Design for carbon–carbon bond forming reactions under ambient conditions†
Goutam Brahmachari
*
Laboratory of Natural Products & Organic Synthesis, Department of Chemistry, Visva-Bharati (a Central University), Santiniketan-731235, West Bengal, India. E-mail: brahmg2001@yahoo.co.in; goutam.brahmachari@visva-bharati.ac.in; Fax: +91-3463-261526; Tel: +91-3463-261526
First published on 28th June 2016
Abstract
The carbon–carbon (C–C) bond forms the ‘backbone’ of nearly every organic molecule, and lies at the heart of the chemical sciences! This transformation has always been one of the most useful and fundamental reactions in the development of organic chemistry. Currently, the concept of ‘green chemistry’ is globally acclaimed and has already advanced quite significantly to emerge as a distinct branch of chemical sciences. Among the principles of ‘green chemistry’, one principle is dedicated to the “design of energy efficiency” – that is to develop synthetic strategies that require less/minimum amounts of energy to carry out a specific reaction with optimum productivity. And the most effective way to save energy is to develop strategies/protocols that are capable enough to carry out the transformations at ambient temperature and pressure! As part of the on-going developments in green synthetic strategies, designing for reactions under ambient conditions coupled with other green aspects is, thus, an area of current choice. This review is aimed at offering an up-to-date development on the design of carbon–carbon bond forming protocols to access a wide variety of organic molecules of topical significance under ambient conditions. The account highlights the brilliant applications of reaction conditions such as the use of solvents or no solvent, catalysts or no catalyst, and the use of green tools like ball-milling, ultrasonication and visible light in achieving the goal!
1. Introduction
Carbon–carbon (C–C) bond formation is the basis for the biogenesis of nature's essential molecules and the C–C bond forms the ‘backbone’ of nearly every organic molecule. Hence, it lies at the heart of the chemical sciences and is regarded as the key transformation in organic synthesis to set up the carbon backbone of organic molecules.1 Currently, a plethora of organic compounds are known that find a wide range of applications as fine chemicals, medicinal and pharmaceutical agents, agrochemicals, and many others.2–16 In the development of organic chemistry, carbon–carbon bond formation has always been one of the most useful and fundamental reactions, and as a result, there is an ever-growing number of methods for carbon–carbon bond formation.17–28With the advent of the 21st century, the public is equally aware of the hazardous substances used and generated by chemical processes, and eventually the concept of ‘green and sustainable chemistry’ has evolved.29–34 The main essence of this concept is to develop a sustainable chemical enterprise that will find creative ways to minimize human exposure to, and the environmental impact of, harmful chemicals while enhancing scientific progress. Current trends of green chemistry practice encompass a number of agenda, such as the avoidance of extensive use of toxic and hazardous reagents and solvents, harsh reaction conditions, and expensive and sophisticated catalysts.35–40 The last decade has seen a tremendous effort toward savings in energy consumption, use of eco-friendly catalysts and solvents, proficiency in atom economy, and minimization of wastes from reactions in order to design novel green synthetic protocols for organic compounds of interest.41 Among various energy-efficient processes, the most effective way to save energy is to develop strategies/protocols that are capable enough to carry out the transformations at ambient temperature. Designing reactions under ambient conditions coupled with other green aspects is, thus, a current area of emphasis.42 The concept of developing reaction strategies at ambient conditions is now an emerging field of research in organic chemistry and is progressing considerably. The purpose of this review is to offer an up-to-date development of carbon–carbon bond forming reactions of topical significance under ambient temperature and pressure. However, no results of any spectroscopic or analytical techniques are supplemented as they are very adequately defined in the original papers and are out of the scope of this article.
2. Carbon–carbon bond forming reactions at ambient conditions
2.1. Carbon–carbon bond forming reactions in solvent medium
This section covers carbon–carbon bond forming reactions occurring in various solvent media under ambient conditions.2.1.1.1. Under catalyst-free conditions. Role of catalysts in organic synthesis is obvious and thus they find huge applications and uses. Efforts have been made to reduce toxicity level of catalysts by multidirectional modifications, but the most fruitful way is to design an organic reaction without catalyst(s), if feasible! If this important achievement can be coupled with energy consideration, one of the most alarming issues in the 21st century, then it would be the great as far green chemistry practice and sustainability are concerned. Recently, research endeavors directed toward catalyst-free reactions have been the subject of numerous studies.84–87 This sub-section summarizes catalyst-free C–C bond forming reactions in aqueous media occurring at room temperature and pressure as reported over the last decade.
2.1.1.1.1. Condensation-addition reaction. Knoevenagel condensation and Michael addition are the fundamental reactions in forming C–C bonds in organic synthesis. A catalyst-free tandem Knoevenagel condensation and Michael addition in water was reported by Yu et al.88 to synthesize a wide range of substituted bis(6-amino-1,3-dimethyluracil-5-yl)methanes 3 from the reaction of aldehydes (1) with cyclic-1,3-diketones (2) at room temperature (Scheme 1). Tetraketonic derivatives (3) are key intermediates for the preparation of diverse biologically relevant heterocycles such as xanthenediones, acridindiones and 4H-1-benzopyrans;89–91 in addition such compounds are also reported to possess tyrosinase inhibitory properties.92
In addition to acting as a solvent, water is also supposed to promote enolization process of 1,3-diketone (2) through hydrogen bonding with enolic OH, thereby enhancing the nucleophilic character of methylene carbon of this C–H activated acid.88 Besides, water also increases the electrophilic character of the carbonyl carbon reacting with aldehyde 1 by forming hydrogen bonds with the carbonyl oxygen. A tandem Knoevenagel condensation and Michael addition ultimately affords the desired tetraketone 3 (Scheme 2).88
There are several reports available in literature for the efficient synthesis of tetraketones most of which suffer from the use of catalysts, surfactants, and aqueous organic solvent as co-solvent.89,92,93 Conversely, the present catalyst-free protocol developed by Yu et al. is not only eco-friendly and high yielding but operationally simple and highly efficient in pure water just at ambient temperature.
Bayat et al.94 developed an efficient protocol for the synthesis of 2,2′-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives (6) from the reaction of dimedone (2) with various aromatic aldehydes (5) in water at room temperature (Scheme 3). The work-up procedure is simple and does not require any column chromatographic purification.
 | ||
| Scheme 3 One-pot synthesis of 2,2′-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives 6. | ||
The authors proposed a plausible mechanism for this transformation (Scheme 4).94 A first molecule of dimedone (2) undergoes enolization in water medium and the enolized species (2a) participates in Knoevenagel condensation with aldehyde 5 generating an intermediate 7. Enolized form of a second molecule of dimedone thereafter undergoes Michael addition with the intermediate 7 through nucleophilic attack, followed by tautomerization to afford the desired product 6.
2.1.1.1.2. C–C coupling via nucleophilic substitution reaction. A catalyst-free ‘on-water’ direct C–C coupling via nucleophilic substitution reaction between indoles (10) and 1,4-benzoquinones (11) affording both 3-indolyl-1,4-benzoquinones (13) and bis(indolyl)-1,4-benzoquinones (14) at room temperature was developed by Li and his group (Scheme 5).95 3-Indolylquinones, particularly bis(indolyl)quinones, constitute an important structural unit in many natural products with biological and pharmaceutical properties including antitumor, anti-HIV and antidiabetic activities.96–101
2.1.1.1.3. C–C coupling via nucleophilic addition. Thakur and Meshram demonstrated an “on water” C–C coupling involving nucleophilic addition of thiazolidinediones (16) or oxindoles (18) with isatins (15) for the diastereoselective synthesis of a novel class of diverse 3-(thiazolidinedione or oxindole)-substituted-3-hydroxy-2-oxindole scaffolds (17 and 19) without the aid of catalyst and column chromatographic purification just at room temperature (Scheme 6).102 A variety of functionalized isatins as well as thiazolidinedione and oxindole derivatives underwent the reaction smoothly with excellent yields from readily available starting materials. 3-(Thiazolidinedione or oxindole) substituted-3-hydroxy-2-oxindole frameworks are regarded as key structural motifs in a large array of alkaloid class of natural products with diverse biological activities such as antioxidant, anticancer, anti-HIV and neuroprotective properties,103–110 and compounds bearing such a structural motif are widely used as targets for drug design.111–113
The same group of investigators reported in the same year another efficient synthesis of a novel class of diverse 3-(2-pyrazolin-5-one)-substituted-3-hydroxy-2-oxindole scaffolds 21 from the reaction of isatins (15) with 2-pyrazolin-5-one derivatives (20) following a catalyst-free method at room temperature (Scheme 7).114 No column chromatographic purification was required in this process.
As proposed by the authors, 3-substituted-2-pyrazolin-5-one (20) first undergoes tautomerization under the influence of water and the resulting tautomer 20′ then takes part in nucleophilic addition-type reaction with isatin 15 to form the adduct 22, followed by its aromatization to furnish the desired product 21 (Scheme 8).
In another report, Olyaei et al.115 described the synthesis of a series of N-heteroaryl-α-arylglycines (25) from the one-pot three-component condensation reaction β-naphthols (22), glyoxylic acid (23) and heteroaryl amines (24) in water at ambient temperature in the absence of any catalyst with moderate to high yields (Scheme 9). From their experimental observations, the investigators also assumed that 2-naphthol undergoes nucleophilic addition with the iminoacid generated in situ from a condensation reaction between amine and glyoxylic acid affording the desired α-naphthylglycine compound. Both C–C and C–N bond formations took place in this transformation.
Strecker synthesis of α-aminonitriles follows similar kind of strategy; such organic compounds are regarded as possible prebiotic precursors to porphyrins, corrins, nicotinic acids, and nucleic acids,116 and find immense applications in the synthesis of a wide range of pharmaceutically relevant natural and unnatural molecules of interest.117 α-Aminonitriles are the key precursors of diverse α-amino acids for synthesizing proteins,118 and also as chiral building blocks in pharmaceutical industries.119 A catalyst-free protocol for the synthesis of racemic α-aminonitriles (29) from the reactions of varying carbonyl compounds (26), amines (27), and acetone cyanohydrin (28) via one-pot Strecker reaction in water was reported by Galletti et al. (Scheme 10).120 A good number of entries were found to proceed very efficiently at ambient conditions with high selectivity. Moreover, the mild reaction conditions and the operational simplicity of this atom-economic cyanation process offer a possibility for the large-scale syntheses of pharmaceutically important natural and synthetic α-amino acids in a cost-effective, cleaner and environmentally friendlier alternative.
Both aliphatic and aromatic aldehydes, and cyclic ketones, in combination with primary and secondary amines were shown to undergo smooth reaction. In some cases, pure α-amino nitriles can be obtained just by direct separation from water. An unusual application of the Strecker reaction to 1,2-diamines to obtain 1,2-diamino nitriles, and to cyclic secondary amines was also reported. However, dialkyl and alkyl aryl ketones practically did not undergo this reaction; in contrast cyclic ketones afforded excellent yields, which is in accordance to the difference in the reactivity and internal strain effect (I-strain) of linear versus cyclic ketones in nucleophilic addition reactions.120
2.1.1.1.4. Catalyst-free one-pot multicomponent reactions. In recent past, several catalyst-free multicomponent reactions occurring at ambient conditions were reported so as to design one-pot synthesis of biologically relevant organic scaffolds, and these transformations involve formation of both carbon–carbon and carbon–heteroatom bonds. Such a novel one-pot eco-friendly protocol for the synthesis of 4,5-disubstituted 2-benzazepine derivatives 32 in water under catalyst-free conditions from a domino reaction between 4-chloro-3-formyl coumarin (30) and benzyl amines (31) at room temperature was demonstrated by Kumar and his group (Scheme 11).121 Both C–C and C–N bonds are formed in this transformation. Benzazepine is a fused N-heterocyclic moiety present as a key structural fragment in various biologically active natural and synthetic molecules.122–128
The investigators proposed a plausible mechanism for the synthesis of 4,5-disubstituted 2-benzazepine (32) out of domino reaction of 4-chloro-3-formyl coumarin (30) with benzyl amines (31) as outlined in Scheme 12. The method does not involve complicated work-up procedures and avoids the use of organic solvents as well. In addition, the investigators also attempted a mixed substrate combination (use of two different benzyl amines) in one exemplary case with a hope to broadening the scope of the reaction to achieve higher product diversity and they became successful in their attempt.
Quinoxalines and their derivatives are an important class of benzoheterocycles exhibiting a broad spectrum of biological activities129–137 and also found applications as dyes138,139 and as building blocks in the synthesis of organic semiconductors.140,141 In 2012, Shaabani et al.142 developed a novel and efficient one-pot multicomponent reaction for the synthesis of 1,6-dihydro-6,6-dimethylpyrazine-2,3-dicarbonitriles (38) from the reaction between alkyl or aryl isocyanides (35), 2,3-diaminomaleonitrile (36) and 3-oxopentanedioic acid (37) at room temperature (Scheme 13). The transformation involves formation of both C–C and C–N bonds.
A possible mechanism for the formation of product 38 was suggested by the authors (Scheme 14). Initially, there occurs a condensation reaction between 2,3-diaminomaleonitrile (36) and 3-oxopentanedioic acid (37) to form an imine derivative 39 which immediately undergoes decarboxylation leading to the formation of another imine derivative 40. In the next step, an intermediate 41 is produced by a nucleophilic attack of isocyanide 35 on 40, followed by an intramolecular nucleophilic attack by the –NH2 group at the activated nitrile moiety to give intermediate 42. Finally, imine–enamine tautomerization of intermediate 42 affords the 1,6-dihydropyrazine-2,3-dicarbonitrile derivative 38. Easy reaction set-up, easy work-up procedure, catalyst-free and mild reaction conditions, no column chromatographic purification, high yields are the notable features of this present protocol; however, the investigators synthesized just six compounds of this series. Scope of this present protocol should be explored.
Ramazani et al.143 reported an operationally simple, mild and water-mediated synthesis of highly functionalized γ-iminolactone derivatives (45) via one-pot three-component reaction of alkyl isocyanides (35), dialkyl acetylenedicarboxylates (43) and phenacyl halides (44) at room temperature (Scheme 15). This transformation involves both C–C and C–O bond formations. In their report, the authors discussed that syntheses of such heterocyclic compounds were accomplished previously using various catalysts. Besides, this present protocol offers other significant advantages such as operational simplicity, mild reaction conditions, enhanced rates, ease of isolation of products, cleaner reaction profiles, and water as solvent.
The authors proposed a plausible mechanism for the condensation reaction (Scheme 16).143 Initially, a zwitterionic species (46) is formed from the reaction between isocyanide 35 and dialkyl carboxylate 43. In the next step, the carbanion part of this zwitterion intermediate attacks the electron-deficient carbonyl carbon of phenacyl halide 44, leading to a dipolar species 47. Cyclization of 47 eventually yields the γ-iminolactone 45.
The quinoline scaffold is prevalent in a variety of pharmacologically active synthetic and natural compounds,144 and are historically among the most important antimalarial drugs ever used.145 Taran et al.146 developed an efficient one-pot three-component synthesis of novel α-(acyloxy)-α-(quinolin-4-yl)acetamides (50) from the reaction of isocyanides (35), quinoline-4-carbaldehyde (48), and arylcarboxylic acids (49) at room temperature using water as reaction medium involving the formation of both C–C and C–O bonds (Scheme 17). Operational simplicity, mild reaction conditions, ease of isolation of products, cleaner reaction profiles, and excellent yields are the key advantages of this method.
