Study on the synthesis and properties of an eco-friendly sugar-based anionic–nonionic surfactant
Tian-Hong Zhaoa,
Jiong-Yi Gu*a,
Wan-Fen Pu*b,
Zhi-Ming Donga and
Rui Liub
aSchool of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu, People's Republic of China. E-mail: gjy105618@163.com
bSchool of Petroleum and Natural Gas Engineering, Southwest Petroleum University, Chengdu, People's Republic of China. E-mail: pwf58@163.com
First published on 11th July 2016
Abstract
An eco-friendly sugar-based anionic–nonionic surfactant (DAGA-ES) with well-established structure was successfully synthesized and characterized by electrospray ionization-time of flight mass spectrography (ESI-TOFMS), 1H nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectrometer (FT-IR). Its adsorption morphology on surface and micelle size in aqueous solution were observed using scanning electron microscope (SEM) and a static laser scattering (SLS) instrument. The surface activities of the solution in the absence or presence of electrolyte were investigated by surface tension measurements. The properties of oil displacement including the ability of reducing oil/water interfacial tension (IFT) and wetting alteration were determined by the rotating drop and contact angle method, respectively. The enhanced oil recovery (EOR) experiment was used to evaluate displacement efficiency. The results indicated that the net-structure could be observed on the surface and the micellar diameter in aqueous solution is 197.2 nm. The fundamental parameters such as critical micelle concentration (CMC), the surface tension at the CMC (γCMC), the hydrophile–lipophile balance (HLB) values, maximum surface excess (Γmax) and minimum surface area (Amin) were obtained via surface tension measurement. In addition, the consequence of the oil displacement property showed that IFT could be reduced to 10−2 mN m−1 in an electrolyte solution without any surfactant additives. Furthermore, with the DAGA-ES concentration increasing, the hydrophilicity for both of the hydrophilic surface and lipophilic surface was gradually enhanced. Wettability reversal was achieved just on the lipophilic surface. The oil displacement experiment showed that the oil recovery increased by 16.29% using DAGA-ES versus 12.82% using AES.
Introduction
Today, eco-friendly chemical materials have drawn world wide attention. Green chemistry will attract wide publicity because a portion of environmental pollution is attributed to the chemical industry. As a renewable resource, glucose has the advantages of non-toxicity, degradation, and is a cheap raw material. For these reasons, it has been widely used in many fields such as detergents, cosmetics, garments, medicinal, and petrochemical fields.1–4The pollution from chemical flooding in the oil industry is also very serious. For instance, alkali that can reduce the oil/water interfacial tension (IFT) significantly not only hurts the reservoir but empoisons the groundwater in combination flooding. If the alkali is absent, multifunctional surfactants will be expected. Surfactants used in the enhanced oil recovery (EOR) industry displaces oil by reducing oil/water IFT, forming an emulsion, and achieving wetting inversion on the surface of rock.5 In order to meet the needs of oil displacement, they should have both the properties of heat resistance and salt tolerance to adapt to the reservoir conditions. Anion groups such as salfacid groups,6 carboxylic acid groups,7 sulfate groups,8 and phosphoric acid groups9 possess the thermal stability while nonionic groups such as polyoxyethylene and polyalcohol10 have good salt-bearing abilities. Therefore, anionic–nonionic surfactants have become a hotspot of current research. Generally, epoxide (ethylene oxide (EO) or propylene oxide (PO)) is used to synthesize nonionic surfactants via ring-opening reaction with active hydrogen.11–14 However, EO or PO is harmful to nature and the development of society owing to its toxicity and explosion risk. From this standpoint, sugar groups as a kind of polyalcohol are widely performed to synthetize various types of surfactants including straight chain,4,15,16 dicephalic,17 Y-shaped,18 gemini,19,20 and polysaccharide ring.21
The sugar-based anionic–nonionic surfactants that have been studied include the following groups: (1) the synthesis of sodium methyl 2-acylamido-2-deoxy-6-O-sulfo-D-glucopyranoside surfactants followed by research on their adsorption and micelle formation.22,23 (2) Dodecyl glucopyranoside carboxylate was obtained in one step with dodecyl glucopyranoside and sodium chloroacetate as raw materials.24 (3) The alkyl D-mannopyranosiduronate surfactants were synthesized by four steps, including Fischer glycosylation, esterification, alkyl exchange reaction and alkaline hydrolysis using alginate as the starting materials. Then, the physico-chemical properties of the surfactants were determined.25
The study of surfactants based on glucose and dodecylamine, which could not meet the needs because of its poor water solubility, have been reported previously.26,27 In this paper, the synthesis and properties of an eco-friendly sugar-based anionic–nonionic surfactant (DAGA-ES) is reported. Glucose, used as nonionic building blocks, reacted with 2-chloride ethyl sulfonic acid sodium as anionic groups. In the reaction process, ethanol, methanol and water were employed as solvents that conform to the principles of green chemistry. Moreover, adsorption morphology, micelle size distribution, surface activity, the oil/water IFT, wettability, and displacement efficiency were determined and the results indicated that this kind of surfactant had significant potential in EOR processes.
