DOI:
10.1039/C6RA11727A
(Paper)
RSC Adv., 2016,
6, 64665-64675
Facile preparation of amidoxime-functionalized fiber by microwave-assisted method for the enhanced adsorption of chromium(VI) from aqueous solution†
Received
5th May 2016
, Accepted 16th June 2016
First published on 16th June 2016
Abstract
In this study, a facile and highly efficient approach, the microwave-assisted (MW-aid) method, was applied for the synthesis of amidoxime-functionalized fibrous adsorbent (PANMW-AO fibers), which exhibited enhanced adsorption capacities for Cr(VI) in aqueous solution. The preparation condition was optimized under four independent variables including: MW power, mass of NH2OH·HCl, time and bath ratio, based on the Box-Behnken design with response surface methodology (RSM). A period of 1 × 5 min was determined to be the optimum microwave (MW) ageing time for the synthesis of PANMW-AO, which is dramatically faster than using conventional heating methods. Effects of pH, contact time, initial concentration of Cr(VI) and temperature on adsorption were investigated systematically by bath adsorption experiments. Nonlinear solutions of a pseudo-second-order kinetic model and Langmuir isotherm model were found to provide the closest fit to the experimental data for the adsorption process of Cr(VI), which indicated that chemisorption is the controlling mechanism and monolayer adsorption is dominant. Thermodynamic parameters revealed the spontaneity of the adsorption process, and higher temperature favored adsorption. The formation of PANMW-AO and Cr(VI)-adsorbed PANMW-AO has been characterized by FTIR, SEM/EDX and XPS instrumentations, which demonstrate that Cr(VI) was adsorbed by the amidoxime group via a surface complexation mechanism. The adsorption of Cr(VI) ions was hardly affected by common coexisting ions such as SO42−, NO3− and Cl−. High desorption efficiency (>90%) of Cr(VI) was achieved using 0.1 M H2SO4 as effluent, by which the investigated adsorbent could be used repeatedly five times with a small decrease in sorption capacity. Rapidity of synthesis and low cost, coupled with highly efficient and rapid adsorption of Cr(VI) ions, make PANMW-AO fibers an attractive adsorbent in the potential application for wastewater treatment.
1 Introduction
Three Cr forms, Cr(0), Cr(III) and Cr(VI), are used commercially and are thermodynamically stable when present in aquatic environments. However, only Cr(VI) has been shown to cause cancers in laboratory animals and occupationally exposed workers as it can diffuse as HCrO4− or CrO42− through cell membranes.1 Sources for Cr(VI) contaminated water originate from industrial effluents such as electroplating, mining extraction, leather tanning and pigment production, causing various health effects ranging from skin irritation to lung cancer, as well as kidney, liver, and gastric damage in humans.2,3 Because of its high toxicity, the discharge of Cr(VI) to surface water is regulated to below 0.5 mg L−1 according to the Integrated Wastewater Discharge Standard of China (GB 8978-1996) while the total Cr [including Cr(III) and Cr(VI)] is regulated to below 1.5 mg L−1.4,5 Noteworthily, due to its oxyanion forms like Cr2O7−, HCrO4− and CrO42− in aquatic environments, it is poorly adsorbed by negatively charged soil particles, resulting in high mobility in the water.6 Thus, it is mandatory to develop and implement an effective and reliable technology to remove Cr(VI) from the water on account of human health security.
The techniques being applied for the removal of Cr(VI) include precipitation,7 adsorption,8 ion exchange,9 reverse osmosis,10 electrodialysis11 and others.12 Among the various methods, adsorption is one of the simplest, economically beneficial methods that can remove the metal more effectively.13,14 Heretofore, due to their high capacity and selectivity, adsorbents immobilized with amidoxime groups were considered to be the most promising materials for the removal of metal ions.15 Many types of adsorbents consisting of amidoxime groups have been prepared and their adsorption performances towards metal ions were reported. Chen et al.16 prepared silica gel supported amidoxime adsorbents with “heterogeneous” and “homogeneous” methods, which exhibited excellent adsorption selectivity for Hg(II). Shaaban et al.17 studied Cu(II), Ni(II) and Pb(II) adsorption by a new amidoxime chelating resin (PAO-AM) and the maximum adsorption capacities for these three metal ions were 146.17, 113.85 and 236.21 mg g−1, respectively. The recovery of uranium(VI) from seawater by amidoxime functionalized adsorbents has attracted abundant attention during recent decades,18–20 while few studies have covered research on Cr(VI) adsorption from wastewater. Ramazan Coskun et al.21 prepared a fibrous adsorbent especially including double amidoxime groups through free radical reaction for the removal of Cr(VI) from wastewater, and the maximum adsorption capacity reached 125 mg g−1 at pH 2.
Nevertheless, the adsorption capacity as well as the amidoxime grafting rate have become the major challenges for the prepared adsorbents, and improvements are needed to be proposed. Currently, conventional heating is the universal method to fulfill the functionalization process, which has a lack of efficiency and wastes energy. The microwave-assisted (MW-aid) method as a highly efficient heating method has wide applications in chemosynthesis due to its characteristics of rapid volumetric heating, high reaction rate, short reaction time, enhanced reaction selectivity and energy saving.22–24 As is well known, the effect of the MW-aid method is created by the interaction of the dipole moment of the molecules with the high-frequency electromagnetic radiation.25,26 The water molecule is one of the best solvents for MW-aid owing to its large dipole moment and total nontoxicity.27
Herein, we introduced a rapid and facile method to prepare amidoxime functionalized polyacrylonitrile (PAN) fiber by an environmentally friendly MW-aid method. This work is to serve as not only an expansion of the MW-aid method in applications of polymer synthesis, but also an expansion of the PAN-based fibrous material as a high-efficient adsorbent for Cr(VI) removal from aqueous solution. In order to find the optimum synthesis conditions, a comprehensive study in association with the factors of MW power, mass of NH2OH·HCl, time and bath ratio was performed by using the response surface methodology (RSM). Kinetic, equilibrium isotherm models and thermodynamic parameters were explored to disclose the process of Cr(VI) adsorption. The regeneration of modified PAN fiber was evaluated in its application. Moreover, the adsorption mechanism was discussed.
2 Experimental
2.1 Materials and apparatus
The polyacrylonitrile fiber (PANF) made of 100% acrylonitrile with a diameter of 10 ± 0.5 μm was purchased from Beijing Rongnai Industry Material Co., Ltd. (Beijing, PR China). Hydroxylamine hydrochloride (NH2OH·HCl) and sodium carbonate (Na2CO3) were both supplied by Aladdin Chemical Reagent Co., Ltd. (Shanghai, PR China). The Cr(VI) solution was prepared by dissolving an appropriate amount of potassium dichromate (K2Cr2O7, Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, PR China) in deionized water. All of the reagents were used without further purification.