A possible mechanism for the formation of product 50 was suggested by the authors (Scheme 18). Initially, protonation occurs at the aldehydic carbonyl oxygen by the carboxylic acid (49) to generate an electron-deficient species 48a, which is then attacked by isocyanide (35) through its electron-rich carbon centre to form intermediate 51. The carboxylate ion 49a adds to the intermediate 51 in the next step via nucleophilic attack leading to the formation of intermediate 52 that undergoes a rearrangement reaction to give ultimately the desired product 50.
 | ||
| Scheme 18 Plausible mechanism for the construction of α-(acyloxy)-α-(quinolin-4-yl)acetamide moiety 50. | ||
In 2009, Kumaravel and Vasuki reported a rapid synthesis of a series of such scaffolds 57 via one-pot four-component reaction of ethyl acetoacetate (53), hydrazine hydrate (54), malononitrile (55) and 2-hydroxybenzaldehydes (56) involving both C–C and C–N bond formations (Scheme 19).147 This reaction protocol offers an easy access to a combinatorial library of these compounds. In addition to C–C, also C–N and C–O bond formations take place in this reaction. Molecular skeleton of a 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile scaffold (57) integrates a chromene and a pyrozolone moiety, both of which possess immense pharmacological and biological efficacies.148–154
 | ||
| Scheme 19 Four-component synthesis of 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile derivatives (57). | ||
The authors proposed the following plausible mechanism for their reaction which involves a series of the tandem reactions: (i) reaction between hydrazine hydrate (54) and ethyl acetoacetate (53) resulting in instantaneous formation of pyrazolone A that tautomerizes to B, (ii) Knoevenagel condensation between 2-hydroxybenzaldehyde (56) and malononitrile (55) forming 2-imino-2H-chromene-3-carbonitrile intermediate (C) by 6-exo-dig cyclization, and (iii) Michael addition of B to D, followed by subsequent rearrangement. The overall reaction is shown step-wise (Scheme 20).
2.1.1.2. Under catalytic conditions. This sub-section summarizes C–C bond forming reactions in water under the influence of both homo- and heterogeneous catalysts at ambient conditions.
2.1.1.2.1. Knoevenagel condensations. In general, reversible dehydration condensation in water is a difficult transformation because the large excess of water pushes the equilibrium in favor of the hydrated compounds;44,48,59,80,155–158 however, certain naturally elaborated enzymes are capable enough to accomplish homogeneous dehydration in water even under neutral conditions at ambient temperature. Such a special attribute of enzyme catalysis motivated Fujita and his group159 to introduce a cationic coordination cage-A (a 12 + charged M6L4 cage) [cage components: (ethylene diamine)Pd(NO3)2 and 2,4,6-tripyridyl-1,3,5-triazine] that was found to dramatically accelerate the Knoevenagel condensation of aromatic aldehydes with Meldrum's acid in water under neutral conditions (Scheme 21). The addition of a nucleophile to the aldehyde to generate anionic intermediates seems to be facilitated by the cationic environment of the cavity. The products are ejected from the cage as a result of the host-guest size discrepancy.
This technique demonstrated a unique dehydration condensation under neutral conditions in water catalyzed by the water-soluble synthetic cationic host cage-A. An aromatic aldehyde substrate (5; an electron-rich guest) first became efficiently encapsulated into the host's (cage-A) hydrophobic cavity, which after then attacked by the enolate of Meldrum's acid (58) to generate oxyanion intermediate. The condensation reaction seems to be facilitated by the anionic intermediate in the cationic environment of the cage. The eventual loss of water molecule occurs smoothly within the hydrophobic cavity to form the dehydrated product (59) which is too large for the cavity and is spontaneously released from the cage, and a new incoming substrate molecule (5) occupies the position. The overall phenomenon follows the tricks of enzyme-like catalysis.159
Recently, Brahmachari has reported that coumarin-3-carboxylic acids (60) can efficiently be synthesized via Knoevenagel condensation between substituted salicylaldehydes (56) and Meldrum's acid (58) in one-pot at room temperature using water for the first time as a green and eco-friendly solvent and commercially available potassium carbonate or sodium azide as inexpensive and less-toxic catalyst (Scheme 22).160 The present method is not only cost-effective and environmentally benign, but also experimentally safe and simple, easy to handle, clean, and efficient also for the large-scale synthesis eliminating the use of any toxic organic solvent and tedious operation of column chromatographic purification. The feasibility of the present method was also examined for a somewhat scaled-up (on the gram scale) experiment and found to be satisfactory.
 | ||
| Scheme 22 Potassium carbonate or sodium azide-catalyzed one-pot synthesis of coumarin-3-carboxylic acids (60) in water. | ||
The transformation involves both C–C and C–O bond formations and a plausible mechanism has been suggested in Scheme 23. Interestingly, azide ion does not act as a nucleophile in this reaction in spite of its use in a 0.5 equivalency. Mild reaction conditions, good to excellent yields, operational simplicity, avoidance of organic solvent, use of water as reaction medium, absence of tedious separation procedures, clean reaction profiles, and energy-efficiency as well as the use of inexpensive and environmentally benign catalysts are the advantages of the present method.
 | ||
| Scheme 23 Plausible mechanism for the base-catalyzed one-pot synthesis of coumarin-3-carboxylic acids 60 in water. | ||
Coumarin-3-carboxylic acids represent a pronounced group of coumarin-heterocyclic compounds with a wide range of applications.161–165 Literature survey reveals that these compounds find applications as synthons of numerous natural and semi-synthetic pharmacological agents like β-lactams,166 isoureas,167 and tetrahydropyridones.168 Ester and amide derivatives of coumarin-3-carboxylic acid have been evaluated to possess efficient inhibitory activity against cancer cell invasion in vitro and tumor growth in vivo.169 Apart from these applications, coumarin-3-carboxylic acids have been widely used as fluorescent probes170 and triplet oxygen sensitizers.171
2.1.1.2.2. Michael addition. In the field of organocatalysis, amine-based catalysts are found to carry out a variety of organic transformations so far;172,173 however, most of the reactions were typically performed in organic solvents with a few aminocatalysts could be used in water.174–180 Under this purview, Wu et al.181 thought to design carbohydrate-based compounds capable to exhibit hydrogen bonding interactions for possible organocatalysts to activate organic reaction in water. The present investigators reported for the first-time that carbohydrate-based tolylsulfonyl hydrazines can effectively accomplish Michael addition of indoles (65) to electron-deficient olefins (66) in water just at room temperature (Scheme 24). This method provides a green process for the synthesis of biologically relevant 3-substituted indole derivatives 68 under mild conditions with high yield and with good substrate scope.
 | ||
| Scheme 24 Carbohydrate-based tolylsulfonyl hydrazine-catalyzed synthesis of 3-substituted indoles 68. | ||
2.1.1.2.3. Matsuda–Heck coupling. In 2012, Gaikwad and Pore introduced a modified eco-friendly and simple method for Matsuda–Heck coupling reaction of olefins with arenediazonium tetrafluoroborate salt catalyzed by in situ generated palladium-nanoparticles in the presence of Triton X-100 as surfactant in water at ambient temperature.182 A variety of arenediazonium tetrafluoroborate salts were coupled with olefins under ligand-free and aerobic conditions to afford arylated products with high yields and stereoselectivity (Scheme 25).
The key advantages of this method include no requirement for additional base, ligand and nitrogen atmosphere that obviously simplify the reaction conditions; in addition the modified method is also associated with excellent yields and high stereoselectivity.
2.1.2.1. Knoevenagel condensations. Inokuchi and Kawafuchi183 developed the first E-selective Knoevenagel condensation of acetoacetic derivatives of TEMPO (2,2,6,6-tetramethylpiperidin-1-yl-3-oxobutanoate) with aldehydes bearing electron-withdrawing substituents using piperidine as catalyst in ethanol at room temperature. Alternatively, they also accomplished the Z-selective Knoevenagel condensation of N-methoxy-N-methyl-3-oxobutanamide with those aldehydes using piperidine/acetic acid as catalyst in the same solvent under identical conditions (Scheme 26).183
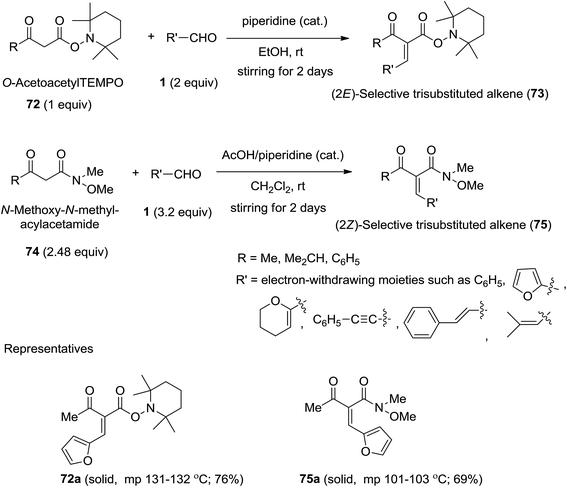 | ||
| Scheme 26 Synthesis of trisubstituted E or Z-2-alkenes via E- and Z-selective Knoevenagel condensation. | ||
In another report, Lakshmi Kantam et al.184 used modified Mg–Al hydrotalcite (MHT) as a heterogeneous reusable catalyst for carrying out Knoevenagel condensations involving various aromatic carbonyl compounds, aliphatic ketone and cyclohexanone with malononitrile/ethyl cyanoacetate in toluene/DMF at room temperature (Scheme 27).
Recently, an eco-friendly one-pot synthetic protocol for 3,3-bis(indol-3-yl)indolin-2-one (16) along with 2,2-bis(indol-3-yl)acenaphthylen-1(2H)-one (18) derivatives has been developed by Brahmachari and Banerjee185 from the pseudo-multicomponent reaction of indole (14, 2 equiv.), respectively with isatin (15) or acenaphthaquinone (17) in aqueous ethanol at room temperature in the presence of a catalytic amount of sulfamic acid (Scheme 28). Bis(indolyl)indolin-2-ones are found to possess various pharmaceutical properties such as anti-inflammatory,186 anti-HIV,187 antitumor,188 spermicidal potential,189 anticancer,190 and cytotoxic191 properties. Interestingly, certain bis(indolyl)indolin-2-one derivatives have been reported to exhibit strong cytotoxicity against a series of cancer cell lines but not against the normal cells.191 The present protocol offers a number of benefits such as mild reaction conditions at ambient temperature and pressure, excellent yields, operational simplicity and absence of tedious separation procedures, reusability of catalyst, energy-efficiency and high atom-economy as well as the use of inexpensive and environmentally benign catalyst. Moreover, reusability of the reaction media is an added advantage to this protocol. A plausible mechanism for this sulfamic acid-catalyzed transformation has also been suggested (Scheme 29).
 | ||
| Scheme 28 One-pot synthesis of functionalized 3,3-bis(indol-3-yl)indolin-2-ones (77) and 2,2-bis(indol-3-yl)acenaphthylen-1(2H)-one derivatives (79). | ||
 | ||
| Scheme 29 Proposed mechanism for the sulfamic acid-catalyzed synthesis of 3,3-bis(indol-3-yl)indolin-2-ones (77). | ||
6-Aminouracil and its derivatives play a key structural part in numerous natural and synthetic bioactive compounds, and also regarded as a versatile building block for several nitrogen-containing heterocycles possessing a wide range of pharmacological potentials.192–195 Brahmachari and his group reported a straightforward and efficient pseudo three-component one-pot synthesis of a series of substituted bis(6-aminouracil-5-yl)methanes 86 in good yields using either ceric ammonium nitrate (CAN)196 or sulfamic acid197 as commercially available, inexpensive and eco-friendly catalyst from the reaction of 6-aminouracils (85) and diverse aldehydes (1) in aqueous ethanol at room temperature (Scheme 30). Both procedures satisfy many green chemistry parameters, such as mild reaction conditions, good to excellent yields, operational simplicity and absence of tedious separation procedures, high atom-economy as well as the use of inexpensive and environmentally benign catalysts.
 | ||
| Scheme 30 CAN/sulfamic acid-catalyzed one-pot pseudo-multicomponent synthesis of substituted bis(6-aminouracil-5-yl)methanes (86). | ||
Lawsone (2-hydroxy-1,4-naphthoquinone) is a major chemical constituent of the medicinal plant, Lawsonia inermis Linn. (synonyms: L. alba, L. spinosa; also known as Henna or Mhendi; family: Lythraceae), different parts of which are traditionally used all over the world as cosmetics (hair dye, body paint and tattoo dye) and herbal remedies in treating various ailments.198–200 This phenolic quinone compound has been evaluated to possess a wide range of biological and pharmacological activities such as antioxidant,201 antibacterial,202 antifungal,203 cytotoxic,204 trypsin inhibitor,205 anticoagulant,206 advanced glycated end products (AGEs) formation inhibitor,207 and anti-acute pancreatitis.208 Under this purview, Brahmachari has recently developed a simple, convenient, clean and highly efficient protocol for the one-pot synthesis of functionalized bis-lawsone derivatives 88 from the pseudo-multicomponent reaction of lawsone (i.e. 2-hydroxynaphthalene-1,4-dione; 87) and diverse aldehydes (1) in aqueous ethanol at room temperature using commercially available sulfamic acid as an inexpensive organocatalyst (Scheme 31).209 The plausible mechanism for this transformation involving a Knoevenagel-type condensation is presented in Scheme 32. Mild reaction conditions, excellent yields, operational simplicity, absence of tedious separation procedures, clean reaction profiles, energy-efficiency and high atom-economy, as well as the use of inexpensive and environmentally benign catalyst are the key advantages of the present method.
2.1.2.2. Addition reactions. Hagiwara et al.210 reported a facile Mannich reaction of arylaldimine 89 with methylsilyl enol ether 90 catalyzed by lithium chloride in dimethylformamide (DMF) at room temperature resulting the synthesis of a series of β-amino esters 91 with good yields (Scheme 33). The reaction is mild enough to apply to aldimines having the AcO–, TBDMSO–, or MeS– group. The present reaction offers a mild, practical, environmentally and economically benign method for synthesizing β-amino carbonyl compounds, which are versatile building blocks for the synthesis of various biologically important products.210
Wang et al.211 reported 2-dicyclohexylphosphino-2,4,6-triisopropylbiphenyl (XPhos, a phosphine derivative, 93)-catalyzed Michael addition of α-trifluoromethylated ester (92) with α,β-unsaturated ketone (26) in N,N-dimethylformamide (DMF) under an aerobic atmosphere at room temperature resulting to the generation of a series of γ-substituted α-trifluoromethylated ester derivatives (94) with moderate to good yields (Scheme 34). Although limited to active Michael acceptors and highly acidic trifluoromethylated nucleophiles, these reactions with α,β-unsaturated carbonyl compounds such as (hetero)aryl vinyl ketones, alkyl vinyl ketones, and phenyl acrylate proceeded efficiently at room temperature, providing many fluorinated compounds with a CF3-containing quaternary carbon center. Fluorinated compounds are of interest because fluorine often offers organic molecules with many important properties such as high lipophilicity, bioavailability, and metabolic stability.212–217
In 2012, Kamalraja et al.218 synthesized a series of 3-(aminomethylene)oxindoles (97) via one pot Michael addition of α-azido ketones (96) and isatylidene malononitriles (95) followed by the conversion of azides to amines using piperidine as an efficient catalyst in ethanol at room temperature with good product diastereoselectivity (Scheme 35). The investigators proposed a plausible mechanism for the transformation (Scheme 36). Initially piperidine reacts with α-azido ketone 96 to form enamine 98, which undergoes Michael addition with isatylidene malononitrile 95 to form adduct 99. Elimination of malononitrile from the adduct 99 results in the formation of vinyl azide 100. Intramolecular cyclization of vinyl azide 100 may take place to form a oxatriazine 102, which transforms to intermediate 103 by the addition of water. Finally the intermediate 103 rearranges to form product 97 (Scheme 36).
The Henry (nitroaldol) reaction is one of the most atom-economical carbon–carbon bond-forming reactions in synthetic chemistry. The resulting β-hydroxy nitro compounds have been used in various beneficial transformations to provide chiral β-amino alcohols and α-hydroxy carboxylic acids. To provide the raw materials for research into these biologically significant building blocks, attention has recently been focused on the development of catalytic, asymmetric versions of the Henry reaction.219–221 The most impressive work of heterobimetallic lanthanoid catalysis222 has stimulated the successful development of various types of asymmetric catalyst.223–234 Among them, the Cu-catalyzed Henry reaction performed at room temperature has received much attention in recent years.223,225,231–234 As part of the ongoing research in this domain, Arai et al.235 designed and developed a new chiral diamine ligand 105 using a cheap building block and its Cu(OAc)2 complex is air-stable and can smoothly catalyze the Henry reaction from a variety of aldehydes and nitromethane in n-propyl alcohol at room temperature in high yield (up to >99%) with excellent enantiomeric excess (over 90%) (Scheme 37). All these points contribute to the practicality and usefulness of this catalytic system.