Experimental details
Materials
Anhydrous glucose, methanol, ethanol, acetone, hexane, and sodium hydroxide were of analytical grade (AR), purchased from Chengdu Kelong Chemical Reagent Factory (China); n-dodecylamine was provided by Tianjin Kwangfu Fine Chemical Industry Research Institute (AR, China); and 2-chloride ethyl sulfonic acid sodium was purchased from Aladdin (AR, US). All of the supplementary materials were AR grade chemicals and used as received; alcohol ether sulfate (AES) was provided by China Commodity Chemical Industry Research Institute (98%, China); crude oil was provided by Seven East area of Xinjiang Oilfield, China and its basic properties were summarized in Table 1.| Viscosity (mPa s) | Density (g cm−3) | SARA fractions (%) | Acid value (mg KOH g−1) | ||||
|---|---|---|---|---|---|---|---|
| Saturated hydrocarbon | Aromatic hydrocarbon | Resin | Asphaltene | Paraffin | |||
| 5.13 | 0.852 | 71.05 | 8.04 | 5.9 | 0.8 | ∼5.0 | ∼0.14 |
Synthesis of N-dodecyl glucosamine (DAGA)
DAGA was synthesized according to methods in the literature.26 Anhydrous glucose (6.00 g, 33.6 mmol), dodecylamine (8.63 g, 47.7 mmol) and methanol were added to a dried 3-necked flask (250 mL) equipped with a condenser and magnetic stirring. Subsequently, the reaction was allowed to rest at 35 °C and the temperature was increased to 55 °C for 3 h only after a white solid generated. The reaction solution was cooled to room temperature and a massive cottony solid precipitated gradually. The crude product was washed with mixed a solvent of acetone and hexane (volume ratio 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and recrystallized with ethanol twice. DAGA (9.17 g) was obtained with a yield of 79.3% after drying at 40 °C.
3) and recrystallized with ethanol twice. DAGA (9.17 g) was obtained with a yield of 79.3% after drying at 40 °C.
Synthesis of sugar-based anionic–nonionic surfactant (DAGA-ES)
DAGA (3 g, 8.65 mmol) and ethanol (100 mL) were added to a dried 3-necked flask (250 mL) equipped with a condenser and magnetic stirring. The mixture was agitated until the DAGA dissolved completely in ethanol at 50 °C. The excessive 2-chloride ethyl sulfonic acid sodium (1.49 g, 10.4 mmol), diluted in distilled water (10 mL), was added to the system slowly dropwise. The reaction was allowed at 50 °C for 7 h with vigorous stirring. In this reaction process, sodium hydroxide solution was added dropwise in order to neutralize the hydrochloric acid produced by the reaction. The crude product, which was a reddish brown and syrupy liquid, was obtained after the mixture underwent vacuum distillation to remove ethanol and a small amount of water. The white and waxy solid was precipitated with 20 mL acetone. The solid was dissolved in 30 mL methanol at room temperature and the insoluble matter, including excessive 2-chloride ethyl sulfonic acid sodium and sodium chloride generated in reaction process, was filtered. The methanol solution was concentrated and extracted with 20 mL acetone. The process, including dissolution, filtration, concentration, and extraction, was repeated for three times. After the white flocculus precipitated from acetone, it was dried in vacuum and pure DAGA-ES (3.70 g) was obtained with a yield of 68.4%.Characterizations of DAGA and DAGA-ES
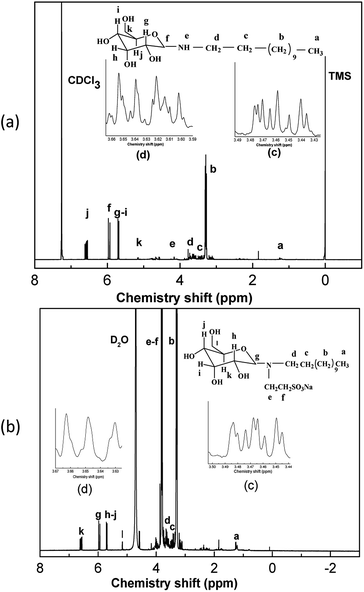
The chemical structures of DAGA and DAGA-ES were characterized by ESI-TOFMS, 1H NMR and FT-IR sequentially. TOFMS was carried out on an Agilent 6224 (Agilent, U.S.) under two modes (positive and negative) using chloroform and distilled water as solvents with a resistivity of 18.0 MΩ cm respectively. 1H NMR was recorded with a Bruker AVANCE III-400 MHz spectrometer (Brook, Switzerland) using CCl3D and D2O as solvent, respectively. FT-IR was carried out on a WQF-520 (Ruili, Beijing) using KBr as the background.Determination of properties for DAGA-ES
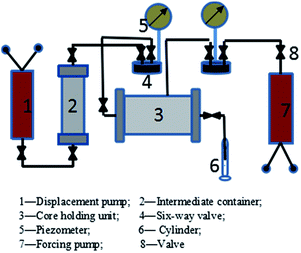