2.2 Preparation of amidoxime functionalized PAN fiber
The polyacrylonitrile fiber was dried in the oven overnight before use and cut into 5 cm lengths, to prevent its becoming entwined while stirring. The preparation procedure is simple and described as follows: first, hydroxylamine hydrochloride and sodium carbonate were dissolved in deionized water, which was placed in a 250 mL three-neck flask. Subsequently the PANF was added into the solution and the mixture was subjected to microwave irradiation. A specialized commercial microwave reactor (COOLPEX-E) with a maximum power range of 1200 W was used for the reaction. Fig. S1 of ESI† shows the components of the MW reactor. After reaction, the modified fiber was washed several times with hot distilled water until neutral and dried in a vacuum at 343 K overnight.
The grafting rate percentage (GP%) was calculated by gravimetry through the following equation:
| |
 | (1) |
where
m0 and
m1 are the weights of raw polyacrylonitrile fiber and amidoxime-functionalized fiber, respectively. The modified PAN fiber was named as PAN
MW-AO.
2.3 Experimental response surface methodology (RSM)
RSM is an efficient technique for the optimization of a multivariable system.28 In this study, the optimal GP% of PANMW-AO was obtained by RSM using Design Expert 7.0. The four independent parameters chosen in this study were MW power (X1), mass of NH2OH·HCl (X2), time (X3) and bath ratio (X4), with the GP% of PANMW-AO fibers as the response. The low, center and high levels of each variable were designated as −1, 0 and +1, respectively, as illustrated in Table 1. Box-Behnken Design (BBD) is the most frequently used form of RSM,29–31 which was employed to evaluate the influence of the four independent variables in 29 sets of experiments. The fundamental assumptions of RSM and more detailed information have been discussed elsewhere.
Table 1 Experimental range and levels of the independent variables
| Variables |
Symbol |
−1 |
0 |
+1 |
| MW power (W): A |
X1 |
300 |
500 |
700 |
| Mass of NH2OH·HCl (g): B |
X2 |
2.0 |
3.0 |
4.0 |
| Time (min): C |
X3 |
1 × 3 |
1 × 5 |
1 × 7 |
| Bath ratio (g g−1): D |
X4 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
A second-order polynomial equation was used to fit the experimental results of BBD as follows:
| | |
Y = b0 + b1X1 + b2X2 + b3X3 + b4X4 + b12X1X2 + b13X1X3 + b14X1X4 + b23X2X3 + b24X2X4 + b34X3X4 + b11X12 + b22X22 + b33X32 + b44X42
| (2) |
where
Y represents the response variable (GP%),
bi,
bii and
bij are the regression coefficients for linear and quadratic effects and the coefficients of the interaction parameters, respectively, and
Xi are the independent variables.
2.4 Characterization and analysis
The concentration of metal ions was determined using an inductively coupled plasma optimal emission spectrometer (ICP-OES; PerkinElmer Optimal 8300). Zeta potential measurements were conducted with a Zeta voltmeter (Zetasizer Nano ZS90). Fourier transform infrared spectroscopy (FT-IR) experiments on PANMW-AO were performed via an FT-IR spectrometer (PerkinElmer spectrum 100), and the spectra were recorded at wave numbers ranging from 400 to 4000 cm−1. The surface morphology of PANMW-AO was observed by scanning electron microscope-energy dispersive X-ray spectroscopy (SEM/EDX; FEI QUANTA 200). In order to observe the changes of surface morphology after adsorption, an SEM/EDX experiment on PANMW-AO after adsorbing Cr(VI) was performed. The X-ray photoelectron spectroscopy (XPS) measurements were conducted using an XPS spectrometer (Thermo Fisher Scientific, ESCALAB 250Xi), with monochromatized Al Kα X-rays.
2.5 Batch adsorption experiments
The prepared PANMW-AO fibers were used as adsorbents for the removal of Cr(VI) from aqueous systems. All batch adsorption experiments were performed on a model SHA-C shaker (Ronghua Instrumental Manufactory Co., Ltd., China) with a shaking speed of 100 rpm. The effect of pH value on adsorption was studied as pH ranged from 1 to 10 with the initial concentration of 300 mg L−1 at room temperature (20 °C). Coexisting anion solutions were prepared by including different amounts of Na2SO4, NaNO3 and NaCl in the Cr(VI) solution. The kinetic adsorption of Cr(VI) was studied by adding 0.05 g PANMW-AO into a 50 mL volume of Cr(VI) solution (300 mg L−1), for a preset time at pH 2. Adsorption isotherms for each heavy metal were conducted with the pH value at 2 and initial concentrations ranging from 20 to 320 mg L−1 at 293 K, 298 K, and 303 K respectively. The amount of adsorbed (qe) on the modified fiber can be obtained by knowing the equilibrium concentration, Ce, remaining in the solution phase, the aqueous phase volume, V (50 mL), and the initial concentration of hexavalent chromium (C0) using the relation:| |
 | (3) |
2.6 Desorption and regeneration experiments
For regeneration of the prepared adsorbents, the PANMW-AO fibers were first placed into contact with 300 mg L−1 Cr(VI) for 12 h at optimum pH and 293 K, and then rinsed with distilled water to remove any residual solution and dried at room temperature overnight. After that, the Cr(VI) binding fibers were immersed into 0.1 M H2SO4 and shaken at 293 K for 30 min. Subsequently, the desorbed Cr(VI) ions were determined by ICP-OES and the desorption efficiency (%) was calculated based on the percentage of the ratio between the desorbed and preadsorbed amounts of the ions. The adsorption/desorption cycle was repeated five times using the same PANMW-AO fibers to estimate the regeneration performance.
3 Results and discussion
3.1 Analysis of response surface design
The total set of experiments in this study based on a 3 level and 4 factor experimental design is elucidated in Table S1 of ESI.† In accordance with the experimental design, a second-order polynomial equation on the basis of actual factors is applied to elucidate the empirical relationships between the independent variables and the response:
| GP% = 35.78 + 3.86X1 + 3.00X2 + 1.58X3 − 0.21X4 − 2.45X1X2 − 0.50X1X3 + 1.63X1X4 − 2.52X2X3 − 1.38X2X4 + 0.28X3X4 − 8.99X12 − 0.67X22 − 2.05X32 + 0.76X42 |
Analysis of variance (ANOVA) was applied to evaluate the adequacy of the model. From the ANOVA of the empirical second-order polynomial model (Table 2), the F value and p value for the model are 20.18 and < 0.0001 respectively, indicating that the model is significant. There is only a 0.01% chance that the “model F value” could occur due to noise.32 Additionally, the “R-squared” of 0.9528 is in reasonable agreement with the “adj R-square” of 0.9056, confirming the good prediction capability of the model. Moreover, the values of p > F less than 0.05 indicate that the model terms are significant, while values greater than 0.05 indicate that the model terms are not significant. For this reason, many values are not significant at the 95% confidence level (a = 5%) and are dropped from the model to build a reduced model with better prediction capabilities.