α-Trichloromethylamines are pharmacologically as well as synthetically interesting compounds.236–243 Recently, Wahl et al.244 reported a nucleophilic addition of trimethyl(trichloromethyl)silane (108) to N-phosphinoyl benzaldimines 107 outlining a tetrabutylammonium difluorotriphenylsilicate (TBAT)-catalyzed route to N-phosphinoyl-α-(trichloromethyl)benzylamines 109 in tetrahydrofuran (THF) with good yields within just one hour at room temperature (Scheme 38).
Hydroxymethylation reactions are among the most important C–C bond-forming reactions in organic synthesis. Kokubo and Kobayashi explored scandium(III) fluoride (ScF3) as a novel catalyst for such a hydroxymethylation reaction of dimethylsilyl (DMS) enolates (111) using aqueous formaldehyde solution in aqueous THF media to give the corresponding β-hydroxy ketones 112 in good to excellent yields at room temperature condition (Scheme 39).245
Zhang246 demonstrated a magnesium-catalyzed allylation addition of aldehydes (aromatic, unsaturated and aliphatic) with allyltributylstannane in the presence of MgI2·(OEt)n etherate in dichloromethane at room temperature, affording homoallylic alcohols with moderate to good yields (Scheme 40). The process is mild, efficient, operationally simple and highly selective.
2.1.2.3. Cycloaddition reactions. Angucyclinones are a large group of naturally occurring quinones that have a benz[a]anthracenequinone framework and exhibit a broad range of biological activities.247 Hsu and Huang performed Diels–Alder reaction of juglone (115) with various styrenes (116) in the presence of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) in dichloromethane catalyzed by boron triacetate at room temperature to generate such derivatives 117 in good yields and with excellent regioselectivity (Scheme 41).248 The investigators successfully applied strategy to the total syntheses of tetrangulol249 and anhydrolandomycinone250 in the present report as well.
Challa et al.251 also developed a metal-free one-pot cascade annulation of acyclic substrates such as dienaminodioate (118), cinnamaldehydes (119) and allyl amine (120) using trifluoroacetic acid as a catalyst for the synthesis of polyfunctional biaryl-2-carbaldehydes 121 at ambient conditions (Scheme 42). The reaction proceeds through Diels–Alder pathway. The investigators also demonstrated synthetic applications of the resulting biaryl-2-carbaldehyde by their conversion into an array of diverse molecules with biological and materials chemistry relevance.
 | ||
| Scheme 42 Acid-catalyzed metal-free one-pot synthesis of polyfunctional biaryl-2-carbaldehydes (121) through Diels–Alder pathway. | ||
Han et al.252 synthesized biologically potent cyclohepta[b]indoles (123)through one-pot three-component [4 + 3]-cycloaddition reaction of indoles (10), carbonyl compounds (26) and cyclic/acyclic dienes (122) using gallium(III) salts (GaBr3/Ga(OTf)3) as catalytic system in dichloromethane at room temperature (Scheme 43); this is the first instance of a (4 + 3) cycloaddition where the 2π component is derived from indole.
 | ||
| Scheme 43 Ga(III)-catalyzed synthesis of cyclohepta[b]indoles 123 through one-pot three-component [4 + 3]-cycloaddition. | ||
2.1.2.4. Substitution reactions. Recently, Cacciuttolo et al.253 have reported bismuth(III) triflate-catalyzed intermolecular reaction between differently substituted electron-rich arenes (124) and unactivated 1,3-dienes (125) at room temperature leading to the efficient synthesis of substituted indane derivatives 126 through a tandem bis-hydroarylation involving C–C bond formation reaction (Scheme 44). This process is highly atom-economic and regioselective.
p-Tolenesulfonic acid (PTSA), a simple Brønsted acid was found to catalyze smoothly C3-selective tert-alkylation of indoles using tertiary propargylic (127) and benzylic alcohols in acetonitrile at room temperature resulting to the functionalization of indoles with a quaternary carbon at the propargylic position (Scheme 45).254 The availability of the reagents used, mild conditions and the fact that only water was generated as a side product, make this method an attractive and environmentally friendly process for the synthesis of C3-substituted indoles 128 and 130.
Xu et al.255 also reported on the substitution reaction of ferrocenyl alcohols 131 with various nucleophiles 132 catalyzed by cerium ammonium nitrate (CAN) at ambient conditions (Scheme 46); this methodology offers an efficient and direct C–C bond formation from alcohols and nucleophiles in acetonitrile at room temperature condition leading to the synthesis of ferrocene functionalities 133 with moderate to high yields.
2.1.2.5. C–H activations. Zhang et al.256 developed a convenient and efficient method for the synthesis of heteroaryl-group-containing compounds via the palladium-catalyzed C–H activation of heteroarenes leading to direct conjugate addition of heteroarenes (134) to α,β-unsaturated ketones (135) under mild reaction conditions to afford Michael adducts 136 in moderate to excellent yields (Scheme 47).
A new and straightforward catalytic route to 2-bicyclo[2.2.1]hept-2-ylidenebicyclo[2.2.1]-heptane 138 involving C–H bond activation of bicyclo[2.2.1]hept-2-ene (137; norbornene) using a tungsten(II) carbonyl complex [(CO)4W(μ-Cl)3W(SnCl3)(CO)3] as a catalyst in dichloromethane was developed by Malinowska et al.257 at ambient conditions (Scheme 48).
 | ||
| Scheme 48 Tungsten(II) carbonyl complex-catalyzed synthesis of 2-bicyclo[2.2.1]hept-2-ylidenebicyclo[2.2.1]-heptane 138. | ||
Substituted β,β′-tricarbonyl derivatives 141 were prepared by Veronese et al.258 from reaction of β,β′-tricarbonyls (139) with isocyanates (140) using cobalt acetylacetonate [Co(acac)2] catalyst in dichloromethane at room temperature (Scheme 49). The catalyst accomplished the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct involving the formation of a new C–C bond between the intercarbonylic methylene of 139 and the isocyanato group. This is also an example of C–H activation reaction occurring at ambient conditions.
1 adduct involving the formation of a new C–C bond between the intercarbonylic methylene of 139 and the isocyanato group. This is also an example of C–H activation reaction occurring at ambient conditions.
The quinone moiety is a privileged structure in medicinal chemistry for the discovery of pharmaceutical leads, and quinone-based compounds find immense applications in pharmaceutical chemistry.101,259–267 Lamblin et al.268 developed a facile route for 2-aryl-1,4-benzoquinones (142) via direct C–H arylation of p-quinone (11) with anilines (24) in the presence of tert-butyl nitrite in aqueous DMSO at room temperature under neutral, additive-free and metal-free conditions (Scheme 50). The investigators proposed that the reaction proceeds through in situ formation of a diazonium hydroxide 143, which on homolytic decomposition generates free radical species and such radical intermediate, in turn, takes part in the arylation of quinone (Scheme 51).
2.1.2.6. Coupling reactions. Wang and Falck269 reported the first example of a rapid, open-flask, single-pot, and scalable process in which aryl radicals derived from aryl triazenes 148 were coupled with heteroarenes 149 via C–H functionalization to produce heterobiaryls 150 in moderate to good yields in dichloromethane–water (3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v) at room temperature (Scheme 52). Best results were obtained with electron-deficient heteroarenes, while both electron donating and withdrawing substituents in the triazene moieties were tolerated.
5 v/v) at room temperature (Scheme 52). Best results were obtained with electron-deficient heteroarenes, while both electron donating and withdrawing substituents in the triazene moieties were tolerated.
Fluorinated biphenyl derivatives are fundamental building blocks in fluorinated liquid crystals. Guo et al.270 synthesized a variety of fluorinated biphenyl derivatives (153) in good yields via Pd-catalyzed Suzuki coupling reaction of aryl bromides and fluorinated phenylboronic acids in aqueous THF solvent at room temperature (Scheme 53). This approach with high activity, good selectivity, mild reaction condition and aqueous phase reaction, as well as potential recycling of the catalytic species offers an environmentally sustainable chemical processes and provides a practical procedure for the synthesis of fluorinated liquid crystals in industry applications.
2-Aryl- and 2-alkyl-1,3,4-oxadiazoles (154) were found to efficiently undergo direct alkynylation upon treatment with readily accessible alkynyl bromides in the presence of a copper catalyst at room temperature, offering a facile approach to the synthesis of biologically interesting substituted oxadiazoles 156 with an oxadiazole core attached with π-conjugated systems (Scheme 54).271
The Sonogashira coupling reaction of terminal acetylenes with aryl and vinyl halides provides a powerful method for synthesizing conjugated alkynes, an important class of molecules that have found application in diverse areas ranging from natural product chemistry to materials science.272–278 In 2000, Hundertmark et al.279 observed that Pd(PhCN)2Cl2/P(t-Bu)3 can as an efficient and a versatile catalyst for room-temperature Sonogashira reactions of aryl bromides 151 with terminal alkynes 157 in the presence of N,N-di-isopropylamine/dioxane in dioxane (Schemes 54 and 55).
However, Finke et al.280 developed an optimized conditions for a general and convenient copper-free Sonogashira cross-coupling between aryl bromides and alkynes using Pd(I)-dimer as catalyst (158) and ZnCl2 as promoter in the presence of N,N-di-isopropylamine/THF at room temperature to generate substituted alkynes in good yields (Scheme 56).
Copper-free Sonogashira coupling of aryl bromide with alkynes using (AllylPdCl)2/P(t-Bu)3 catalyst in the presence of piperidine or DABCO in acetonitrile at room temperature was also reported by Soheili et al.281 Another mild protocol for the copper-free Sonogashira coupling of aryl iodides with terminal acetylenes in water under aerobic conditions was accomplished using 1 mol% PdCl2 in the presence of pyrrolidine at room temperature.282
2.1.2.7. Ring-expansion. Medium- and large-size ring systems are widespread, ranging from naturally occurring compounds to macrocyclic synthetic receptors or ligands, and their synthesis is still a challenging job for organic chemists.283–288 Huang et al.289 successfully demonstrated the ring-expansion reaction of bicyclic vinylidenecyclopropanes (160) in the presence of titanium chloride (TiCl4) as a Lewis acid catalyst in dichloromethane, providing an efficient method for the synthesis of naphthalenes with annulated carbocycles of various ring sizes (161) in good to excellent yields under mild conditions at room temperature (Scheme 57).
2.2. Carbon–carbon bond forming reactions in solvent-free/neat conditions
Solvent-free reaction protocols offer several advantages like reduced pollution, lower cost, and simplicity in processing, which are beneficial to the industry as well as to the environment.8,36,290–296 In recent years, mechanochemistry (i.e. chemical synthesis using mechanical force) has attracted much attention because it allows promotion of reactions under solvent-free conditions by grinding reactants together.297,298 Mechanochemical synthesis affords many advantages, such as greater efficiency with regard to time, materials, and energy usage, as well as the discovery of new or improved reactivity and products, as an alternative approach to synthesis. Planetary ball mill has now also come-up as an effective tool to carrying out a plethora of organic transformations with substantial improvements in reaction time, selectivity and energy efficiency, compared to conventional solution-based synthesis.299–303 The present section focuses on the applications of various solvent-free protocols in implementing carbon–carbon bond forming reactions reported in recent times. | ||
| Scheme 58 CTAOH-catalyzed one-pot synthesis of substituted nitroalkanes 163 and β-nitroalcohols 164. | ||
An efficient solvent-free protocol for Friedel–Crafts acylation of aromatic compounds using nontoxic, inexpensive and reusable ZnO powder as solid catalytic surface was developed by Sarvari and Sharghi305 under ambient conditions. The advantages of this environmentally benign and safe protocol include a simple reaction setup not requiring specialized equipment, very mild reaction conditions, high product yields, very short reaction times, and the elimination of solvents (Scheme 59).
In another report, Lakshmi Kantam et al.306 demonstrated a simple and recyclable room temperature protocol for direct alkylation of nitrogen-heterocycles such as indoles and pyrroles with epoxides via Friedel–Crafts reaction by using ionic liquid [bmim][OTf] as an efficient catalyst and reaction medium (Scheme 60). The process takes place with high regio- and chemoselectivity. The synthesized compounds are of pharmacological and biological importance.307–313
 | ||
| Scheme 60 Ionic liquid-catalyzed synthesis of alkylated N-heterocycles 169 and 1171 via Friedel–Crafts reaction. | ||
Zhang et al.314 also reported that Friedel–Crafts alkylation of indoles by tert-enamides (172) proceeds effectively in the presence of acetic acid to afford the pharmacologically and biologically active 2-oxo-1-pyrrolidine derivatives 173 in moderate to good yields under neat condition at room temperature (Scheme 61).
Palmieri et al.319 developed an eco-friendly simple and heterogeneous synthetic approach for the preparation of potentially interesting (Z)-α-bromonitroalkene compounds (179) with overall good yields via a two-step transformation involving Henry reaction under solvent-free conditions at room temperature (Scheme 64). It is to be noted that the final dehydration of nitroalkanol proceeds in this process under acidic conditions, contrary to the standard procedures that usually employ basic conditions.
Molecular iodine has been found to be as an efficient, inexpensive, and easy-to-handle catalyst for C-3 alkylation/alkenylation of diverse indoles with 1,3-dicarbonyl compounds at room temperature under solvent-free conditions with excellent yields (Scheme 65).320
 | ||
| Scheme 66 L-Proline-catalyzed three-component one-pot synthesis of gem-(β-dicarbonyl)arylmethanes 184. | ||
A plausible mechanism for this transformation has also been suggested (Scheme 67). The initially formed iminium salt (164) arising out of the reaction between aldehyde (1) and L-proline, undergoes Mannich reaction with C–H activated acid (162) to generate intermediate 166 which immediately takes part in Michael addition with the indole moiety (10) affording the desired product 163. L-Proline releases out for the next cycle.
 | ||
| Scheme 67 Proposed mechanism for the L-proline catalyzed multicomponent synthesis of the gem-(β-dicarbonyl)arylmethanes 184. | ||
Sulfamic acid has been reported to behave as a highly efficient organocatalyst for the condensation of indoles with various aldehydes or ketones. An et al.328 demonstrated an efficient and cost-effective protocol for the synthesis of bis(indol-3-yl)methanes 189 at room temperature under neat conditions (Scheme 68). Mild reaction conditions, easy product separation, reusability of the catalyst are some of the major advantages of this protocol.
Kumar et al.329 developed L-proline catalyzed efficient protocol for one-pot synthesis of 3-amino-alkylated indoles 187 via a three-component Mannich-type reaction of secondary amines (186), aldehyde (1) and indoles (10) under solvent-free conditions at room temperature (Scheme 69). The notable advantage of this methodology is an improved condition for the synthesis of such compounds without the formation of bis-indoles.
A solvent-free, environmentally clean and a general protocol was developed by Mulla et al.330 for the efficient synthesis of 1-amido- and 1-carbamato-alkyl naphthols/phenols 194 in good to excellent yield via one-pot three-component condensation of various aldehyde, amide/urea/carbamate, and naphthols/phenols using ethylammonium nitrate (EAN) as reusable ionic liquid catalyst under neat reaction condition at ambient temperature (Scheme 70). EAN was recovered and recycled several times without loss of catalytic activity.