The adsorption morphology of the DAGA-ES surface was observed by the freeze etching method using SEM (Quanta 450, U.S.). A drop of DAGA-ES solution with a concentration of CMC was deposited on a rivet that was placed in an ultra-low temperature and high vacuum environment. The micelle size in an aqueous solution with a different concentration was performed on SLS (Brookhaven, U.S.) at a scattering angle of 90°. The influence of the presence, or absence, of sodium chloride in the aqueous solution on the surface tension was measured by the Wilhelmy plate method using a BZY-1 automatic Surface Tension Meter (Shanghai Equity Instruments Factory, China) at room temperature. The IFT between the solution of the surfactant and crude oil was determined using a TX500C rotating drop ultra-low oil/water interfacial tension instrument (Kenuo, U.S.) at 6000 rpm and 35 °C. The wettability on hydrophilic and lipophilic surfaces (glass sheets were soaked in distilled water and silicone oil, respectively, for about one week) in a region of concentration was evaluated by the contact angle method using a HARKE-SPCA contact angle measuring instrument (Haako, China). Contact angles with different sizes were formed when the oil drops contacted with the hydrophilic or hydrophobic surface. The oil flooding experiments were carried out at 35 °C with a constant concentration of surfactants (0.30 wt%) using the core displacement model as shown in Fig. 10. The experiments were implemented mainly following two steps. One was the preparation of the oil-saturated core; the other was flooding tests using simulated water and surfactants. With the activated water slug of DAGA-ES and AES injected and water drive going on again thereafter, the injection pressure and average oil recovery were determined. The displacement rate remained constant (0.5 mL min−1) and the injected volume of the surfactant solution equaled to the void volume.Results and discussion
Synthesis of DAGA and DAGA-ES
The synthesis process of DAGA is summarized in Fig. 1(a). As a relatively active group, aldehyde groups react with laurylamine via reductive hydrogenation and can generate glucose polyimide by losing one water molecule. Finally, glucose polyimide converts to glucose amine. It has poor solubility in water at pH ≥ 7 whereas it well dissolves in water at pH < 7.The synthesis of DAGA-ES is summarized in Fig. 1(b). DAGA reacts with 2-chloride ethyl sulfonic acid sodium by SN2 substitution. DAGA-ES can be obtained after the hydrogen chloride generated in the reaction process is neutralized by sodium hydroxide. The obtained products dissolve in water and methanol while they precipitate in such solvents as ethanol, acetone and ether.
Characterization results
Based on several references,30,31 and compared with the spectrum of the DAGA, DAGA-ES shows that the peaks for –SO3− shifts to 1180 cm−1, 1066 cm−1, 609 cm−1 and 543 cm−1 and C–N shifts to 1320 cm−1. This proves that the sulfonic group has been introduced to DAGA successfully.
After integrating the three characterization methods (ESI-MS, 1H-NMR and FT-IR), the chemical structures of DAGA and DAGA-ES can be confirmed.
Adsorption morphology on the surface and micelle size in aqueous solutions
The SEM results under different magnification were obtained. A net-structure can be observed clearly from Fig. 5. The synthetic compounds containing two hydrophilic groups (numerous hydroxyl and amino groups) arrange neither closely nor flatly instead of with heeling and winding on interface although in saturated adsorption state.32 Furthermore, intermolecular and intramolecular hydrogen bonds will be formed because of the presence of hydroxyl and amino groups. The synergism between the above two factors are attributed to the formation of the net-structure. | ||
| Fig. 5 The SEM of surface adsorption morphology: (a) magnification is 300; (b) magnification is 5000. | ||
SLS was used to determine the micelle size distribution for DAGA-ES. The relationship between intensity and diameter calculated by the NNLS programmed algorithm is shown in Fig. 6. Where, d is the diameter and c(d) is the cumulative distribution of diameter. There are two peaks: peak 1 could correspond to a single molecule and peak 2 is likely to be a spherical micelle.11 It is observed that peak 1 is extremely weak in Fig. 6(b), very sharp in Fig. 6(a) and medium in Fig. 6(c). The reason why there is a great difference between Fig. 6(b) and (a) or Fig. 6(c) is that nearly all of the surfactant molecules are assembled together forming micelles at 5 mmol L−1. In Fig. 6(a), the strong peak 1 is because most of the surfactant molecules are present in the as single molecule at a concentration of 3 mmol L−1. When the surfactant concentration is 7 mmol L−1, which exceeds CMC, there are a small fraction of the single molecule surfactants in solution. It can be concluded that the main micelle diameter is 197.2 nm.