Table 2 ANOVA for analysis of variance and adequacy of the quadratic model
| Source |
Sum of squares |
Degree of freedom |
Mean square |
F-value |
Prob > F |
| Model |
958.38 |
14 |
68.46 |
20.18 |
<0.0001 |
| X1 |
178.64 |
1 |
178.64 |
52.66 |
<0.0001 |
| X1 |
108.00 |
1 |
108.00 |
31.84 |
<0.0001 |
| X1 |
30.08 |
1 |
30.08 |
8.87 |
0.0100 |
| X1 |
0.52 |
1 |
0.52 |
0.15 |
0.7011 |
| X1X2 |
24.01 |
1 |
24.01 |
7.08 |
0.0186 |
| X22 |
2.94 |
1 |
2.94 |
0.87 |
0.3676 |
Table 2 reports the sum of squares, together with their F value and corresponding p value. According to the monomial coefficient values of the regression model, p(X1) < 0.001, p(X2) < 0.001, p(X3) = 0.0100, p(X4) = 0.7011, the bath ratio is an insignificant factor, while time is a significant factor. By contrast, power of radiation (X1) and mass of NH2OH·HCl (X2) play the most influential roles in the grafting percentage of modified fiber. The combined effect of MW power and mass of NH2OH·HCl on the grafting percentage of PANMW-AO fibers is shown in Fig. 1 as response surface plots. The GP% increases up to 40.6% with the increase of both MW power and mass of NH2OH·HCl.
 |
| | Fig. 1 Response surface plot for GP% of PANMW-AO fibers showing interaction between power of radiation (W) and mass of NH2OH·HCl (g) (time 1 × 5, bath ratio at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | |
The mathematic model generated during RSM implementation was validated by conducting experiments for a given optimal medium setting. To confirm the adequacy of the model for predicting the maximum grafting percentage of PANMW-AO fibers, a verification experiment was carried out using the optimum conditions (500 W, 4 g NH2OH·HCl, 1 × 5 min and bath ratio at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The average maximum GP% of 39.8% that is obtained from three replicate experiments is close to the predicted value of 40.5%, which proves the validity of the model for simulating the GP% of PANMW-AO fibers because of the good agreement between the predicted value and the experimental value.
1). The average maximum GP% of 39.8% that is obtained from three replicate experiments is close to the predicted value of 40.5%, which proves the validity of the model for simulating the GP% of PANMW-AO fibers because of the good agreement between the predicted value and the experimental value.
3.2 MW-aid mechanism of the preparation
In order to give an insight into the MW-aid mechanism for the preparation of PANMW-AO fibers, the products were prepared by conventional heating using a thermostatic magnetic heating agitator (CL-3, Gongyi City Yuhua Instrument Co., Ltd., China) and the prepared product was named PANCV-AO. The preparation parameters and the grafting results are shown in Table 3. It is obvious to observe that the consumed time is profoundly reduced and the obtained fibers from the MW-aid method show more than twice the GP% of those from conventional heating. Consequently, the adsorption capacity of the PANMW-AO fibers is considerably higher than PANCV-AO. Moreover, the mechanical strength of PANMW-AO is much more extensively maintained than PANCV-AO in terms of the remarkably reduced time of the functionalization process and thus is more suitable for practical use in water.
Table 3 Comparison of different heating types for preparation of PANMW-AO fiber
| Products |
Energy source |
Parameters |
GP% |
Adsorption capacity (mg g−1) |
| A |
B |
C |
D |
| PANMW-AO fibers |
MW-aid method |
500 W |
4.0 |
5 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40.6 |
219.8 |
| PANCV-AO fibers |
Conventional heating |
373 K |
4.0 |
720 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
19.8 |
92.6 |
MW irradiation offers a fast, inexpensive and convenient method of heating, which is an alternative way to provide energy to chemical reaction systems. Microwave effects in chemical reactions are related to short-range molecular friction, due to the continuous polarization of molecules caused by MW irradiation.33 This process of ordered (with electromagnetic field) and disordered (without the field) dipoles enhances the molecular attrition, increasing the local temperature and reaction rates.34,35 Moreover, it is believed that MW can provide a specific effect (non-thermal effect), which is generally connected to the selective adsorption of MW energy by polar molecules.36 In our reactive system, the matrix of PAN fibers as well as the solvent (water) are both polar and thus could interact with MW, leading to the reduction of the activation energies of the reaction. These might cause the higher grafting rate of amidoxime groups, which would be favorable for the uptake of Cr(VI).
3.3 Adsorption behaviour
3.3.1 Effect of pH on adsorption capacity. Commonly, the change in the external pH value mainly affects the surface charges of the adsorbents and the existence form of the ions.37 To explore the relationship between the external pH value and the adsorption capacity, the zeta potentials of the PANMW-AO and PAN fibers were determined over a range of pH values from 1 to 10. As shown in Fig. 2, the zeta potential of PANMW-AO fibers is positive at pH < 5.8, while that of the PAN fibers is positive at pH < 3.7, indicating that the zero point of the zeta potential for the PANMW-AO fibers is higher after the surface amidoxime reaction. The ascending zero point of the zeta potential may be attributed to the protonated –NH3+ of the amidoxime groups on the fibers and thus can provide better sorption amounts for negative Cr(VI) ions via electrostatic attraction. Furthermore, the effect of pH on Cr(VI) removal was examined. Expectedly, as depicted in Fig. 2, PANMW-AO fibers show distinctly higher Cr(VI) adsorption capacity than PAN fibers throughout the pH range. For PANMW-AO fibers, the highest Cr(VI) adsorption amount is observed at pH 2. Under this condition, the capacity of PANMW-AO fibers is as high as 219.8 mg g−1, more than 40 times that of PAN fibers. The Cr(VI) species are highly dependent on the pH value. H2CrO4 is the predominant species at pH below 1.0, which cannot be electrostatically attracted by the protonated amidoxime groups. HCrO4− and Cr2O72− are predominant in the pH range of 2.0–6.0, while CrO42− is the major species when pH > 6.38 At a lower pH (approximately 2), the Cr(VI) species are mostly in their monovalent forms (HCrO4−), which require only one exchange site and are more likely to be adsorbed. Noteworthily, the wastewater effluent from chromium industries is usually acid, which gives an edge to the application of PANMW-AO fibers with high adsorption capacity in treating Cr(VI) under this condition.