The investigators proposed a plausible mechanism for the transformation. The reaction involves first the activation of carbonyl group of aldehyde by EAN (intermediate 195) followed by the nucleophilic addition of beta-naphthol to obtain complex 196. The removal of water from complex 196 produces intermediate 197 as an ortho-quinonemethide. The subsequent activation of 197 by EAN to generate 197′ as a Michael acceptor is followed by in situ Michael addition of amide or carbamate or urea to release instantly the corresponding product 194 (Scheme 71).
 | ||
| Scheme 71 Proposed mechanism for EAN-catalyzed synthesis of 1-amido- and 1-carbamato-alkyl naphthols/phenols 194. | ||
Kshirsagar et al.331 reported for the first time on one-pot synthesis of functionalized cyclohexenes 199 through a three-component reaction in the presence of a solid catalyst; the investigators demonstrated that Mg–Al hydrotalcite (Mg–Al HT calcined at 500 °C) acts as an efficient solid heterogeneous catalyst for solvent-free one-pot three-component synthesis of a series of 2-amino-5-nitro-4,6-diarylcyclohex-1-ene-1,3,3-tricarbonitriles (199) from the condensation of aryl aldehyde, malononitrile, and nitromethane at ambient temperature (Scheme 72). The catalyst can be easily separated and is recyclable.
Liu et al.332 developed a solvent-free and fast one pot synthesis of spiropyranyl-oxindoles 200 in the presence of NaHCO3 under grinding at room temperature with good to excellent yields (Scheme 73). Short reaction times, good yields, operational simplicity, and eco-friendliness are the key features of this procedure for the synthesis of a series of spiro[indoline-3,4′-pyrano[2,3-c]pyrazol]-2-ones, spiro-oxindole framework of which is an important structural motif in a number of bioactive natural products and pharmaceuticals.333–336
2.3. Carbon–carbon bond forming reactions under ultrasonication
Ultrasonication is a process of irradiating a liquid sample with ultrasonic (>20 kHz) waves resulting in agitation, and this technique is now-a-days a well-regarded eco-environmental technology in green chemistry being advantageous over the traditional thermal methods as enhanced reaction rates, formation of purer products, improved yields, increased selectivities, easier experimental procedures, and use of milder conditions both in case of homogeneous and heterogeneous reactions.337–344 Successful applications of ultrasound in synthetic organic chemistry have been demonstrated in recent years in carrying out a handful of organic transformations with enhanced reaction rates, yields and selectivity just at ambient conditions which otherwise require drastic conditions of temperature and pressure.334–362On propagation into the liquid media, sound waves result in alternating high-pressure (compression) and low-pressure (rarefaction) cycles. During rarefaction, high-intensity sonic waves create small vacuum bubbles in the liquid, and the rapid nucleation, growth and collapse of these micrometer scale bubbles constitute the phenomenon of cavitation, generating very high local temperatures in short times. This is the driving force of smooth chemical transformations under ultrasound irradiation.363–371 Number of organic reactions promoted by ultrasonication have been revisited in recent times.63,372–375 The present section just offers a highlight on certain recently reported carbon–carbon bond forming reactions occurred under the influence of ultrasound irradiation at ambient conditions.
Narayanaperumal et al.380 developed an efficient solvent-free conjugate Michael addition of 2,4-pentanedione (203) to various nitroalkenes (202) in the presence of a catalytic amount of 1-methyl-3-(2-(piperidin-1-yl)ethyl)-1H-imidazol-3-ium chloride, a base-behavior task-specific ionic liquid (TSIL), under ultrasonication furnishing the desired conjugate adducts 204 in good to excellent yields (Scheme 75). The investigators reported on the successful recovery and recycling of the ionic liquid in further reactions for at least four successive runs without observing significant decrease in yield.
Xanthene derivatives are useful pharmacological as well as optical properties.383–386 Khaligh and Shirini387 have recently designed a room temperature and solvent-free one-pot synthesis of a series of such compounds under the influence of ultrasound irradiation from the reaction of aromatic aldehydes (1; 1 mmol) and a varying substrates such as 2-naphthol (22; 2 mmol), 2-hydroxynaphthalene-1,4-dione (87; 2 mmol), mixture of 2-naphthol (1 mmol) and indane-1,3-dione (206; 1 mmol) or 2-hydroxynaphthalene-1,4-dione (1 mmol) and indane-1,3-dione (1 mmol) using a heterogeneous and reusable N-sulfonic acid poly(4-vinylpyridinium) hydrogen sulfate (NSPVPHS) catalyst (Scheme 78). Compared with traditional methods, the present methodology offers several advantages such as the accelerated reaction rate and good yields, minimization of the energy consumption, ease of preparation and handling of the catalyst, simple experimental procedure, and mild reaction conditions. The transformation involves formation of C–C and C–O bonds. Previously, Venkatesan et al.388 also reported on the use of ultrasonication in synthesizing 1,8-dioxo-octahydroxanthene derivatives in the presence of the ionic liquid, [Hbim]BF4 (IL) as a catalyst and reaction medium with methanol as co-solvent at ambient temperature.
 | ||
| Scheme 78 N-Sulfonic acid poly(4-vinylpyridinium) hydrogen sulfate (NSPVPHS)-catalyzed one-pot solvent-free synthesis of xanthenes. | ||
Datta and Pasha389 reported a one-pot multicomponent synthesis of a series of 2-amino-3-cyanao-4H-chromenes (209) from the reaction of diverse aromatic aldehydes (5), malononitrile (55) and resorcinol (208) at 28–30 °C in water under ultrasonication, using glycine as a cost effectiveness and non-toxic organocatalyst (Scheme 79).
 | ||
| Scheme 79 Glycine-catalyzed one-pot multicomponent synthesis of 2-amino-3-cyano-4H-chromenes (209) in water. | ||
Ziarati et al.390 developed an efficient protocol for the synthesis of 2-aryl-5-methyl-2,3-dihydro-1H-3-pyrazolones (211) with the aid of synergetic effect of ultrasonication and Cu-nano catalysis from a four-component one-pot condensation reaction of hydrazines, ethyl acetoacetate, aldehydes and β-naphthol in water (Scheme 80). The authors commented that US can increase the surface area of the catalyst and supply additional activation through efficient mixing and enhanced mass transport. This multicomponent protocol in water gave excellent yields in short reaction times and showed a wide range of applicability as it could be used with different substrates, including aromatic aldehydes and hydrazines to provide the corresponding pyrazolones in good yields.390
2.4. Carbon–carbon bond forming reactions driven by visible-light
Application of visible-light as a clean, inexpensive, and sustainable alternative energy source to promote chemical transformations has received increasing attention from the chemistry community in recent times. Actually, a hundred years ago G. Ciamician had already recognized that sunlight could be utilized as an inexpensive, abundant, clean and renewable energy source for organic chemistry.391 The ability to convert solar energy into chemical energy in an efficient manner, thus, continues to be an important issue from the viewpoint of environmental sustainability. However, due to inability of most common organic substances to absorb light in the visible region, the major part of photochemical reactions of organic substrates described to date involve the use of high-energy UV light.392 UV-light-induced reactions not only require the use of specialized light sources, but they are also accompanied by many side reactions that limit their broad application. Under this purview, sincere drives have been made so far to develop visible-light photocatalysts to channel energy from the visible light spectrum to organic molecules capable to mediate the desired chemical transformations. This approach originates from the unique property of metal complexes and organic dyes to become engage in single-electron-transfer (SET) processes with organic substrates upon photoexcitation with visible light. As a result, many of the most commonly employed visible light photocatalysts are thus polypyridyl complexes of ruthenium and iridium393,394 or organic dyes.395 Generally, irradiation of the photocatalyst with visible light produces a redox-active excited state, which can be either reduced or oxidized by single electron transfer (SET) from or to the substrate; in addition, the photoexcited states of photocatalysts can serve as energy donors, activating organic substrates through an energy transfer process.396,397 A number of good reviews are already in literature summarizing the developments in the field of photocatalysts and their useful applications in synthetic organic chemistry.398–412 The present section summarizes most recent developments in visible-light-driven carbon–carbon bond forming reactions at ambient conditions.Recently, Woo and Kim448 have developed a visible-light-induced photoredox-catalyzed rapid protocol for the synthesis of CF3-substituted cyclic ketones (217) via trifluoromethylation and 1,2-carbon migration of 1-(1-arylvinyl)cyclobutanol derivatives 215 using Ru(phen)3Cl2 as photocatalyst and Umemoto's reagent 216 as a source of CF3-moiety at ambient temperature (Scheme 82).
 | ||
| Scheme 82 Synthesis of CF3-substituted cyclic ketones (217) via photocatalytic trifluoromethylation/1,2-carbon migration sequences. | ||
In the same year (2015), Zheng et al.449 have reported on the development of a viable visible-light-induced method for smooth implementation of trifluoromethylarylation/1,4-aryl shift/desulfonylation cascade reaction of α,β-unsaturated imide alkenes (218) using CF3SO2Cl as CF3 source in the presence of Ru(bpy)3Cl2 as the photocatalyst at room temperature, thereby yielding trifluoromethyl isoquinolinediones (219), trifluoromethyl oxindoles (220) and α-aryl-β-trifluoromethylamides (221) under varying conditions in moderate to good yield (Scheme 83). Operational simplicity, low catalyst loading (5% catalyst), and less additives are the advantages of this method.
In another report, Deng et al.450 have recently described an efficient method for photocatalytic oxytrifluoromethylation reaction of N-allylamides (222/224)for the first time under the influence of visible light leading to the synthesis of biologically relevant CF3-containing diversely functionalized oxazolines (223) and benzoxazines (225) at room temperature (Scheme 84). The transformation involves formation of both C–C and C–O bonds.
Oh et al.451 developed a new photoredox-catalyzed protocol for vicinal chlorotrifluoromethylation of alkenes (226) in the presence of Ru(Phen)3Cl2 as photocatalyst using CF3SO2Cl (227) as a source for the CF3 radical and chloride ion under visible light irradiation (Scheme 85). Various terminal and internal alkenes were transformed to their vicinal chlorotrifluoromethylated derivatives 228.
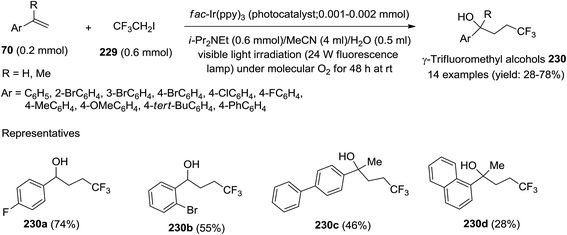
Very recently, Li et al.452 have reported visible-light-induced photoredox difunctionalization reactions of diverse substituted styrenes 70 with 1,1,1-trifluoro-2-iodoethane (229) under an oxygen atmosphere in the presence of water yielding a series of γ-trifluoromethyl alcohols 230 (Scheme 86). They used fac-Ir(ppy)3 as a photocatalyst in this light-induced radical reaction, and it has also been demonstrated that the oxygen atom in the product originates from molecular oxygen. The investigators have observed that water plays a key role in this reaction, and instead of the desired product, 2,2,2-trifluoroethanol has been found to form in the absence of water; no difunctionalization of styrenes is observed.452
α,β-Epoxy ketones are regarded as important intermediates and precursors in synthetic organic chemistry.454–456 Li and Wang457 have recently demonstrated the visible-light induced straightforward protocol for the synthesis of such compounds 232 from a range of styrenes and benzaldehydes under the influence of Ru(bpy)3Cl2 (as photocatalyst), tert-butyl hydroperoxide (t-BuOOH) and cesium carbonate as a base at room temperature (Scheme 88). The investigators have proposed that the process proceeds through visible-light-enabled photocatalytic generations of acyl radicals as key intermediates as depicted in Scheme 89.457 The transformation involves the formation of both C–C and C–O bonds.
In recent time, an effort to merging photoredox and nickel catalysis for better dual catalytic activation mode in exploring C–C bond formations has been initiated. In a recent report, Noble et al.458 have successfully accomplished decarboxylative Csp3–Csp2 cross-coupling of diverse carboxylic acids (49′) with vinyl halides (233) through the synergetic merger of photoredox and nickel catalysis under mild conditions under the influence of visible light at room temperature (Scheme 90). The investigators have extended their new methodology to a variety of α-oxy- and α-amino acids, as well as simple hydrocarbon-substituted acids; diverse vinyl iodides and bromides yielded vinylation products in high efficiency under mild, operationally simple reaction conditions.
Recently, Primer et al.459 have developed visible-light induced single-electron-mediated alkyl transfer as a novel mechanism for transmetalation, enabling a general Csp3–Csp2 cross-coupling of secondary alkyltrifluoroborates (symmetrical and both unsymmetrical and sterically encumbered) 235 with an array of substituted aryl bromides effected by an Ir-photoredox catalyst and a Ni cross-coupling catalyst under mild conditions at room temperature (Scheme 90). The investigators have claimed their method as operationally simple and superior to previously reported such cross-coupling protocols (Scheme 91).
Kaldas et al.460 have developed a light-mediated process for the generation of organic free radicals with unactivated bromoalkanes/arenes (237) using dimeric Au(I) photocatalyst under basic conditions at room temperature for functionalization of substituted indoles (238) (Scheme 92). This method may be a mild and relatively safe alternative to organostannanes and pyrophoric initiators used for accessing high energy radicals that were previously inaccessible through catalytic or stoichiometric means.
3. Concluding remarks and outlook
Practice of green and sustainable chemistry, thus, encompasses so many criteria during carrying out organic transformations, and those which can satisfy these criteria to a greater extent are welcome! Under this purview, designing of methods for promising and useful organic reactions under ambient conditions coupled with other green aspects is, thus, a hot area in current trends in green chemistry research. Carbon–carbon (C–C) bond forms the ‘backbone’ of nearly every organic molecule, and is essentially regarded as the key transformation in organic synthesis to set up the carbon backbone of organic molecules. It is needless to mention that the carbon–carbon bond formation has always been one of the most useful and fundamental reactions in the development of organic chemistry, and continues to be. The present review offers an up-to-date development on the design of carbon–carbon bond forming protocols to access a wide variety of organic molecules of topical interest under ambient temperature and pressure. The account categorically focuses on the brilliant applications of reaction conditions such as the use of solvents or no solvent, catalysts or no catalyst, and the use of green tools like ball-milling, ultrasonication and visible light in achieving the goal! These reported protocols for developing carbon–carbon frame-work are associated with a handful of advantages such as mild reaction conditions, good yields, operational simplicity and absence of tedious separation procedures, clean reaction profiles, high atom-economy, inexpensive starting materials, and environmentally benign catalysts and their reusability. The author of this article hopes that this account would motivate the young minds of the coming generation in chemistry as well as the experts and professionals practicing green and sustainable chemistry at large.Acknowledgements
The author is thankful to the Science and Engineering Research Board (SERB), Department of Science & Technology (DST), New Delhi for financial support (Grant No. EMR/2014/001220).References
- E. J. Corey and X. M. Cheng, The Logic of Chemical Synthesis, John Wiley & Sons, New York, 1989 Search PubMed
.
- P. J. Parsons, C. S. Penkett and A. J. Shell, Chem. Rev., 1996, 96, 195 CrossRef CAS PubMed
.
- G. Mehta and A. Srikrishna, Chem. Rev., 1997, 97, 671 CrossRef CAS PubMed
.
- A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945 CrossRef CAS PubMed
.
- E. J. Kang and E. Lee, Chem. Rev., 2005, 105, 4348 CrossRef CAS PubMed
.
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS PubMed
.
- M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 2008, 108, 2015 CrossRef CAS PubMed
.
- M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140 CrossRef CAS PubMed
.
- B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef PubMed
.
- G. Brahmachari, Handbook of Pharmaceutical Natural Products, Wiley-VCH, Weinheim, 2010, vol. 1 and 2 Search PubMed
.
- Bioactive Natural Products: Opportunities and Challenges in Medicinal Chemistry, ed. G. Brahmachari, World Scientific Publishing Co., Singapore, 2011 Search PubMed
.
- Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds, ed. G. Brahmachari, CRC Press/Taylor Francis Group, LLC, USA, 2013 Search PubMed
.
- K. E. O. Ylijoki and J. M. Stryker, Chem. Rev., 2013, 113, 2244 CrossRef CAS PubMed
.
- J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Rev., 2015, 115, 5301 CrossRef CAS PubMed
.
