A. Musa, Met. Mater. Int., 2014, 20, 459–467 CrossRef CAS
.
- P. Arellanes-Lozada, O. Olivares-Xometl, D. Guzmán-Lucero, N. V. Likhanova, M. A. Domínguez-Aguilar, I. V. Lijanova and E. Arce-Estrada, Materials, 2014, 7, 5711–5734 CrossRef CAS PubMed
.
- S. S. Abd El Rehim, M. A. Amin, S. O. Moussa and A. S. Ellithy, Mater. Chem. Phys., 2008, 112, 898–906 CrossRef CAS
.
- R. Rosliza, W. B. Wan Nik and H. B. Senin, Mater. Chem. Phys., 2008, 107, 281–288 CrossRef CAS
.
- S. A. Umoren, O. Ogbobe, P. C. Okafor and E. E. Ebenso, J. Appl. Polym. Sci., 2007, 105, 3363–3370 CrossRef CAS
.
- A. Musa, A. Mohamad, A. Kadhum and Y. Tabal, J. Mater. Eng. Perform., 2011, 20, 394–398 CrossRef CAS
.
- S. John and A. Joseph, Mater. Corros., 2013, 64, 625–632 CrossRef CAS
.
- S. John, K. M. Ali and A. Joseph, Bull. Mater. Sci., 2011, 34, 1245–1256 CrossRef CAS
.
- K. F. Khaled, Corros. Sci., 2010, 52, 2905–2916 CrossRef CAS
.
- M. A. Amin, Q. Mohsen and O. A. Hazzazi, Mater. Chem. Phys., 2009, 114, 908–914 CrossRef CAS
.
- A. S. Fouda, M. Abdallah and M. Eissa, Afr. J. Pure Appl. Chem., 2013, 7, 394–404 Search PubMed
.
- A. S. Fouda, M. F. El-Sherbiny and M. M. Motawea, Desalin. Water Treat., 2011, 30, 207–216 CrossRef CAS
.
- A. S. Fouda, M. Abdallah, I. S. Ahmed and M. Eissa, Arabian J. Chem., 2012, 5, 297–307 CrossRef CAS
.
- M. A. Amin, J. Appl. Electrochem., 2009, 39, 689–696 CrossRef CAS
.
- K. M. Devender, K. U. Rajesh and C. Alok, Rev. Roum. Chim., 2010, 55, 227–232 CAS
.
- E. E. Ebenso, P. C. Okafor and U. J. Ekpe, Anti-Corros. Methods Mater., 2003, 50, 414–421 CrossRef CAS
.
- P. S. Desai and R. T. Vashi, Anti-Corros. Methods Mater., 2011, 58, 70–75 CrossRef CAS
.
- P. S. Desai and R. T. Vashi, J. Indian Chem. Soc., 2008, 85, 92–96 CAS
.
- P. S. Desai and R. T. Vashi, J. Indian Chem. Soc., 2009, 86, 547–550 CAS
.
- A. Nithya and S. Rajendran, Indian J. Appl. Res., 2013, 3, 1–3 Search PubMed
.
- H. Ashassi-Sorkhabi, Z. Ghasemi and D. Seifzadeh, Appl. Surf. Sci., 2005, 249, 408–418 CrossRef CAS
.
- S. M. Sayyah, S. S. Abd El-Rehim, M. M. El-Deeb and S. M. Mohamed, The corrosion inhibition of aluminium by some of 3-alkyloxyaniline monomeric surfactants and their analogues polymers in 0.5 M HCl solution in Developments in Corrosion Protection, 2014, DOI:10.5772/57350
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11818f |
| This journal is © The Royal Society of Chemistry 2016 |