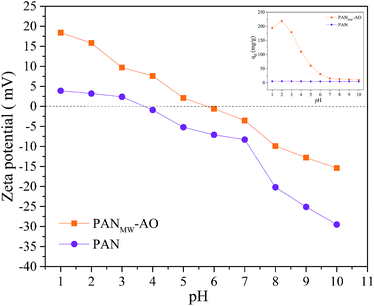 |
| | Fig. 2 Effect of pH on the adsorption of Cr(VI) onto the PANMW-AO fibers. | |
3.3.2 Adsorption kinetics. Adsorption kinetics are important constants for the evaluation of a good adsorbent. As can be seen from Fig. 3, the Cr(VI) uptake on PANMW-AO fibers is rapid in the first 30 min, contributing to about 70% of the ultimate adsorption amount for Cr(VI), and then ascends gradually. In this study, the adsorption equilibrium is achieved within about 2 h.
 |
| | Fig. 3 Adsorption kinetics curve of Cr(VI) on PANMW-AO at 298 K. | |
Kinetic models are a significant aspect of adsorption studies and define the efficiency of the adsorption process. In order to convincingly investigate the adsorption behaviors of Cr(VI) with the adsorbents, pseudo-first-order kinetics and pseudo-second-order kinetics have been employed to simulate the experimental data and the best fit model was selected based on both the nonlinear regression correlation coefficient (R2) and the calculated χ2 values. The value of χ2 represents the similarity between the data from the model and the data from the experiment test. A small χ2 value indicates similarities, while a larger number represents variation from the experimental data.39–41
The equations for these two kinetic models and χ2 are presented respectively as follows:
| |
 | (5) |
| |
 | (6) |
where
qe and
qt are the amounts of heavy metal ions adsorbed (mg g
−1) at equilibrium and at time
t (min), respectively, while
k1,
k2 and
k3 are the rate constants of the pseudo-first-order model (min
−1) and pseudo-second-order model (mg g
−1 min
−1). The
qe,cal (
qe,calculated) equilibrium capacity was calculated from the model (mg g
−1) and
qe,exp. (
qe,experimental) was the equilibrium capacity obtained from the experimental data (mg g
−1). As seen from the results listed in
Table 4, the pseudo-second-order provides better coefficients (
R2 = 0.9991) than the pseudo-first-order model (
R2 = 0.9758). Simultaneously, the good agreement between the
qe,cal (217.1 mg g
−1) and the
qe,exp (219.8 mg g
−1) suggests that the adsorption kinetics closely follows the pseudo-second-order rather than the pseudo-first-order model. Moreover, the
χ2 value of the pseudo-second-order model is smaller, which also proves its better fit with the experimental data. The pseudo-second-order model was developed on the assumption that the rate-determining step may be chemisorption promoted by covalent forces through electron sharing between adsorbent and adsorbate. Thus, the result suggests that the adsorption of Cr(
VI) on the PAN
MW-AO fibers is mainly controlled by chemically reactive adsorption.
Table 4 Kinetic model constants for the adsorption of Cr(VI) onto PANMW-AO fibers
| qe,exp. (mg g−1) |
Pseudo-first-order model |
Pseudo-second-order model |
Intra-particle diffusion model |
| qe,cal. (mg g−1) |
k1 (min−1) |
R2 |
χ2 |
qe,cal. (mg g−1) |
k2 × 104 (mg g−1 min−1) |
R2 |
χ2 |
k3 (mg g−1 min−1/2) |
C (mg g−1) |
R2 |
| 219.8 |
210.5 |
0.037 |
0.9758 |
10.5 |
217.1 |
1.76 |
0.9991 |
3.89 |
15.34 |
36.62 |
0.9152 |
Furthermore, an intra-particle diffusion model was also used to analyze the rate-limiting step in adsorption.42 The model can be expressed by the Weber–Morris equation as follows:
where
k3 is the intra-particle diffusion rate constant and the intercept
C (mg g
−1) is a constant related to the thickness of the boundary layer. It is postulated that the intra-particle diffusion is considered to be the rate-limiting step when the plot of
qt vs. t0.5 yields a straight line that passes through the original data.
The plot of qt vs. t0.5 is given in Fig. 4. The adsorption plots of Cr(VI) do not pass through the origin, which suggests the intra-particle diffusion is not the only rate-limiting step. This behavior indicates that the Cr(VI) adsorption process by PANMW-AO fibers involves more than one single kinetic stage. The initial rapid adsorption may be governed by boundary layer diffusion and the subsequent slow uptake is attributed to the intra-particle diffusion effect. As listed in Table 3, the obtained high values of k3 and C indicate that the PANMW-AO fibers exhibit a fast removal rate for Cr(VI) from aqueous solution.
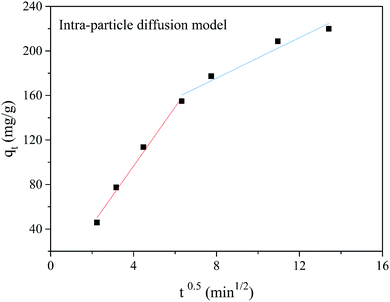 |
| | Fig. 4 Intra-particle diffusion model for the adsorption of Cr(VI) onto PANMW-AO fibers. | |
3.3.3 Adsorption isotherms. The effect of initial concentration on the uptake amount of PANMW-AO fibers for Cr(VI) was studied at 293 K, 298 K and 303 K. As illustrated in Fig. 6, the amount of metal ions adsorbed onto the PANMW-AO fibers is increased with increasing Cr(VI) concentration, and then the plateau value is reached. The maximum adsorption capacity for Cr(VI) was found to be 218.6 mg g−1 at 303 K.The adsorption isotherms of PANMW-AO fibers were investigated by the Langmuir, Freundlich and Temkin models, respectively. The Langmuir model assumes that a monomolecular layer is formed when adsorption takes place with an interaction between the adsorbed molecules.
The Freundlich isotherm is an empirical equation assuming that the adsorption process takes place on heterogeneous surfaces and the adsorption capacity is related to the concentration of adsorbate at equilibrium. The Temkin isotherm assumes that the heat of adsorption of the molecules decreases linearly due to sorbent–sorbate interaction.43 The Langmuir, Freundlich and Temkin models can be expressed as follows:
| |
 | (8) |
| |
 | (9) |
| |
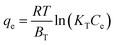 | (10) |
where
qe is the amount of metal ions adsorbed at equilibrium by the adsorbent (mg g
−1),
Ce is the equilibrium concentration (mg L
−1),
qm is the theoretical saturation adsorption capacity (mg g
−1),
Kd (L mg
−1) is the equilibrium Langmuir constant, and
Kf (mg g
−1) and
n are constants representing the adsorption capacity and intensity of adsorption.
KT (L g
−1) is the equilibrium binding constant corresponding to the maximum binding energy,
BT (kJ mol
−1) is the Temkin constant related to the heat of adsorption,
R (8.314 J mol
−1 K
−1) is the universal gas constant and
T (K) is the absolute temperature.