- Bioactive Natural Products: Chemistry and Biology, ed. G. Brahmachari, Wiley-VCH, Weinheim, 2015 Search PubMed
.
- A. M. Armaly, Y. C. DePorre, E. J. Groso, P. S. Riehl and C. S. Schindler, Chem. Rev., 2015, 115, 9232 CrossRef CAS PubMed
.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS
.
- R. Franzén, Can. J. Chem., 2000, 78, 957 CrossRef
.
- R. Franzén and Y. Xu, Can. J. Chem., 2005, 83, 266 CrossRef
.
- L. Yin and J. Liebscher, Chem. Rev., 2006, 107, 133 CrossRef PubMed
.
- J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Nat. Chem., 2009, 1, 494 CrossRef CAS PubMed
.
- P. Clapés, W.-D. Fessner, G. A. Sprenger and A. K. Samland, Curr. Opin. Chem. Biol., 2010, 14, 154 CrossRef PubMed
.
- D. G. Gillingham, P. Stallforth, A. Adibekian, P. H. Seeberger and D. Hilvert, Nat. Chem., 2010, 2, 102 CrossRef CAS PubMed
.
- J. Soloducho, J. Cabaj, K. Idzik, A. Nowakowska-Oleksy, A. Swist and M. Lapkowski, Curr. Org. Chem., 2010, 14, 1234 CrossRef CAS
.
- V. Resch, J. H. Schrittwieser, E. Siirola and W. Kroutil, Curr. Opin. Biotechnol., 2011, 22, 793 CrossRef PubMed
.
- M. Pérez, M. Fañanás-Mastral, P. H. Bos, A. Rudolph, S. R. Harutyunyan and B. L. Feringa, Nat. Chem., 2011, 3, 377 CrossRef PubMed
.
- A. Bhunia, R. S. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC
.
- G. Brahmachari, Chem. Rec., 2016, 16, 98 CrossRef CAS PubMed
.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed
.
- J. H. Clark, Green Chem., 1999, 1, 1 RSC
.
- M. Poliakoff and P. Licence, Nature, 2007, 450, 810 CrossRef CAS PubMed
.
- Green Chemistry Education: Changing the Course of Chemistry, ACS Symposium Series, ed. P. T. Anastas, I. J. Levy and K. E. Parent, American Chemical Society, USA, 2009, vol. 1011 Search PubMed
.
- J. H. Clark, R. Luque and A. S. Matharu, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 183 CrossRef CAS PubMed
.
- R. Cernansky, Nature, 2015, 519, 379 CrossRef PubMed
.
- R. Sheldon, Nature, 1999, 399, 636 CrossRef CAS PubMed
.
- K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025 CrossRef CAS PubMed
.
- K. Kaneda and T. Mizugaki, Energy Environ. Sci., 2009, 2, 655 CAS
.
- B. M. Trost, Acc. Chem. Res., 2002, 35, 695 CrossRef CAS PubMed
.
- G. Brahmachari and B. Banerjee, Curr. Green Chem., 2015, 2, 274 CrossRef CAS
.
- A. Farrán, C. Cai, M. Sandoval, Y. Xu, J. Liu, M. J. Hernáiz and R. J. Linhardt, Chem. Rev., 2015, 115, 6811 CrossRef PubMed
.
- Green Synthetic Approaches for Biologically Relevant Heterocycles, ed. G. Brahmachari, Elsevier, Amsterdam, 2014 Search PubMed
.
- G. Brahmachari, Room Temperature Organic Synthesis, Elsevier, Amsterdam, 2015 Search PubMed
.
- C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS
.
- P. A. Grieco, Organic Synthesis in Water, Blackie Academic and Professional, London, 1998 Search PubMed
.
- R. Breslow, Acc. Chem. Res., 2004, 37, 471 CrossRef CAS PubMed
.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC
.
- M. Carril, R. SanMartin, I. Tellitu and E. Dominguez, Org. Lett., 2006, 8, 1467 CrossRef CAS PubMed
.
- C. J. Li and L. Chen, Organic Chemistry in Water, Chem. Soc. Rev., 2006, 35, 68–82 RSC
.
- Organic Reactions in Water, ed. U. M. Lindstrom, Blackwell Publishing, Oxford, 2007 Search PubMed
.
- H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114 CrossRef CAS
.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed
.
- Y. Hirai and Y. Uozumi, Chem. Commun., 2010, 46, 1103 RSC
.
- M. M. Savant, A. M. Pansuriya, C. V. Bhuva, N. Kapuriya, A. S. Patel, V. B. Audichya, P. V. Pipaliya and Y. T. Naliapara, J. Comb. Chem., 2010, 12, 176 CrossRef CAS PubMed
.
- F. Zhou and C.-J. Li, Nat. Commun., 2014, 5, 4254, DOI:10.1038/ncomms5254
.
- K. Park and S. Lee, Org. Lett., 2015, 17, 1300 CrossRef CAS PubMed
.
- P. Tundo, P. Anastas, D. S. Black, J. Breen, T. Collins, S. Memoli, J. Miyamoto, M. Polyakoff and W. Tumas, Pure Appl. Chem., 2000, 72, 1207 CAS
.
- T. Tsukinoki, S. Nagashima, Y. Mitoma and M. Tashiro, Green Chem., 2000, 2, 117 RSC
.
- F. Bigi, M. L. Conforti, R. Maggi, A. Piccinno and G. Sartori, Green Chem., 2000, 2, 101 RSC
.
- K. Manabe, S. Iimura, X. M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2002, 124, 11971 CrossRef CAS PubMed
.
- D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS
.
- S. Otto, W. Blokzijl and J. B. F. N. Engberts, J. Org. Chem., 1994, 59, 5372 CrossRef CAS
.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed
.
- G. Sartori, G. Enderlin, G. Hervé and C. Len, Synthesis, 2012, 44, 767 CrossRef CAS
.
- G. Sartori, G. Hervé, G. Enderlin and C. Len, Synthesis, 2013, 45, 330 CrossRef CAS
.
- A. Decottignies, A. Fihri, G. Azemar, F. Djedaini-Pilard and C. Len, Catal. Commun., 2013, 32, 101 CrossRef CAS
.
- A. Hassine, S. Sebti, A. Solhy, M. Zahouily, C. Len, M. N. Hedhili and A. Fihri, Appl. Catal., A, 2013, 450, 13 CrossRef CAS
.
- S. Gallagher-Duval, G. Hervé, G. Sartori, G. Enderlin and C. Len, New J. Chem., 2013, 37, 1989 RSC
.
- H. Saggadi, D. Luart, N. Thiebault, I. Polaert, L. Estel and C. Len, Catal. Commun., 2014, 44, 15 CrossRef CAS
.
- G. Hervé, G. Sartori, G. Enderlin, G. Mackenzie and C. Len, RSC Adv., 2014, 4, 18558 RSC
.
- H. Saggadi, D. Luart, N. Thiebault, I. Polaert, L. Estel and C. Len, RSC Adv., 2014, 4, 21456 RSC
.
- G. Hervé and C. Len, RSC Adv., 2014, 4, 46926 RSC
.
- T. Lussier, G. Hervé, G. Enderlin and C. Len, RSC Adv., 2014, 4, 46218 RSC
.
- A. Hassine, M. Bouhrara, S. Sebti, A. Solhy, R. Mahfouz, D. Luart, C. Len and A. Fihri, Curr. Org. Chem., 2014, 18, 3141 CrossRef CAS
.
- K. Dänoun, I. Jioui, M. Bouhrara, M. Zahouily, A. Solhy, M. Jouiad, C. Len and A. Fihri, Curr. Org. Chem., 2015, 19, 2132 CrossRef
.
- H. Saggadi, I. Polaert, D. Luart, C. Len and L. Estel, Catal. Today, 2015, 255, 66 CrossRef CAS
.
- S. L. Guenic, C. Ceballos and C. Len, Catal. Lett., 2015, 145, 1851 CrossRef
.
- S. L. Guenic, F. Delbecq, C. Ceballos and C. Len, J. Mol. Catal. A: Chem., 2015, 410, 1 CrossRef
.
- G. Cravotto, E. Borretto, M. Oliverio, A. Procopio and A. Penoni, Catal. Commun., 2015, 63, 2 CrossRef CAS
.
- S. L. Guenic, C. Ceballos and C. Len, J. Mol. Catal. A: Chem., 2016, 411, 72 CrossRef
.
- U. M. Lindstrom, Chem. Rev., 2002, 102, 2751 CrossRef PubMed
.
- Y. Hayashi, Angew. Chem., Int. Ed., 2006, 45, 8103 CrossRef CAS PubMed
.
- K. Taira and S. J. Benkovic, J. Med. Chem., 1988, 31, 129 CrossRef CAS PubMed
.
- J. K. Beattie, C. S. P. McErlean and C. B. W. Phippen, Chem.–Eur. J., 2010, 16, 8972 CrossRef CAS PubMed
.
- J. Zhang, Z. W. Cui, F. Wang, Y. Wang, Z. W. Miao and R. Y. Chen, Green Chem., 2007, 9, 1341 RSC
.
- M. Y. Wu, K. Li, N. Wang, T. He and X. Q. Yu, Synthesis, 2011, 1831 CAS
.
- M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC
.
- M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, ChemSusChem, 2014, 7, 24 CrossRef CAS PubMed
.
- J. J. Yu, L. M. Wang, J. Q. Liu, F. L. Guo, Y. Liu and N. Jiao, Green Chem., 2010, 12, 216 RSC
.
- B. A. Marco, T. A. Manuel, P. M. Itzia, M. M. Francisco, E. Georgina, M. Elies and E. Espinosa, J. Chem. Crystallogr., 1999, 29, 759 CrossRef
.
- P. Shanmugasundaram, K. J. Prabahar and V. T. Ramakrishnan, J. Heterocycl. Chem., 1993, 30, 1003 CrossRef CAS
.
- N. Srividya, P. Ramamurthy, P. Shanmugasundaram and V. T. Ramakrishnan, J. Org. Chem., 1996, 61, 5083 CrossRef CAS
.
- K. M. Khan, G. M. Maharvi, M. T. H. Khan, A. J. Shaikh, S. Perveen, S. Begun and M. I. Choudhary, Bioorg. Med. Chem., 2006, 14, 344 CrossRef CAS PubMed
.
- D. B. Ramachary and M. Kishor, J. Org. Chem., 2007, 72, 5056 CrossRef CAS PubMed
.
- M. Bayat, H. Imanieh and S. H. Hossieni, Chin. Chem. Lett., 2009, 20, 656 CrossRef CAS
.
- H.-B. Zhang, L. Liu, Y.-J. Chen, D. Wang and C.-J. Li, Eur. J. Org. Chem., 2006, 869 CrossRef
.
- D. Harris Jr, A. Nguyen, A. Harald, P. Hirth, G. McMahon and C. Tang, Org. Lett., 1999, 1, 43 CrossRef
and references cited therein.
- A. F. Barrero, E. J. Alvarez-Manzaneda, M. Mar Herrador, R. Chahboum and P. Galera, Bioorg. Med. Chem. Lett., 1999, 9, 2325 CrossRef CAS PubMed
.
- K. Arai, S. Shimizu, Y. Taguchi and Y. Yamamoto, Chem. Pharm. Bull., 1981, 29, 991 CrossRef CAS
.
- S. Shimizu, Y. Yamamoto and S. Koshimura, Chem. Pharm. Bull., 1982, 30, 1896 CrossRef CAS PubMed
.
- A. Kaji, T. Iwata, N. Kiriyama, S. Wakusawa and K. Miyamoto, Chem. Pharm. Bull., 1994, 42, 1682 CrossRef CAS PubMed
.
- B. Zhang, G. Salituro, D. Szalkowski, Z. Li, Y. Zhang, I. Royo, D. Vilella, M. T. Diez, F. Pelaez, C. Ruby, R. L. Kendall, X. Mao, P. Griffin, J. Calaycay, J. R. Zierath, J. V. Heck, R. G. Smith and D. E. Moller, Science, 1999, 284, 974 CrossRef CAS PubMed
.
- P. B. Thakur and H. M. Meshram, RSC Adv., 2014, 4, 5343 RSC
.
- T. Tokunaga, W. E. Hume, T. Umezome, K. Okazaki, Y. Ueki, K. Kumagai, S. Hourai, J. Nagamine, H. Seki, M. Noguchi and R. Nagata, J. Med. Chem., 2001, 44, 4641 CrossRef CAS PubMed
.
- H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36 CrossRef CAS
.
- C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS
.
- J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC
.
- T. Kagata, S. Saito, H. Shigemori, A. Ohsaki, H. Kubota and J. Kobayashi, J. Nat. Prod., 2006, 69, 1517 CrossRef CAS PubMed
.
- C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed
.
- J. J. Badillo, N. V. Hanhan and A. K. Franz, Curr. Opin. Drug Discovery Dev., 2010, 13, 758 CAS
.
- F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS
.
- P. Hewawasam, N. A. Meanwell, V. K. Gribkoff, S. I. Dworetzky and C. G. Boissard, Bioorg. Med. Chem. Lett., 1997, 7, 1255 CrossRef CAS
.
- P. Hewawasam, M. Erway, S. L. Moon, J. Knipe, H. Weiner, C. G. Boissard, D. J. Post- Munson, Q. Gao, S. Huang, V. K. Gribkoff and N. A. Meanwell, J. Med. Chem., 2002, 45, 1487 CrossRef CAS PubMed
.
- N. Boechat, W. B. Kover, V. Bongertz, M. M. Bastos, N. C. Romeiro, M. L. G. Azavedo and W. Wollinger, Med. Chem., 2007, 3, 533 CrossRef CAS
.
- P. B. Thakur and H. M. Meshram, RSC Adv., 2014, 4, 6019 RSC
.
- A. Olyaei, E. C. Parashkuhi, S. Raoufmoghaddam and M. Sadeghpour, Synth. Commun., 2010, 40, 3609 CrossRef CAS
.
- D. Enders and J. P. Shilvock, Chem. Soc. Rev., 2000, 29, 359 RSC
.
- L. Wang, J. Shen, Y. Tang, Y. Chen, W. Wang, Z. Cai and Z. Du, Org. Process Res. Dev., 2007, 11, 487 CrossRef CAS
.
- H. Gröger, Chem. Rev., 2003, 103, 2795 CrossRef PubMed
.
- Y. M. Shafran, V. A. Bakulev and V. S. Mokrushin, Russ. Chem. Rev., 1989, 58, 148 CrossRef
.
- P. Galletti, M. Pori and D. Giacomini, Eur. J. Org. Chem., 2011, 3896 CrossRef CAS
.
- J. Venkata Prasad, M. Prabhakar, K. Manjulatha, D. Rambabu, K. A. Solomon, G. Gopi Krishna and A. A. Kumar, Tetrahedron Lett., 2010, 51, 3109 CrossRef CAS
.
- R. K. Smalley, in Comprehensive Heterocyclic Chemistry, ed. W. Lwowski, Pergamon Press, Bristol, 1984, vol. 7, pp. 545–546 Search PubMed
.
- J. P. K. Meigh, in Science of Synthesis, ed. S. M. Weinreb, Thieme, Stuttgart, New York, 2004, vol. 17, pp. 829–830 Search PubMed
.
- A. P. Guzikowski, E. R. Whittemore, R. M. Woodward, E. Weber and J. F. W. Keana, J. Med. Chem., 1997, 40, 2424 CrossRef CAS PubMed
.
- Y. Murakami, H. Hara, T. Okada, H. Hashizume, M. Kii, Y. Ishihara, M. Ishikawa, M. Shimamura, S. Mihara, G. Kato, K. Hanasaki, S. Hagishita and M. Fujimoto, J. Med. Chem., 1999, 42, 2621 CrossRef CAS PubMed
.
- C. Schultz, A. Link, M. Leost, D. W. Zaharevitz, R. Gussio, E. A. Sausville, L. Meijer and C. Kunick, J. Med. Chem., 1999, 42, 2909 CrossRef CAS PubMed
.