The fitting of the equilibrium data at different temperatures by the Langmuir, Freundlich and Temkin models was conducted and the results are summarized in Fig. 5 and Table 5. In the comparison of R2 and χ2 values at all temperatures, the adsorption isotherm data fit the Langmuir model better than both the Freundlich and Temkin models, which indicates the monolayer adsorption of Cr(VI) by PANMW-AO fibers. Additionally, the maximum adsorption capacities calculated from the Langmuir model are highly consistent with the actual saturated adsorption capacity for Cr(VI) at each temperature.
 |
| | Fig. 5 Adsorption isotherm curves and the variation of RL with initial concentration of Cr(VI) on PANMW-AO at 293 K, 298 K and 303 K. | |
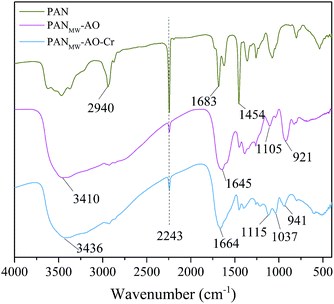 |
| | Fig. 6 FT-IR spectrum of PANF, PANMW-AO and PANMW-AO–Cr(VI). | |
Table 5 Adsorption isotherm parameters for the adsorption of Cr(VI) on PANMW-AO at 293 K, 298 K and 303 K
| T (K) |
Langmuir parameters |
Freundlich parameters |
Temkin parameters |
| qm (mg g−1) |
Kd (L mg−1) |
R2 |
χ2 |
n |
Kf (mg g−1) |
R2 |
χ2 |
KT (L g−1) |
BT (kJ mol−1) |
R2 |
χ2 |
| 293 |
232.70 |
0.0536 |
0.9985 |
7.7 |
2.39 |
33.31 |
0.9555 |
238.5 |
0.588 |
48.94 |
0.9967 |
15.1 |
| 298 |
238.28 |
0.0700 |
0.9992 |
5.0 |
2.53 |
40.29 |
0.9459 |
320.9 |
0.783 |
48.92 |
0.9945 |
28.0 |
| 303 |
250.71 |
0.0841 |
0.9986 |
9.6 |
2.58 |
46.89 |
0.9429 |
384.8 |
1.04 |
48.09 |
0.9914 |
49.4 |
Moreover, the dimensionless separation factor RL could be calculated by applying the Langmuir parameters in the following equation:
| |
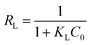 | (11) |
This factor can indicate the favorability of the adsorption process, which is indicated to be either unfavorable (RL > 1), linear (RL = 1), favorable (RL < 1), or irreversible (RL = 0) under each condition.44 For the initial concentration studied at all temperatures, the RL values of Cr(VI) fall between 0 and 0.5, confirming the favorable nature of adsorption by PANMW-AO fibers. Additionally, the values of RL decrease with the increasing of the initial concentration of adsorbate, which indicates that the adsorption process is more favorable at higher concentration.
3.3.4 Thermodynamic analysis. In order to better understand the effect of temperature on the adsorption of Cr(VI) ions onto the PANMW-AO fibers, three basic thermodynamic parameters were studied: the Gibbs free energy change (ΔG), entropy (ΔS) and enthalpy (ΔH). The thermodynamic parameters ΔG, ΔH and ΔS for this adsorption process were determined by using the following equations:| |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (12) |
| |
 | (13) |
where Kd is the adsorption equilibrium constant obtained from the Langmuir model, R is the universal gas constant (8.314 J mol−1 K−1), and T is the temperature (K). The values of ΔH and ΔS could be obtained as the slope and intercept from a linear plot between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus 1/T.
Kd versus 1/T.The corresponding values of the thermodynamic parameters are presented in Table 6, which shows that the ΔH and ΔS are positive for all the experiments and ΔG is negative in all systems.
Table 6 Thermodynamic parameters for the adsorption of Cr(VI) on PANMW-AO
| ΔH (kJ mol−1) |
ΔS (J mol−1 K−1) |
ΔG (kJ mol−1) |
| 293 K |
298 K |
303 K |
| 41.57 |
17.45 |
−2.45 |
−3.20 |
−3.73 |
The negative values of ΔG revealed that the adsorption process was a chemical exothermic reaction in nature, and with the increase of the temperature, the adsorbed amount at equilibrium increased. The positive value of ΔS reveals the increased randomness and a rise in the degrees of freedom at the PANMW-AO fibers–solution interface during the binding of the Cr(VI) ions on the active sites of the adsorbent.39 It is also observed that with an increase of temperature the value of ΔG decreases, indicating that the sorption process is spontaneous and thermodynamically favorable with an increase in temperature.
3.4 Characterization of mechanism study
The FT-IR spectra of the raw PANF and PANMW-AO fibers before and after Cr(VI) adsorption are shown in Fig. 6. A sharp and distinct adsorption band at 2243 cm−1 is attributed to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups in the polyacrylonitrile fiber, which decreases remarkably after the conversion into amidoxime.18 Although the fiber was claimed to be made of 100% acrylonitrile, the adsorption at 1683 cm−1 still confirms the existence of methyl acrylate or methyl methacrylate. From the PANMW-AO spectrum, it can be observed that new peaks appear at 3410 cm−1 (broad, assigned to both N–H and O–H), 1645 cm−1 (assigned to C
N groups in the polyacrylonitrile fiber, which decreases remarkably after the conversion into amidoxime.18 Although the fiber was claimed to be made of 100% acrylonitrile, the adsorption at 1683 cm−1 still confirms the existence of methyl acrylate or methyl methacrylate. From the PANMW-AO spectrum, it can be observed that new peaks appear at 3410 cm−1 (broad, assigned to both N–H and O–H), 1645 cm−1 (assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1105 cm−1 (assigned to C–N) and 921 cm−1 (assigned to N–O), confirming the successful conversion of the nitrile group into the amidoxime group.16 After the adsorption of Cr(VI), the bands at 3410 cm−1, 1645 cm−1, 1105 cm−1 and 921 cm−1 are blue shifted into 3436 cm−1, 1664 cm−1, 1115 cm−1 and 941 cm−1, along with a new peak that occurs at 1037 cm−1. The blue shift of the vibration frequency indicates the formation of coordination bonds between amidoxime groups and metal ions.46 Consequently, these changes are all related to the chemical attachment of Cr(VI) onto the PANMW-AO fibers.
N), 1105 cm−1 (assigned to C–N) and 921 cm−1 (assigned to N–O), confirming the successful conversion of the nitrile group into the amidoxime group.16 After the adsorption of Cr(VI), the bands at 3410 cm−1, 1645 cm−1, 1105 cm−1 and 921 cm−1 are blue shifted into 3436 cm−1, 1664 cm−1, 1115 cm−1 and 941 cm−1, along with a new peak that occurs at 1037 cm−1. The blue shift of the vibration frequency indicates the formation of coordination bonds between amidoxime groups and metal ions.46 Consequently, these changes are all related to the chemical attachment of Cr(VI) onto the PANMW-AO fibers.
The morphologies and surface composition of raw PAN and PANMW-AO fibers before and after Cr(VI) adsorption were characterized by SEM/EDX and the results are shown in Fig. 8. Evidently, the smooth surface of the raw PAN fiber becomes creased, and the diameter of the modified fibers increases to a certain degree. Simultaneously, the EDX pattern provides the elemental information, which confirms the existence of Cr(VI) adsorbed on PANMW-AO fibers.
 |
| | Fig. 7 XPS spectra of high resolution scan of Cr 2p: (a) PANMW-AO fibers before adsorption; (b) PANMW-AO fibers after adsorption. | |
 |
| | Fig. 8 SEM images of (a) PAN, (b) PANMW-AO, (c) PANMW-AO–Cr and (d) EDX analysis of elemental composition of PANMW-AO–Cr(VI). | |
To further explore the possible mechanism of Cr(VI) adsorption by the PANMW-AO fiber, XPS experiments were performed to investigate the surface chemical composition of the PANMW-AO fibers before and after adsorption of Cr(VI). Normally, for the Cr 2p XPS spectrum, the significant binding energies at 577.0–578.0 eV and 586.0–588.0 eV correspond to Cr(III), while those for Cr(VI) are at 580.0–580.5 eV and 589.0–590.0 eV.41 As shown in the survey spectra in Fig. 7(b), the appearance of the Cr 2p spectra evidently confirmed the adsorption of Cr(VI) by the adsorbent. More specifically, two significant bands appear at binding energies of 586.5 eV and 576.6 eV, which are assigned to the Cr 2p3/2 and Cr 2p1/2 orbitals, respectively. The XPS data reveals that the chromium attached to the surface of the adsorbent is the trivalent form, which indicates that the Cr(VI) has been reduced into Cr(III) thoroughly during the adsorption onto the PANMW-AO fibers.
3.5 Effects of coexisting anions on Cr(VI) adsorption
In many cases, natural water sources contain various kinds of anions, which may compete with the Cr(VI) ions in the adsorption process. In the present study, the effect of different anions including NO3− (NaNO3), SO42− (NaSO4), and Cl− (NaCl) on Cr(VI) removal was studied, and the results are shown in Fig. 9. The capacity of Cr(VI) in the presence of 100 mg g−1 SO42− is 82.5% of that for solely Cr(VI) solution, whereas it is 89.4% and 92.3% for 100 mg g−1 NO3− and Cl−. Comparatively, the effects of these coexisting anions on Cr(VI) adsorption by PANMW-AO fibers are smaller than or equal to those of other materials reported in the literature.13,17,45 This could be explained by the different affinities of the ions toward PANMW-AO fibers, which mainly depend on the ion charge density. The Z/r (charge/radius) value of SO42− is larger than those for NO3− and Cl− and multivalent anions are adsorbed more readily than monovalent anions. Consequently, the decreasing trend of Cr(VI) adsorption capacity in the presence of competing anions is observed in the order: Cl− < NO3− < SO42−.
 |
| | Fig. 9 Effects of coexisting anions on the adsorption of Cr(VI). | |
3.6 Reusability test and comparison with literature
To assess their reusability, the used PANMW-AO fibers were separated by filtration after the first run and the extraction of adsorbed Cr(VI) ions, then 0.1 M H2SO4 was used to regenerate metal ions from the adsorbent. It is observed from Fig. 10 that the efficiency of the desorption process is generally above 90.5% and the adsorption capacity for Cr(VI) after five runs still maintains 80% of its original adsorption amount. On account of there being no significant loss of activity during the adsorption process, the PANMW-AO fibers are expected to be a reliable and powerful adsorbent to capture Cr(VI) from aqueous solution. Comparisons with other reported fibrous adsorbents, which were prepared through conventional heating, and with activated carbon, are listed in Table 7. The results reveal that MW irradiation is a facile and highly efficient way to improve the adsorption capacity of the adsorbent for Cr(VI). The consumed time and reagents are reduced while the amidoxime grafting rate is improved significantly. The combination of thermal and non-thermal effects of MW irradiation is believed to expedite the modification procedure and also result in the high grafting ratio of amidoxime groups. Commonly, MW irradiation is instantaneous, which leads to shorter start-up and rapid internal heating. Meanwhile, the non-thermal effects such as overheating, hot spots and selective heating may increase the probabilities of effective contacts between the reactants and thus improve the processes and obtain better yields.
 |
| | Fig. 10 Adsorption capacity of the PANMW-AO fibers after five repeated regenerations. | |
Table 7 Comparison of PANMW-AO fiber adsorption capacities with other fibrous adsorbents and activated carbon
| Adsorbents |
Grafting time (h) |
pH |
Adsorption capacity (mg g−1) |
Reference |
| Hybrid carbon fibers |
>10 |
2.0 |
190.0 |
47 |
| EDTA-anchored PAN-based nanofiber |
2 |
3.0 |
66.24 |
6 |
| Strong alkaline anion exchange fiber |
— |
2.0 |
201.2 |
48 |
| Thiol-modified cellulose nanofiber |
>24 |
4.0 |
76.5 ± 2.0 |
49 |
| Double amidoxime-containing PET fibers |
10 |
2.0 |
22.72 |
21 |
| PAN-NH2 nanofibers |
4 |
2.0 |
137.6 ± 4.1 |
50 |
| Fibrous adsorbent with amino and quaternary ammonium groups |
6 |
4.3 |
98.5 |
46 |
| Activated carbon |
— |
— |
21.34 |
8 |
| PANMW-AO fiber |
0.17 |
2.0 |
219.8 |
Present study |
4 Conclusions
The microwave-assisted method has been proven to be an efficient and rapid approach for the immobilization of amidoxime groups on PAN fibers. A significant reduction of the reaction time and energy consumption and a better adsorption capacity were obtained relative to conventional heating, and both thermal and non-thermal effects of MW irradiation were believed to make contributions to the grafting process. The preparation conditions have been investigated with a response surface methodology based on the Box-Behnken design, and the optimum conditions were found to be MW power at 500 W, 4 g NH2OH·HCl, 1 × 5 min time and bath ratio at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a maximum GP% gain of 40.6%. The obtained PANMW-AO fibers showed a very high affinity to Cr(VI) at pH 2 and the binding mechanism was proven to be surface complexation by the characterization results of FTIR, SEM/EDX and XPS. The pseudo second-order kinetic equation provides the best correlation for the process, suggesting a chemisorption process as the rate limiting step. According to the nonlinear regression correlation coefficient (R2) and the calculated χ2 values of the adsorption isotherm equations, the Langmuir isotherm has been found to be better fitted than the Freundlich and Temkin isotherms. The adsorption thermodynamics demonstrated that the adsorption process is spontaneous and endothermic. Competition from common coexisting ions, such as SO42−, NO3− and Cl−, was negligible. PANMW-AO fibers could be reused repeatedly 5 times with more than 80% of initial adsorption capacity, suggesting that PANMW-AO fibers have promising potential as an adsorbent for the removal of Cr(VI) from aqueous solution.
1, with a maximum GP% gain of 40.6%. The obtained PANMW-AO fibers showed a very high affinity to Cr(VI) at pH 2 and the binding mechanism was proven to be surface complexation by the characterization results of FTIR, SEM/EDX and XPS. The pseudo second-order kinetic equation provides the best correlation for the process, suggesting a chemisorption process as the rate limiting step. According to the nonlinear regression correlation coefficient (R2) and the calculated χ2 values of the adsorption isotherm equations, the Langmuir isotherm has been found to be better fitted than the Freundlich and Temkin isotherms. The adsorption thermodynamics demonstrated that the adsorption process is spontaneous and endothermic. Competition from common coexisting ions, such as SO42−, NO3− and Cl−, was negligible. PANMW-AO fibers could be reused repeatedly 5 times with more than 80% of initial adsorption capacity, suggesting that PANMW-AO fibers have promising potential as an adsorbent for the removal of Cr(VI) from aqueous solution.