- Y. Aramaki, M. Seto, T. Okawa, T. Oda, N. Kanzaki and M. Shiraishi, Chem. Pharm. Bull., 2004, 53, 254 CrossRef
.
- C. Kunick, K. Lauenroth, K. Wieking, X. Xie, C. Schultz, R. Gussio, D. Zaharevitz, M. Leost, L. Meijer, A. Weber, F. S. Jorgensen and T. Lemcke, J. Med. Chem., 2004, 47, 22 CrossRef CAS PubMed
.
- B. A. Bunin and J. A. Ellman, J. Am. Chem. Soc., 1992, 114, 10997 CrossRef CAS
.
- A. Monge, J. A. Palop, J. C. Del Castillo, J. M. Caldero, J. Roca, G. Romero, J. Del Rio and B. Lasheras, J. Med. Chem., 1993, 36, 2745 CrossRef CAS PubMed
.
- A. Gazit, H. App, G. McMahon, A. Chen, A. Levitzki and F. D. Bohmer, J. Med. Chem., 1996, 39, 2170 CrossRef CAS PubMed
.
- L. Lee, W. V. Murray and R. A. Rivero, J. Org. Chem., 1997, 62, 3874 CrossRef
.
- G. A. Morales, J. W. Corbett and W. F. DeGrado, J. Org. Chem., 1998, 63, 1172 CrossRef CAS
.
- L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2002, 45, 5604 CrossRef CAS PubMed
.
- K. Toshima, R. Takano, T. Ozawa and S. Matsumara, Chem. Commun., 2002, 212 RSC
.
- D. Gupta, N. N. Ghosh and R. Chandra, Bioorg. Med. Chem. Lett., 2005, 15, 1019 CrossRef CAS PubMed
.
- C. Venkatesh, B. Singh, P. K. Mahata, H. Ila and H. Junjappa, Org. Lett., 2005, 7, 2169 CrossRef CAS PubMed
.
- A. Katoh, T. Yoshida and J. Ohkando, Heterocycles, 2000, 52, 911 CrossRef CAS
.
- N. D. Sonawane and D. W. Rangnekar, J. Heterocycl. Chem., 2002, 39, 303 CrossRef CAS
.
- D. O'Brien, M. S. Weaver, D. G. Lidzey and D. D. C. Bradley, Appl. Phys. Lett., 1996, 69, 881 CrossRef
.
- S. Dailey, W. J. Feast, R. J. Peace, I. C. Sage, S. Till and E. L. Wood, J. Mater. Chem., 2001, 11, 2238 RSC
.
- A. Shaabani, F. Hajishaabanha, H. Mofakham, M. Mahyari and B. Lali, Helv. Chim. Acta, 2012, 95, 246 CrossRef CAS
.
- A. Ramazani, A. Rezaei, A. T. Mahyari, M. Rouhani and M. Khoobi, Helv. Chim. Acta, 2010, 93, 2033 CrossRef CAS
.
- T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Wiley-VCH, Weinheim, 2nd edn, 2003, pp. 316–336 Search PubMed
.
- K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 3245 CrossRef CAS PubMed
.
- J. Taran, A. Ramazani, S. W. Joo, K. Ślepokura and T. Lis, Helv. Chim. Acta, 2014, 97, 1088 CrossRef CAS
.
- K. Kumaravel and G. Vasuki, Green Chem., 2009, 11, 1945 RSC
.
- E. A. A. Hafez, M. H. Elnagdi, A. G. A. Elagamey and F. M. A. A. Ei-Taweel, Heterocycles, 1987, 26, 903 CrossRef CAS
.
- L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef CAS
.
- G. P. Ellis, in The Chemistry of Heterocyclic Compounds: Chromenes, Chromanes and Chromones, ed. A. Weissberger and E. C. Taylor, Wiley, New York, 1997, ch. II Search PubMed
.
- S. Kasibhatla, H. Gourdeau, K. Meerovitch, J. Drewe, S. Reddy, L. Qiu, H. Zhang, F. Bergeron, D. Bouffard, Q. Yang, J. Herich, S. Lamothe, S. Xiong Cai and B. Tseng, Mol. Cancer Ther., 2004, 3, 1365 CAS
.
- H. Gourdeau, L. Leblond, B. Hamelin, C. Desputeau, K. Dong, I. Kianicka, D. Custeau, C. Bourdeau, L. Geerts, S. Xiong Cai, J. Drewe, D. Labrecque, S. Kasibhatla and B. Tseng, Mol. Cancer Ther., 2004, 3, 1375 CAS
.
- W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, J. Zhao, C. Crogan-Grundy, L. Xu, S. Lamothe, H. Gourdeau, R. Denis, B. Tseng, S. Kasibhatla and S. Xiong Cai, J. Med. Chem., 2007, 50, 2858 CrossRef CAS PubMed
.
- R. Tripathy, A. Ghose, J. Singh, E. R. Bacon, T. S. Angeles, S. X. Yang, M. S. Albom, L. D. Aimone, J. L. Herman and J. P. Mallamo, Bioorg. Med. Chem. Lett., 2007, 17, 1793 CrossRef CAS PubMed
.
- K. Manabe, X.-M. Sun and S. Kobayashi, J. Am. Chem. Soc., 2001, 123, 10101 CrossRef CAS PubMed
.
- S. Kobayashi, S. Iimura and K. Manabe, Chem. Lett., 2002, 10 CrossRef CAS
.
- M. Kunishima, H. Imada, K. Kikuchi, K. Hioki, J. Nishida and S. Tani, Angew. Chem., Int. Ed., 2005, 44, 7254 CrossRef CAS PubMed
.
- C.-J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed
.
- T. Murase, Y. Nishijima and M. Fujita, J. Am. Chem. Soc., 2012, 134, 162 CrossRef CAS PubMed
.
- G. Brahmachari, ACS Sustainable Chem. Eng., 2015, 3, 2350 CrossRef CAS
.
- R. D. H. Murray, Nat. Prod. Rep., 1995, 12, 477 RSC
.
- O. Kayser and H. Kolodziej, Planta Med., 1997, 63, 508 CrossRef CAS PubMed
.
- D. Bhavsar, J. Trivedi, S. Parekh, M. Savant, S. Thakrar, A. Bavishi, A. Radadiya, H. Vala, J. Lunagariya and M. Parmar, Bioorg. Med. Chem. Lett., 2011, 21, 3443 CrossRef CAS PubMed
.
- S. Vazquez-Rodriguez, R. Figueroa-Guíñez, M. J. Matos, L. Santana, E. Uriarte, M. Lapier, J. D. Maya and C. Olea-Azar, Med. Chem. Commun., 2013, 4, 993 RSC
.
- D. Zhi Qiang, J. B. Shi, B. A. Song and X. H. Liu, RSC Adv., 2014, 4, 5607 RSC
.
- L. Bonsignore, A. De Logu, S. M. Lavagna, G. Loy and D. Secci, Eur. J. Med. Chem., 1994, 29, 479 CrossRef CAS
.
- L. Bonsignore, F. Cottiglia, S. M. Lavagna, G. Loy and D. Secci, Heterocycles, 1999, 50, 469 CrossRef CAS
.
- D. Jonsson, M. Erlandsson and A. Unden, Tetrahedron Lett., 2001, 42, 6953 CrossRef CAS
.
- I. Kempen, M. Hemmer, S. Counerotte, L. Pochet, P. de Tullio, J. M. Foidart, S. Blacher, A. Noel, F. Frankenne and B. Pirotte, Eur. J. Med. Chem., 2008, 43, 2735 CrossRef CAS PubMed
.
- E. Peroni, G. Caminati, P. Baglioni, F. Nuti, M. Chelli and A. M. Papini, Bioorg. Med. Chem. Lett., 2002, 12, 1731 CrossRef CAS PubMed
.
- D. P. Specht, P. A. Martic and S. Farid, Tetrahedron, 1982, 38, 1203 CrossRef CAS
.
- P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed
.
- B. List, Acc. Chem. Res., 2004, 37, 548 CrossRef CAS PubMed
.
- T. J. Dickerson and K. D. Janda, J. Am. Chem. Soc., 2002, 124, 3220 CrossRef CAS PubMed
.
- A. Córdova, W. Notz and C. F. Barbas III, Chem. Commun., 2002, 3024–3025 RSC
.
- H. Torii, M. Nakadai, K. Ishihara, S. Saito and H. Yamamoto, Angew. Chem., Int. Ed., 2004, 43, 1983 CrossRef CAS PubMed
.
- T. Hamada, K. Manabe and S. Kobayashi, J. Am. Chem. Soc., 2004, 126, 7768 CrossRef CAS PubMed
.
- S. Luo, X. Mi, S. Liu and J.-P. Cheng, Chem. Commun., 2006, 3687 RSC
.
- N. Mase, Y. Nakai, N. Ohara, H. Yoda, K. Takabe, F. Tanaka and C. F. Barbas III, J. Am. Chem. Soc., 2006, 128, 734 CrossRef CAS PubMed
.
- L. Cheng, X. Wu and Y. Lu, Org. Biomol. Chem., 2007, 5, 1018 CAS
.
- P. Wu, Y. Wan and J. Cai, Synlett, 2008, 1193–1198 CAS
.
- D. S. Gaikwad and D. M. Pore, Synlett, 2012, 23, 2631 CrossRef CAS
.
- T. Inokuchi and H. Kawafuchi, J. Org. Chem., 2006, 71, 947 CrossRef CAS PubMed
.
- M. Lakshmi Kantam, B. M. Choudary, C. Venkat Reddy, K. Koteswara Rao and F. Figueras, Chem. Commun., 1998, 1033 RSC
.
- G. Brahmachari and B. Banerjee, ACS Sustainable Chem. Eng., 2014, 2, 2802 CrossRef CAS
.
- A. Natarajan, Y. H. Fan, H. Chen, Y. Guo, J. Iyasere, F. Harbinski, W. J. Christ, H. Aktas and J. A. Halperin, J. Med. Chem., 2004, 47, 1882 CrossRef CAS PubMed
.
- R. T. Bal, B. Anand, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2005, 15, 4451 CrossRef PubMed
.
- R. Tripathy, A. Reiboldt, P. A. Messina, M. Iqbal, J. Singh, E. R. Bacon, T. S. Angeles, S. X. Yang, M. S. Albom, C. Robinson, H. Chang, B. A. Ruggeri and J. P. Mallamo, Bioorg. Med. Chem. Lett., 2006, 16, 2158 CrossRef CAS PubMed
.
- P. Paira, A. Hazra, S. Kumar, R. Paira, K. B. Sahu, S. Naskar, P. Saha, S. Mondal, A. Maity, S. Banerjee and N. B. Mondal, Bioorg. Med. Chem. Lett., 2009, 19, 4786 CrossRef CAS PubMed
.
- A. Kamal, Y. V. V. Srikanth, M. N. A. Khan, T. B. Shaik and M. Ashraf, Bioorg. Med. Chem. Lett., 2010, 20, 5229 CrossRef CAS PubMed
.
- B. V. S. Reddy, N. Rajeswari, M. Sarangapani, Y. Prashanthi, R. J. Ganji and A. Addlagatta, Bioorg. Med. Chem. Lett., 2012, 22, 2460 CrossRef PubMed
.
- E. Lunt and C. G. Newton, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, 1984, vol. 3, p. 199 Search PubMed
.
- T. K. Bradshaw and D. W. Hutchinson, Chem. Soc. Rev., 1977, 6, 43 RSC
.
- M. S. Barnette, Prog. Drug Res., 1999, 53, 193 CAS
.
- R. Gupta, G. Kumar and R. S. Kumar, Methods Find. Exp. Clin. Pharmacol., 2005, 27, 101 CrossRef CAS PubMed
.
- G. Brahmachari and B. Banerjee, RSC Adv., 2015, 5, 39263 RSC
.
- B. Banerjee and G. Brahmachari, Curr. Organocatal., 2016, 3, 125 CrossRef CAS
.
- R. N. Chopra, S. L. Nayer and I. C. Chopra, Glossary of India medicinal plants, CSIR Publications, New Delhi, 1956, p. 151 Search PubMed
.
- G. Chaudhary, S. Goyal and P. Poonia, Int. J. Pharm. Sci. Drug Res., 2010, 2, 91 Search PubMed
.
- M. Mahkam, M. Nabati and H. R. Kafshboran, Iran. Chem. Commun., 2014, 2, 34 Search PubMed
.
- M. A. Omar, J. Med. Sci., 2005, 5, 163 CrossRef
.
- L. B. Dama, B. N. Poul and B. V. Jadhav, J. Ecotoxicol. Environ. Monit., 1999, 8, 213 Search PubMed
.
- S. N. Dixit, H. S. Srivastava and R. D. Tripathi, Indian Phytopathol., 1980, 31, 131 Search PubMed
.
- D. Wang, R. Sauriasari, Y. Takemura, K. Tsutsui, N. Masuoka, K. Sano, M. Horita, B. L. Wang and K. Ogino, Toxicology, 2007, 235, 103 CrossRef PubMed
.
- S. Yogisha, D. S. Samiulla, D. Prashanth, R. Padmaja and A. Amit, Fitoterapia, 2002, 73, 690 CrossRef CAS PubMed
.
- V. R. Rao, R. A. Kumar and T. V. P. Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1993, 32, 903 Search PubMed
.
- N. Sultana, M. I. Choudhary and A. J. Khan, J. Enzyme Inhib. Med. Chem., 2009, 24, 257 CrossRef CAS PubMed
.
- S. Biradar and B. Veeresh, Indian J. Exp. Biol., 2013, 51, 256 CAS
.
- G. Brahmachari, ACS Sustainable Chem. Eng., 2015, 3, 2058 CrossRef CAS
.
- H. Hagiwara, D. Iijima, B. Z. S. Awen, T. Hoshi and T. Suzuki, Synlett, 2008, 1520–1522 CrossRef CAS
.
- Q. Wang, F. Huan, H. Shen, J.-C. Xiao, M. Gao, X. Yang, S.-I. Murahashi, Q.-Y. Chen and Y. Guo, J. Org. Chem., 2013, 78, 12525 CrossRef CAS PubMed
.
- L. D. Jennings, D. R. Rayner, D. B. Jordan, J. F. Okonya, G. S. Basarab, D. K. Amorose, B. M. Anaclerio, J. K. Lee, R. S. Schwartz and K. A. Whitmore, Bioorg. Med. Chem., 2000, 8, 897 CrossRef CAS PubMed
.
- K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed
.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC
.
- W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed
.
- J. P. Begue and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, John Wiley & Sons, New York, 2008 Search PubMed
.
- Fluorine in Medicinal Chemistry and Chemical Biology, ed. I. Ojima, John Wiley & Sons, West Sussex, 2009 Search PubMed
.
- J. Kamalraja, T. Hari Babu, D. Muralidharan and P. T. Perumal, Synlett, 2012, 23, 1950 CrossRef CAS
.
- M. Shibasaki and H. Gröger, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Berlin, Germany, 1999, vol. III, pp. 1075–1090 Search PubMed
.
- M. Shibasaki, H. Gröger and M. Kanai, in Comprehensive Asymmetric Catalysis, Supplement 1, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, Heidelberg, Germany, 2004, pp. 131–133 Search PubMed
.
- C. Palomo, M. Oiarbide and A. Laso, Eur. J. Org. Chem., 2007, 2561 CrossRef CAS
.
- H. Sasai, T. Suzuki, S. Arai, T. Arai and M. Shibasaki, J. Am. Chem. Soc., 1992, 114, 4418 CrossRef CAS
.
- C. Christensen, C. Juhl and K. A. Jørgensen, Chem. Commun., 2001, 2222 RSC
.
- B. Trost and V. S. C. Yeh, Angew. Chem., Int. Ed., 2002, 41, 861 CrossRef CAS
.
- D. A. Evans, D. Seidel, M. Rueping, H. W. Lam, J. T. Shaw and C. W. Downey, J. Am. Chem. Soc., 2003, 125, 12692 CrossRef CAS PubMed
.