Acknowledgements
The work was supported by State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (2015DX03), Fundamental Research Funds for the Central Universities (HIT. NSRIF. 201671) and National Science Foundation for Post-doctoral Scientists of China (2014M561356).
Notes and references
- M. Owlad, M. K. Aroua, W. A. W. Daud and S. Baroutian, Water, Air, Soil Pollut., 2008, 200, 59–77 CrossRef.
- D. W. O'Connell, C. Birkinshaw and T. F. O'Dwyer, Bioresour. Technol., 2008, 99, 6709–6724 CrossRef PubMed.
- K. C. K. Lai and I. M. C. Lo, Environ. Sci. Technol., 2008, 42, 1238–1244 CrossRef CAS PubMed.
- M. Nuchter, B. Ondruschka, W. Bonrath and A. Gum, Green Chem., 2004, 6, 128–141 RSC.
- X. Lv, J. Xu, G. Jiang, J. Tang and X. Xu, J. Colloid Interface Sci., 2012, 369, 460–469 CrossRef CAS PubMed.
- E. F. C. Chaúque, L. N. Dlamini, A. A. Adelodun, C. J. Greyling and J. Catherine Ngila, Appl. Surf. Sci., 2016, 369, 19–28 CrossRef.
- R. C. Thomson and M. K. Miller, Acta Mater., 1998, 46, 2203–2213 CrossRef CAS.
- Z.-Y. Kong, J.-F. Wei, Y.-H. Li, N.-N. Liu, H. Zhang, Y. Zhang and L. Cui, Chem. Eng. J., 2014, 254, 365–373 CrossRef CAS.
- G. Wójcik, V. Neagu and I. Bunia, J. Hazard. Mater., 2011, 190, 544–552 CrossRef PubMed.
- A. Bódalo, J. L. Gómez, E. Gómez, A. M. Hidalgo and A. Alemán, Desalination, 2005, 180, 277–284 CrossRef.
- T. Ölmez, J. Hazard. Mater., 2009, 162, 1371–1378 CrossRef PubMed.
- K. Y. Wang and T. S. Chung, J. Membr. Sci., 2006, 281, 307–315 CrossRef CAS.
- Q. Li, Y. Qian, H. Cui, Q. Zhang, R. Tang and J. Zhai, Chem. Eng. J., 2011, 173, 715–721 CrossRef CAS.
- C.-J. Li, Y.-J. Li, J.-N. Wang and J. Cheng, Chem. Eng. J., 2013, 220, 294–301 CrossRef CAS.
- C. Gunathilake, J. Gorka, S. Dai and M. Jaroniec, J. Mater. Chem. A, 2015, 3, 11650–11659 CAS.
- J. Chen, R. Qu, Y. Zhang, C. Sun, C. Wang, C. Ji, P. Yin, H. Chen and Y. Niu, Chem. Eng. J., 2012, 209, 235–244 CrossRef CAS.
- A. F. Shaaban, D. A. Fadel, A. A. Mahmoud, M. A. Elkomy and S. M. Elbahy, J. Environ. Chem. Eng., 2014, 2, 632–641 CrossRef CAS.
- G. Bayramoglu and M. Y. Arica, Microporous Mesoporous Mater., 2016, 226, 117–124 CrossRef CAS.
- Y. Zhao, X. Wang, J. Li and X. Wang, Polym. Chem., 2015, 6, 5376–5384 RSC.
- H. Zhao, X. Liu, M. Yu, Z. Wang, B. Zhang, H. Ma, M. Wang and J. Li, Ind. Eng. Chem. Res., 2015, 54, 3101–3106 CrossRef CAS.
- R. Coskun and Y. Dilci, J. Macromol. Sci., Part A: Pure Appl.Chem., 2014, 51, 767–782 CrossRef CAS.
- V. O. Njoku, K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2013, 215–216, 383–388 CrossRef CAS.
- A. Roy and J. Bhattacharya, Chem. Eng. J., 2012, 211–212, 493–500 CrossRef CAS.
- X. J. Wang, Y. Wang, X. Wang, M. Liu, S. Q. Xia, D. Q. Yin, Y. L. Zhang and J. F. Zhao, Chem. Eng. J., 2011, 174, 326–332 CrossRef CAS.
- I. Bilecka and M. Niederberger, Nanoscale, 2010, 2, 1358–1374 RSC.
- C. Oliver Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC.
- S. Deng, P. Wang, G. Zhang and Y. Dou, J. Hazard. Mater., 2016, 307, 64–72 CrossRef CAS PubMed.
- S. Chatterjee, A. Kumar, S. Basu and S. Dutta, Chem. Eng. J., 2012, 181–182, 289–299 CrossRef CAS.
- M. Khayet, A. Y. Zahrim and N. Hilal, Chem. Eng. J., 2011, 167, 77–83 CrossRef CAS.
- I. Dahlan, K. T. Lee, A. H. Kamaruddin and A. R. Mohamed, Environ. Sci. Technol., 2008, 42, 1499–1504 CrossRef CAS PubMed.
- J. Wu, H. Zhang, N. Oturan, Y. Wang, L. Chen and M. A. Oturan, Chemosphere, 2012, 87, 614–620 CrossRef CAS PubMed.
- M. J. K. Bashir, H. A. Aziz, M. S. Yusoff and M. N. Adlan, Desalination, 2010, 254, 154–161 CrossRef CAS.
- L. Perreux, A. Loupy and F. Volatron, Tetrahedron, 2002, 58, 2155–2162 CrossRef CAS.
- L. C. Lopes, M. T. M. Barreto, K. M. Gonçalves, H. M. Alvarez, M. F. Heredia, R. O. M. A. de Souza, Y. Cordeiro, C. Dariva and A. T. Fricks, Enzyme Microb. Technol., 2015, 69, 10–18 CrossRef CAS PubMed.
- M. Zieliński, M. Zielińska and M. Dębowski, Bioresour. Technol., 2013, 127, 223–230 CrossRef PubMed.
- M. A. Herrero, J. M. Kremsner and C. O. Kappe, J. Org. Chem., 2008, 73, 36–47 CrossRef CAS PubMed.
- G. Ndayambaje, K. Laatikainen, M. Laatikainen, E. Beukes, O. Fatoba, N. van der Walt, L. Petrik and T. Sainio, Chem. Eng. J., 2016, 284, 1106–1116 CrossRef CAS.
- P. Miretzky and A. F. Cirelli, J. Hazard. Mater., 2010, 180, 1–19 CrossRef CAS PubMed.