- Y. Kogami, T. Nakajima, T. Ashizawa, S. Kezuka, T. Ikeno and T. Yamada, Chem. Lett., 2004, 33, 614 CrossRef CAS
.
- C. Palomo, M. Oiarbide and A. Laso, Angew. Chem., Int. Ed., 2005, 44, 3881 CrossRef CAS PubMed
.
- B. M. Choudary, K. V. S. Ranganath, U. Pal, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2005, 127, 13167 CrossRef CAS PubMed
.
- T. Marcelli, R. N. S. van der Haas, J. H. van Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2006, 45, 929 CrossRef CAS PubMed
.
- T. Mandal, S. Samanta and C.-G. Zhao, Org. Lett., 2007, 9, 943 CrossRef CAS PubMed
.
- Y. Xiong, F. Wang, X. Huang, Y. Wen and X. Feng, Chem.–Eur. J., 2007, 13, 829 CrossRef CAS PubMed
.
- K. Ma and J. You, Chem.–Eur. J., 2007, 13, 1863 CrossRef CAS PubMed
.
- M. Bandini, F. Piccinelli, S. Tommasi, A. Umani-Ronchi and C. Ventrici, Chem. Commun., 2007, 616 RSC
.
- M. Bandini, M. Benaglia, R. Sinisi, S. Tommasi and A. Umani-Ronchi, Org. Lett., 2007, 9, 2151 CrossRef CAS PubMed
.
- T. Arai, M. Watanabe and A. Yanagisawa, Org. Lett., 2007, 9, 3595 CrossRef CAS PubMed
.
- A. S. Hirwe, R. L. Metcalf and I. P. Kapoor, J. Agric. Food Chem., 1972, 20, 818 CrossRef CAS PubMed
.
- M. S. Singer, US Pat., 3,796,561A, 1974Chem. Abstr., 1974, 81, 10486
.
- M. Takamatsu and M. Sekiya, Chem. Pharm. Bull., 1980, 28, 3098 CrossRef CAS
.
- L. F. Lee, M. G. Dolson, R. K. Howe and B. R. Stults, J. Org. Chem., 1985, 50, 3216 CrossRef CAS
.
- J. M. Cox, J. M. Clough, N. J. Barnes, D. P. J. Pearson, I. R. Matthews, S. K. Vohra, S. C. Smith, G. Mitchell and R. A. Barber, PCT Int. Appl. WO 9,533,719A1, 1995Chem. Abstr., 1996, 124, 261022
.
- P. Riederer, P. Foley, G. Bringmann, D. Feineis, R. Bruckner and G. Manfred, Eur. J. Pharmacol., 2002, 442, 1 CrossRef CAS PubMed
.
- E. Y. Shinkevich, M. S. Novikov and A. F. Khlebnikov, Synthesis, 2007, 225 CAS
.
- K. Abbaspour Tehrani, S. Stas, B. Lucas and N. De Kimpe, Tetrahedron, 2009, 65, 1957 CrossRef CAS
.
- B. Wahl, A. Cabré, S. Woodward and W. Lewis, Tetrahedron Lett., 2014, 55, 5829 CrossRef CAS
.
- M. Kokubo and S. Kobayashi, Synlett, 2008, 1562 CAS
.
- X. Zhang, Synlett, 2008, 65 CrossRef
.
- J. Rohr and R. Thiericke, Nat. Prod. Rep., 1992, 9, 103 RSC
.
- D.-S. Hsu and J.-Y. Huang, J. Org. Chem., 2012, 77, 2659 CrossRef CAS PubMed
.
- T. Matsumoto, T. Sohma, H. Yamaguchi, S. Kurata and K. Suzuki, Tetrahedron, 1995, 51, 7347 CrossRef CAS
.
- T. Henkel, J. Rohr, J. M. Beale and L. Schwenen, J. Antibiot., 1990, 43, 492 CrossRef CAS PubMed
.
- C. Challa, J. Vellekkatt, J. Ravindran and R. S. Lankalapalli, Org. Biomol. Chem., 2014, 12, 8588 CAS
.
- X. Han, H. Li, R. P. Hughes and J. Wu, Angew. Chem., Int. Ed., 2012, 51, 10390 CrossRef CAS PubMed
.
- B. Cacciuttolo, P. Ondet, S. Poulain-Martini, G. Lemière and E. Duñach, Org. Chem. Front., 2014, 1, 765 RSC
.
- R. Sanz, D. Miguel, J. M. Alvarez-Gutierrez and F. Rodriguez, Synlett, 2008, 975 CrossRef CAS
.
- X. Xu, R. Jiang, X. Zhou, Y. Liu, S. Ji and Y. Zhang, Tetrahedron, 2009, 65, 877 CrossRef CAS
.
- X. Zhang, X. Yu, X. Feng and M. Bao, Synlett, 2012, 23, 1605 CrossRef CAS
.
- A. Malinowska, I. Czeluśniak, M. Górski and T. A. Szymańska-Buzar, J. Mol. Catal. A: Chem., 2005, 226, 259 CrossRef CAS
.
- A. C. Veronese, E. Durini, V. Bertolasi, M. Basato and C. Tubaro, Tetrahedron, 2010, 66, 8313 CrossRef CAS
.
- S. J. Gould, Chem. Rev., 1997, 97, 2499 CrossRef CAS PubMed
.
- S. Fotso, R. P. Maskey, I. Grün-Wollny, K.-P. Schulz, M. Munk and H. Laatsch, J. Antibiot., 2003, 56, 931 CrossRef CAS PubMed
.
- J. Marco-Contelles and M. T. Molina, Curr. Org. Chem., 2003, 7, 1433 CrossRef CAS
.
- R. S. Coleman, F.-X. Felpin and W. Chen, J. Org. Chem., 2004, 69, 7309 CrossRef CAS PubMed
.
- Z. Nikolovska-Coleska, L. Xu, Z. Hu, Y. Tomita, P. Li, P. P. Roller, R. Wang, X. Fang, R. Guo, M. Zhang, M. E. Lippman, D. Yang and S. Wang, J. Med. Chem., 2004, 47, 2430 CrossRef CAS PubMed
.
- J. Koyama, Recent Pat. Anti-Infect. Drug Discovery, 2006, 1, 113 CrossRef CAS
.
- G. Viault, D. Grée, S. Das, J. S. Yadav and R. Grée, Eur. J. Org. Chem., 2011, 1233 CrossRef CAS
.
- A. Ortega, Á. Rincón, K. L. Jiménez-Aliaga, P. Bermejo-Bescós, S. Martín-Aragón, M. T. Molina and A. G. Csákÿ, Bioorg. Med. Chem. Lett., 2011, 21, 2183 CrossRef CAS PubMed
.
- I. Abraham, R. Joshi, P. Pardasani and R. T. Pardasani, J. Braz. Chem. Soc., 2011, 22, 385 CrossRef CAS
.
- M. Lamblin, G. Naturale, J. Dessolin and F.-X. Felpin, Synlett, 2012, 23, 1621 CrossRef CAS
.
- R. Wang and J. R. Falck, Org. Chem. Front., 2014, 1, 1029 RSC
.
- M. Guo, F. Jian and R. He, J. Fluorine Chem., 2006, 127, 177 CrossRef CAS
.
- T. Kawano, N. Matsuyama, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2010, 75, 1764 CrossRef CAS PubMed
.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef
.
- L. Cassar, J. Organomet. Chem., 1975, 93, 253 CrossRef CAS
.
- H. A. Dieck and R. F. Heck, J. Organomet. Chem., 1975, 93, 259 CrossRef CAS
.
- K. Sonogashira, in Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon, New York, 1991, vol. 3, ch. 2.4 Search PubMed
.
- R. Rossi, A. Carpita and F. Bellina, Org. Prep. Proced. Int., 1995, 27, 127 CrossRef CAS
.
- K. Sonogashira, in Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, New York, 1998, ch. 5 Search PubMed
.
- L. Brandsma, S. F. Vasilevsky and H. D. Verkruijsse, Application of Transition Metal Catalysts in Organic Synthesis, Springer-Verlag, Berlin, 1998, ch. 10 Search PubMed
.
- T. Hundertmark, A. F. Littke, S. L. Buchwald and G. C. Fu, Org. Lett., 2000, 2, 1729 CrossRef CAS PubMed
.
- A. D. Finke, E. C. Elleby, M. J. Boyd, H. Weissman and J. S. Moore, J. Org. Chem., 2009, 74, 8897 CrossRef CAS PubMed
.
- A. Soheili, J. Albaneze-Walker, J. A. Murry, P. G. Dormer and D. L. Hughes, Org. Lett., 2003, 5, 4191 CrossRef CAS PubMed
.
- B. Liang, M. Dai, J. Chen and Z. Yang, J. Org. Chem., 2005, 70, 391 CrossRef CAS PubMed
.
- B. Dietrich, P. Viout and J. M. Lehn, Macrocyclic Chemistry, Wiley-VCH, Weinheim, 1993 Search PubMed
.
- S. Ma and E. Negishi, J. Am. Chem. Soc., 1995, 117, 6345 CrossRef CAS
.
- J. Breitenbach, J. Boosfeld and F. Vögtle, in Comprehensive Supramolecular Chemistry, ed. J. M. Lehn, Pergamon, New York, 1996, vol. 2, pp. 29–67 Search PubMed
.
- A. Klapars, S. Parris, K. W. Anderson and S. L. Buchwald, J. Am. Chem. Soc., 2004, 126, 3529 CrossRef CAS PubMed
.
- D. Hildebrandt, W. Hüggenberg, M. Kanthak, T. Plöger, M. Müller and G. Dyker, Chem. Commun., 2006, 2260 RSC
.
- S. O'Keeffe, F. Harrington and A. R. Maguire, Synlett, 2007, 2367–2370 Search PubMed
.
- X. Huang, C. Su, Q. Liu and Y. Song, Synlett, 2008, 229 CrossRef CAS
.
- A. L. Garay, A. Pichona and S. L. James, Chem. Soc. Rev., 2007, 36, 846 RSC
.
- B. Rodríguez, A. Bruckmann, T. Rantanen and C. Bolm, Adv. Synth. Catal., 2007, 349, 2213 CrossRef
.
- V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629 CrossRef CAS PubMed
.
- M. Gupta, S. Paul and R. Gupta, Acta Chim. Slov., 2009, 56, 749 CAS
.
- L. Basolo, A. Bernasconi, E. Borsini, G. Broggini and E. M. Beccalli, ChemSusChem, 2011, 4, 1637 CrossRef CAS PubMed
.
- A. Stolle and B. Ondruschka, Pure Appl. Chem., 2011, 83, 1343 CrossRef CAS
.
- H. M. Marvaniya, K. N. Modi and D. J. Sen, Int. J. Drug Dev. Res., 2011, 3, 34 CAS
.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC
.
- T. Friscic, Chem. Soc. Rev., 2012, 41, 3493 RSC
.
- A. Bruckmann, A. Krebs and C. Bolm, Green Chem., 2008, 10, 1131 RSC
.
- F. Schneider, T. Szuppa, A. Stolle, B. Ondruschka and H. Hopf, Green Chem., 2009, 11, 1894 RSC
.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317 RSC
.
- T. Friščić, I. Halasz, V. Štrukil, M. Eckert-Maksić and R. E. Dinnebier, Croat. Chem. Acta, 2012, 85, 367 CrossRef
.
- Ball Milling Towards Green Synthesis: Applications, Projects and Challenges, ed. B. C. Ranu and A. Stolle, RSC Publishing, London, 2014 Search PubMed
.
- R. Ballini, D. Fiorini, M. V. Gil and A. Palmieri, Tetrahedron, 2004, 60, 2799 CrossRef CAS
.
- M. H. Sarvari and H. Sharghi, J. Org. Chem., 2004, 69, 6953 CrossRef CAS PubMed
.
- M. Lakshmi Kantam, R. Chakravarti, B. Sreedhar and S. Bhargava, Synlett, 2008, 1449 CrossRef
.
- B. H. Lipshutz, Chem. Rev., 1986, 86, 795 CrossRef CAS
.
- J. E. Saxton, Nat. Prod. Rep., 1989, 6, 1 RSC
.
- R. A. Glennon, J. Med. Chem., 1997, 40, 1 CrossRef PubMed
.
- H.-C. Zhang, H. Ye, A. F. Moretto, K. K. Brumfield and B. E. Maryanoff, Org. Lett., 2000, 2, 89 CrossRef CAS PubMed
.
- M. E. Jung and F. Slowinski, Tetrahedron Lett., 2001, 42, 6835 CrossRef CAS
.
- T. Walsh, R. B. Toupence, F. Ujjainwala, J. R. Young and M. T. Goulet, Tetrahedron, 2001, 57, 5233 CrossRef CAS
.
- M. G. N. Russell, R. J. Baker, L. Barden, M. S. Beer, L. Bristow, H. B. Howard, B. Broughton, M. Knowles, G. McAllister, S. Patel and J. L. Castro, J. Med. Chem., 2001, 44, 3881 CrossRef CAS PubMed
.
- Y. Zhang, J. Jiang, X.-Q. Chu, R. Jiang, X.-P. Xu, D.-H. Li and S.-J. Ji, Synlett, 2012, 23, 751 CrossRef CAS
.
- D. Romo, S. D. Meyer, D. D. Johnson and S. L. Schreiber, J. Am. Chem. Soc., 1993, 115, 9345 CrossRef
.
- K. C. Nicolaou, D. W. Kim and R. Baati, Angew. Chem., Int. Ed., 2002, 41, 3701 CrossRef CAS
.
- W. Zhou, W. Yan, J.-X. Wang and K. Wang, Synlett, 2008, 137 CAS
.
- J. S. Yadav, D. C. Bhunia, V. K. Singh and P. Srihari, Tetrahedron Lett., 2009, 50, 2470 CrossRef CAS
.
- A. Palmieri, S. Gabrielli and R. Ballini, Synlett, 2013, 24, 114 CAS
.
- N. Singh and K. N. Singh, Synlett, 2012, 23, 2116 CrossRef CAS
.
- A. D. Patil, A. J. Freyer, D. S. Eggleston, R. C. Haltiwanger, M. F. Bean, P. B. Taylor, M. J. Caranfa, A. L. Breen, H. R. Bartus, R. K. Johnson, R. P. Hertzberg and J. W. Westley, J. Med. Chem., 1993, 36, 4131 CrossRef CAS PubMed
.
- A.-u. Rahman and A. Basha, Indole Alkaloids, Harwood Academic Publishers, Chichester, UK, 1998 Search PubMed
.
- S. Hibino and T. Chozi, Nat. Prod. Rep., 2001, 18, 66 RSC
.
- M. P. Lézé, M. L. Borgne, P. Marchand, D. Loquet, M. Kogler, G. L. Baut, A. Palusczak and R. W. Hartmann, J. Enzyme Inhib. Med. Chem., 2004, 19, 549 CrossRef PubMed
.
- M. Somai and F. Yamada, Nat. Prod. Rep., 2005, 22, 73 RSC
.
- H. C. Zhang, L. V. R. Bonaga, H. Ye, C. K. Derian, B. P. Damiano and B. E. Maryanoff, Bioorg. Med. Chem. Lett., 2007, 17, 2863 CrossRef CAS PubMed
.
- G. Brahmachari and S. Das, RSC Adv., 2014, 4, 7380 RSC
.
- L.-T. An, F.-Q. Ding, J.-P. Zou, X.-H. Lu and L.-L. Zhang, Chin. J. Chem., 2007, 25, 822 CrossRef CAS
.
- A. Kumar, M. K. Gupta and M. Kumar, Green Chem., 2012, 24, 290 RSC
.
- S. A. R. Mulla, T. A. Salama, M. Y. Pathan, S. M. Inamdar and S. S. Chavan, Tetrahedron Lett., 2013, 54, 672 CrossRef CAS
.
- S. W. Kshirsagar, N. R. Patil and S. D. Samant, Tetrahedron Lett., 2010, 51, 2924 CrossRef CAS
.
- Y. Liu, Z. Ren, W. Cao, J. Chen, H. Deng and M. Shao, Synth. Commun., 2011, 41, 3620 CrossRef CAS
.