- M. Sayın, M. Can, M. İmamoğlu and M. Arslan, React. Funct. Polym., 2015, 88, 31–38 CrossRef.
- T. S. Anirudhan, J. R. Deepa and Binusreejayan, Chem. Eng. J., 2015, 273, 390–400 CrossRef CAS.
- H. Gu, S. B. Rapole, J. Sharma, Y. Huang, D. Cao, H. A. Colorado, Z. Luo, N. Haldolaarachchige, D. P. Young, B. Walters, S. Wei and Z. Guo, RSC Adv., 2012, 2, 11007–11018 RSC.
- H. Gu, S. B. Rapole, Y. Huang, D. Cao, Z. Luo, S. Wei and Z. Guo, J. Mater. Chem. A, 2013, 1, 2011–2021 CAS.
- F. Gao, H. Gu, H. Wang, X. Wang, B. Xiang and Z. Guo, RSC Adv., 2015, 5, 60208–60219 RSC.
- C. Zhijiang, J. Jianru, Z. Qing and Y. Haizheng, RSC Adv., 2015, 5, 82310–82323 RSC.
- N. Horzum, T. Shahwan, O. Parlak and M. M. Demir, Chem. Eng. J., 2012, 213, 41–49 CrossRef CAS.
- J. Wang, L. Zhao, W. Duan, L. Han and Y. Chen, Ind. Eng. Chem. Res., 2012, 51, 13655–13662 CrossRef CAS.
- W. Zheng, J. Hu, Z. Han, E. Diesel, Z. Wang, Z. Zheng, C. Ba, J. Langer and J. Economy, Chem. Eng. J., 2016, 287, 54–61 CrossRef CAS.
- W. Wang, M. Li and Q. Zeng, Sep. Purif. Technol., 2015, 149, 16–23 CrossRef CAS.
- R. Yang, K. B. Aubrecht, H. Ma, R. Wang, R. B. Grubbs, B. S. Hsiao and B. Chu, Polymer, 2014, 55, 1167–1176 CrossRef CAS.
- M. Avila, T. Burks, F. Akhtar, M. Göthelid, P. C. Lansåker, M. S. Toprak, M. Muhammed and A. Uheida, Chem. Eng. J., 2014, 245, 201–209 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Feature of the MW reactor, experimental design and response value for different conditions. See DOI: 10.1039/c6ra11727a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The average maximum GP% of 39.8% that is obtained from three replicate experiments is close to the predicted value of 40.5%, which proves the validity of the model for simulating the GP% of PANMW-AO fibers because of the good agreement between the predicted value and the experimental value.
1). The average maximum GP% of 39.8% that is obtained from three replicate experiments is close to the predicted value of 40.5%, which proves the validity of the model for simulating the GP% of PANMW-AO fibers because of the good agreement between the predicted value and the experimental value.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1



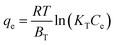

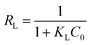
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd
Kd

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd versus 1/T.
Kd versus 1/T.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups in the polyacrylonitrile fiber, which decreases remarkably after the conversion into amidoxime.18 Although the fiber was claimed to be made of 100% acrylonitrile, the adsorption at 1683 cm−1 still confirms the existence of methyl acrylate or methyl methacrylate. From the PANMW-AO spectrum, it can be observed that new peaks appear at 3410 cm−1 (broad, assigned to both N–H and O–H), 1645 cm−1 (assigned to C
N groups in the polyacrylonitrile fiber, which decreases remarkably after the conversion into amidoxime.18 Although the fiber was claimed to be made of 100% acrylonitrile, the adsorption at 1683 cm−1 still confirms the existence of methyl acrylate or methyl methacrylate. From the PANMW-AO spectrum, it can be observed that new peaks appear at 3410 cm−1 (broad, assigned to both N–H and O–H), 1645 cm−1 (assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1105 cm−1 (assigned to C–N) and 921 cm−1 (assigned to N–O), confirming the successful conversion of the nitrile group into the amidoxime group.16 After the adsorption of Cr(VI), the bands at 3410 cm−1, 1645 cm−1, 1105 cm−1 and 921 cm−1 are blue shifted into 3436 cm−1, 1664 cm−1, 1115 cm−1 and 941 cm−1, along with a new peak that occurs at 1037 cm−1. The blue shift of the vibration frequency indicates the formation of coordination bonds between amidoxime groups and metal ions.46 Consequently, these changes are all related to the chemical attachment of Cr(VI) onto the PANMW-AO fibers.
N), 1105 cm−1 (assigned to C–N) and 921 cm−1 (assigned to N–O), confirming the successful conversion of the nitrile group into the amidoxime group.16 After the adsorption of Cr(VI), the bands at 3410 cm−1, 1645 cm−1, 1105 cm−1 and 921 cm−1 are blue shifted into 3436 cm−1, 1664 cm−1, 1115 cm−1 and 941 cm−1, along with a new peak that occurs at 1037 cm−1. The blue shift of the vibration frequency indicates the formation of coordination bonds between amidoxime groups and metal ions.46 Consequently, these changes are all related to the chemical attachment of Cr(VI) onto the PANMW-AO fibers.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a maximum GP% gain of 40.6%. The obtained PANMW-AO fibers showed a very high affinity to Cr(VI) at pH 2 and the binding mechanism was proven to be surface complexation by the characterization results of FTIR, SEM/EDX and XPS. The pseudo second-order kinetic equation provides the best correlation for the process, suggesting a chemisorption process as the rate limiting step. According to the nonlinear regression correlation coefficient (R2) and the calculated χ2 values of the adsorption isotherm equations, the Langmuir isotherm has been found to be better fitted than the Freundlich and Temkin isotherms. The adsorption thermodynamics demonstrated that the adsorption process is spontaneous and endothermic. Competition from common coexisting ions, such as SO42−, NO3− and Cl−, was negligible. PANMW-AO fibers could be reused repeatedly 5 times with more than 80% of initial adsorption capacity, suggesting that PANMW-AO fibers have promising potential as an adsorbent for the removal of Cr(VI) from aqueous solution.
1, with a maximum GP% gain of 40.6%. The obtained PANMW-AO fibers showed a very high affinity to Cr(VI) at pH 2 and the binding mechanism was proven to be surface complexation by the characterization results of FTIR, SEM/EDX and XPS. The pseudo second-order kinetic equation provides the best correlation for the process, suggesting a chemisorption process as the rate limiting step. According to the nonlinear regression correlation coefficient (R2) and the calculated χ2 values of the adsorption isotherm equations, the Langmuir isotherm has been found to be better fitted than the Freundlich and Temkin isotherms. The adsorption thermodynamics demonstrated that the adsorption process is spontaneous and endothermic. Competition from common coexisting ions, such as SO42−, NO3− and Cl−, was negligible. PANMW-AO fibers could be reused repeatedly 5 times with more than 80% of initial adsorption capacity, suggesting that PANMW-AO fibers have promising potential as an adsorbent for the removal of Cr(VI) from aqueous solution.