- M. J. Kornet and A. P. Thio, J. Med. Chem., 1976, 19, 892 CrossRef CAS PubMed
.
- C. B. Cui, H. Kakeya and H. Osada, Tetrahedron, 1996, 52, 12651 CrossRef CAS
.
- H. B. Rasmussen and J. K. Macleod, J. Nat. Prod., 1997, 60, 1152 CrossRef CAS
.
- R. M. Williams and R. J. Cox, Acc. Chem. Res., 2003, 36, 127 CrossRef CAS PubMed
.
- T. J. Mason, Chemistry with Ultrasound, Elsevier, London, 1990 Search PubMed
.
- J. P. Lorimer and T. J. Mason, Chem. Soc. Rev., 1987, 16, 239 RSC
.
- S. V. Le and C. M. R. Low, Ultrasound in Synthesis, Springer-Verlag, Berlin, 1989 Search PubMed
.
- T. J. Mason, Chem. Soc. Rev., 1997, 26, 443 RSC
.
- J. L. Luche, Synthetic Organic Sonochemistry, Plenum Press, NewYork, 1998 Search PubMed
.
- Advances in Sonochemistry, ed. T. J. Mason, JAI Press, London and Greenwich, CT, 1990, vol. 1 Search PubMed
.
- M. Sillanpää, T.-D. Pham and R. A. Shrestha, Ultrasound Technology in Green Chemistry, Springer Science + Business Media, 2011 Search PubMed
.
- S. Puri, B. Kaur, A. Parmar and H. Kumar, Curr. Org. Chem., 2013, 17, 1790 CrossRef CAS
.
- H. Bremner, Ultrason. Sonochem., 1994, 1, S119 CrossRef
.
- V. V. Boldyrev, Ultrason. Sonochem., 1995, 2, S143 CrossRef CAS
.
- R. R. Deshmukh, R. Rajagopal and K. V. Srinivasan, Chem. Commun., 2001, 1544 RSC
.
- G. J. Price, Ultrason. Sonochem., 2003, 10, 277 CrossRef CAS PubMed
.
- F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2005, 61, 11771 CrossRef CAS
.
- G. Cravotto, G. Palmisano, S. Tollari, G. M. Nano and A. Penoni, Ultrason. Sonochem., 2005, 12, 91 CrossRef CAS PubMed
.
- R. Cella and H. A. Stefani, Tetrahedron, 2006, 62, 5656 CrossRef CAS
.
- S. Tangestaninejad, M. Moghadam, V. Mirkhani and H. Kargar, Ultrason. Sonochem., 2006, 13, 32 CrossRef CAS PubMed
.
- A. C. Silva, A. L. F. de Souza and O. A. C. Antunes, J. Organomet. Chem., 2007, 692, 3104 CrossRef CAS
.
- A. K. Sinha, V. Kumar, A. Sharma and R. Kumar, Tetrahedron, 2007, 63, 11070 CrossRef CAS
.
- M. M. Mojtahedi, M. Javadpour and M. S. Abaee, Ultrason. Sonochem., 2008, 15, 828 CrossRef CAS PubMed
.
- H. P. Hemantha, G. Chennakrishnareddy, T. M. Vishwanatha and V. V. Sureshbabu, Synlett, 2009, 407 CAS
.
- B. Vasantha, H. P. Hemantha and V. V. Sureshbabu, Synthesis, 2010, 2990 CAS
.
- Q. Liu, H. Ai and Z. Li, Ultrason. Sonochem., 2011, 18, 477 CrossRef CAS PubMed
.
- R. G. Soengas and A. M. S. Silva, Synlett, 2012, 23, 873 CrossRef CAS
.
- E. Ruiz, H. Rodriguez, J. Coro, V. Niebla, A. Rodriguez, R. Martinez-Alvarez, H. N. de Armas, M. Suarez and N. Martin, Ultrason. Sonochem., 2012, 19, 221 CrossRef CAS PubMed
.
- N. G. Khaligh, Ultrason. Sonochem., 2013, 20, 1062 CrossRef CAS PubMed
.
- J. Safari and L. Javadian, Ultrason. Sonochem., 2015, 22, 341 CrossRef CAS PubMed
.
- T. J. Mason and J. P. Lorimer, Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing, Wiley-VCH, Germany, 2002 Search PubMed
.
- T. J. Mason and D. Peters, Practical Sonochemistry: Power Ultrasound Uses and Applications, Woodhead Publishing, Cambridge, 2nd edn, 2003 Search PubMed
.
- M. A. Margulis, High Energy Chem., 2004, 38, 135 CrossRef CAS
.
- G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180 RSC
.
- G. Cravotto and P. Cintas, Angew. Chem., Int. Ed., 2007, 46, 5476 CrossRef CAS PubMed
.
- T. J. Mason, Ultrason. Sonochem., 2007, 14, 476 CrossRef CAS PubMed
.
- J. H. Bang and K. S. Suslick, Adv. Mater., 2010, 22, 1039 CrossRef CAS PubMed
.
- P. Cintas, G. Palmisano and G. Cravotto, Ultrason. Sonochem., 2011, 18, 836 CrossRef CAS PubMed
.
- M. Ashokkumar, Ultrason. Sonochem., 2011, 18, 864 CrossRef CAS PubMed
.
- F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047 CrossRef CAS
.
- R. Cella and H. A. Stefani, Tetrahedron, 2009, 65, 2619 CrossRef CAS
.
- R. Patil, P. Bhoir, P. Deshpande, T. Wattamwar, M. Shirude and P. Chaskar, Ultrason. Sonochem., 2013, 20, 1327 CrossRef CAS PubMed
.
- M. A. Schiel, A. B. Chopa, G. F. Silbestri, M. B. Alvarez, A. G. Lista and C. E. Domini, Use of Ultrasound in the Synthesis of Heterocycles of Medicinal Interest, in Green Synthetic Approaches for Biologically Relevant Heterocycles, ed. G. Brahmachari, Elsevier, Amsterdam, The Netherland, 2014, ch. 21, pp. 571–601 Search PubMed
.
- Y. Yamamoto and N. Asao, Chem. Rev., 1993, 93, 2207 CrossRef CAS
.
- S. E. Denmark and J. Fu, Chem. Rev., 2003, 103, 2763 CrossRef CAS PubMed
.
- M. Yus, J. C. González-Gómez and F. Foubelo, Chem. Rev., 2011, 111, 7774 CrossRef CAS PubMed
.
- J. J. R. Freitas, T. R. Couto, I. H. Cavalcanti, J. C. R. Freitas, R. A. Oliveira and P. H. Menezes, Ultrason. Sonochem., 2014, 21, 1609 CrossRef CAS PubMed
.
- S. Narayanaperumal, R. C. da Silva, K. S. Feu, A. F. de la Torre, A. G. Corrêa and M. W. Paixão, Ultrason. Sonochem., 2013, 20, 793 CrossRef CAS PubMed
.
- K. Saïd, Y. Moussaoui, M. Kammoun and R. B. Salem, Ultrason. Sonochem., 2011, 18, 23 CrossRef PubMed
.
- J.-T. Li, M.-X. Sun, G.-Y. He and X.-Y. Xu, Ultrason. Sonochem., 2011, 18, 412 CrossRef CAS PubMed
.
- J. P. Poupelin, G. Saint-Rut, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67 CAS
.
- G. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683 CrossRef PubMed
.
- M. Ahmad, T. A. King, D.-K. Ko, B. H. Cha and J. Lee, J. Phys. D: Appl. Phys., 2002, 35, 1473 CrossRef CAS
.
- B. Banerjee and G. Brahmachari, J. Chem. Res., 2014, 38, 745 CrossRef CAS
.
- N. G. Khaligh and F. Shirini, Ultrason. Sonochem., 2015, 22, 397 CrossRef CAS PubMed
.
- K. Venkatesan, S. S. Pujari, R. J. Lahoti and K. V. Srinivasan, Ultrason. Sonochem., 2008, 15, 548 CrossRef CAS PubMed
.
- B. Datta and M. A. Pasha, Ultrason. Sonochem., 2012, 19, 725 CrossRef CAS PubMed
.
- A. Ziarati, J. Safaei-Ghomi and S. Rohani, Ultrason. Sonochem., 2013, 20, 1069 CrossRef CAS PubMed
.
- G. Ciamician, Science, 1912, 36, 385 CAS
.
- N. Hoffmann, Chem. Rev., 2008, 108, 1052 CrossRef CAS PubMed
.
- K. Kalyanasundaram, Coord. Chem. Rev., 1982, 46, 159 CrossRef CAS
.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85 CrossRef CAS
.
- D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97 RSC
.
- T. J. Meyer, Acc. Chem. Res., 1989, 22, 163 CrossRef CAS
.
- B. Happ, A. Winter, M. D. Hager and U. S. Schubert, Chem. Soc. Rev., 2012, 41, 2222 RSC
.
- N. Serpone and E. Pelizzetti, Photocatalysis: fundamentals and applications, Wiley, New York, 1989 Search PubMed
.
- A. Albini and M. Fagnoni, ChemSusChem, 2008, 1, 63 CrossRef CAS PubMed
.
- K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785 CrossRef CAS PubMed
.
- T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed
.
- J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC
.
- F. Teplý, Collect. Czech. Chem. Commun., 2011, 76, 859 CrossRef
.
- J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed
.
- J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed
.
- L. Shi and W.-J. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC
.
- S. Maity and N. Zheng, Synlett, 2012, 23, 1851 CrossRef CAS PubMed
.
- M. A. Ischay and T. P. Yoon, Eur. J. Org. Chem., 2012, 3359 CrossRef CAS
.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed
.
- J. Xuan, L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Eur. J. Org. Chem., 2013, 6755 CrossRef CAS
.
- Y. Xi, H. Yi and A. Lei, Org. Biomol. Chem., 2013, 11, 2387 CAS
.
- D. P. Hari and B. König, Angew. Chem., Int. Ed., 2013, 52, 4734 CrossRef CAS PubMed
.
- H. Yi, X. Zhang, C. Qin, Z. Liao, J. Liu and A. Lei, Adv. Synth. Catal., 2014, 356, 2873 CrossRef CAS
.
- D. L. Boger, C. W. Boyce, R. M. Garbaccio and J. A. Goldberg, Chem. Rev., 1997, 97, 787 CrossRef CAS PubMed
.
- P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity Applications, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed
.
- S. M. Weinreb, Chem. Rev., 2006, 106, 2531 CrossRef CAS PubMed
.
- K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013 CrossRef CAS
.
- R. M. Wilson and S. J. Danishefsky, Angew. Chem., Int. Ed., 2010, 49, 6032 CrossRef CAS PubMed
.
- P. V. Ramachandran, Asymmetric fluoroorganic chemistry, ACS Symposium Series, American Chemical Society, Washington, DC, 2000, vol. 746 Search PubMed
.
- J. W. Corbett, S. S. Ko, J. D. Rodgers, L. A. Gearhart, N. A. Magnus, L. T. Bacheler, S. Diamond, S. Jeffrey, R. M. Klabe, B. C. Cordova, S. Garber, K. Logue, G. L. Trainor, P. S. Anderson and S. K. Erickson-Viitanen, J. Med. Chem., 2000, 43, 2019 CrossRef CAS PubMed
.
- S. Caron, N. M. Do, J. E. Sieser, P. Arpin and E. Vazquez, Org. Process Res. Dev., 2007, 11, 1015 CrossRef CAS
.
- V. A. Petrov, Fluorinated Heterocyclic Compounds: Synthesis Chemistry and Applications, DuPont Central Research and Development, Wilmington, 2009 Search PubMed
.
- A. A. Gakh and K. L. Kirk, Fluorinated heterocycles, ACS Symposium Series, American Chemical Society, Washington, DC, 2009, vol. 1003 Search PubMed
.
- G. Chaume, N. Lensen, C. CaupAne and T. Brigaud, Eur. J. Org. Chem., 2009, 5717 CrossRef CAS
.
- Y.-L. Liu, J.-L. Chen, G.-H. Wang, P. Sun, H.-Y. Huang and F.-L. Qing, Tetrahedron Lett., 2013, 54, 5541 CrossRef CAS
.
- T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed
.
- O. A. Tomashenko and V. V. Grushin, Aromatic Trifluoromethylation with Metal Complexes, Chem. Rev., 2011, 111, 4475–4521 CrossRef CAS PubMed
.
- J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed
.
- D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef CAS PubMed
.
- T. Besset, C. Schneider and D. Cahard, Angew. Chem., Int. Ed., 2012, 51, 5048 CrossRef CAS PubMed
.
- A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950 CrossRef CAS PubMed
.
- X.-G. Zhang, H.-X. Dai, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 11948 CrossRef CAS PubMed
.
- X.-F. Wu, H. Neumann and M. Beller, Chem.–Asian J., 2012, 7, 1744 CrossRef CAS PubMed
.
- L. Zhang, K. Chen, G. Chen, B. Li, S. Luo, Q. Guo, J. Wei and Z. Shi, Org. Lett., 2013, 15, 10 CrossRef CAS PubMed
.
- H. Egami, R. Shimizu, S. Kawamura and M. Sodeoka, Angew. Chem., Int. Ed., 2013, 52, 4000 CrossRef CAS PubMed
.
- W. Kong, M. Casimiro, N. Fuentes, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 13086 CrossRef CAS PubMed
.
- H. Liu, Z. Gu and X. Jiang, Adv. Synth. Catal., 2013, 355, 617 CrossRef CAS
.
- T. Koike and M. Akita, J. Fluorine Chem., 2014, 167, 30 CrossRef CAS
.
- E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC
.
- H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed
.
- C. Zhang, Org. Biomol. Chem., 2014, 12, 6580 CAS
.
- L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS PubMed
.
- X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed
.
- X. H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed
.
- J. Charpentier, N. Fruh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed
.
- C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed
.
- X.-Y. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed
.
- S. B. Woo and D. Y. Kim, J. Fluorine Chem., 2015, 178, 214 CrossRef CAS
.
- L. Zheng, C. Yang, Z. Z. Xu, F. Gao and W. Xia, J. Org. Chem., 2015, 80, 5730 CrossRef CAS PubMed
.
- Q.-H. Deng, J.-R. Chen, Q. Wei, Q.-Q. Zhao, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2015, 51, 3537 RSC
.
- S. H. Oh, Y. R. Malpani, N. Ha, Y.-S. Jung and S. B. Han, Org. Lett., 2014, 16, 1310 CrossRef CAS PubMed
.
- L. Li, M. Huang, C. Liu, J.-C. Xiao, Q.-Y. Chen, Y. Guo and Z.-G. Zhao, Org. Lett., 2015, 17, 4714 CrossRef CAS PubMed
.
- G. Pratsch and L. E. Overman, J. Org. Chem., 2015, 80, 11388 CrossRef CAS PubMed
.
- W. Adam, C. R. Saha-Möller and P. A. Ganeshpure, Chem. Rev., 2001, 101, 3499 CrossRef CAS PubMed
.
- Q. Xia, H. Ge, C. Ye, Z. Liu and K. Su, Chem. Rev., 2005, 105, 1603 CrossRef CAS PubMed
.
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072 CrossRef CAS PubMed
.
- J. Li and D. Z. Wang, Org. Lett., 2015, 17, 5260 CrossRef CAS PubMed
.
- A. Noble, S. J. McCarver and D. W. C. MacMillan, J. Am. Chem. Soc., 2015, 137, 624 CrossRef CAS PubMed
.
- D. N. Primer, I. Karakaya, J. C. Tellis and G. A. Molander, J. Am. Chem. Soc., 2015, 137, 2195 CrossRef CAS PubMed
.
- S. J. Kaldas, A. Cannillo, T. McCallum and L. Barriault, Org. Lett., 2015, 17, 2864 CrossRef CAS PubMed
.
Footnote |
| † This article is dedicated to Professor Srinivasan Chandrasekaran on the occasion of his 70th birthday |
| This journal is © The Royal Society of Chemistry 2016 |