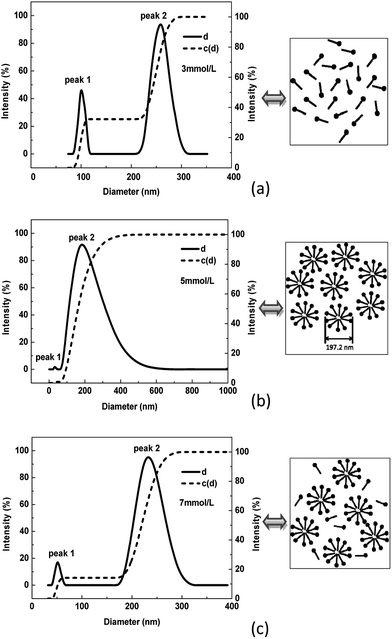 | ||
| Fig. 6 The intensity-weighted size distributions of different concentrations of surfactant: (a) 3 mmol L−1; (b) 5 mmol L−1; (c) 7 mmol L−1. | ||
Equilibrium surface tension and CMC results
A BZY-1 automatic surface tension meter was used to determine the equilibrium surface tension and CMC at room temperature. The CMC is gained by fitting the curve of the surface tension against the logarithm of the surfactant concentration. The two equilibrium surface tension curves of DAGA-ES solution diluted using distilled water and 3 mol L−1 NaCl solution are shown in Fig. 7.It can be clearly seen from Fig. 7 that the surface tension decreases gradually with increasing concentration and this trend is more obvious in sodium chloride solution. However, when the concentration exceeds CMC, the surface tension remains constant because of micelle formation. The trend is in agreement with conventional surfactants.33,34 The surface tension at the CMC is called γCMC. The CMC and γCMC of DAGA-ES in distilled water are 5 mmol L−1 and 1 mmol L−1, respectively, and in 3 mol L−1 sodium chloride are 24.9 mN m−1 and 22.3 mN m−1, respectively. When NaCl is added, the γCMC has a small change but the CMC decreases significantly. Like traditional anionic surfactants, the electrostatic repulsion between anionic head groups has been weakened owing to the combination of opposite sodium ions with sulfonate ions, which is conducive to micelles formation, hence, the CMC reduces.35,36 The surface activities of other surfactants that have similar a structure to DAGA-ES are summarized in Table 2. A comparison of the results shows DAGA-ES to have superior surface activity.
Fundamental surfactant parameters
The HLB value formula of the sulfonate anionic surfactant is shown as follows:37
| HLB = A × log|CMC| + B | (1) |
It is seen from Fig. 7 that the value of CMC is 5 mmol L−1 in distilled water. According to eqn (1), the HLB value of DAGA-ES is 11.72. Surfactant with stronger solubility corresponds to higher HLB values. The value of HLB is 11.72, which is between 10 and 13 (aqueous solution should be translucent or transparent). The dissolution test result shows that the aqueous solution of DAGA-ES is transparent. It can be concluded that the HLB value obtained by the CMC method is in agreement with the solution properties.
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c is the slope of the surface tension isotherm near the CMC. The value of n depends on the type of surfactant and the salinity of the solvent. In this paper, the value of n is 2 in distilled water because of a 1
c is the slope of the surface tension isotherm near the CMC. The value of n depends on the type of surfactant and the salinity of the solvent. In this paper, the value of n is 2 in distilled water because of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ionic surfactants without extra electrolyte.40 However, n = 1 in the presence of 3 mol L−1 NaCl.41,42 In Fig. 7, it appears that dr/d
1 ratio of ionic surfactants without extra electrolyte.40 However, n = 1 in the presence of 3 mol L−1 NaCl.41,42 In Fig. 7, it appears that dr/d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c is −2.45 in distilled water and the calculated Γmax is 0.5 ± 0.1 μmol m−2 while dr/d
c is −2.45 in distilled water and the calculated Γmax is 0.5 ± 0.1 μmol m−2 while dr/d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c is −4.29 in 3 mol L−1 sodium chloride solution and the calculated Γmax is 1.7 ± 0.1 μmol m−2. The polarity of the sodium chloride solution is higher than that of distilled water; therefore, the DAGA-ES trends to adsorb onto the air/water interface due to hydrophobic interactions. The concentration of DAGA-ES on the air/water interface is higher than that in the solution with electrolyte present, so the Γmax is more obvious in 3 mol L−1 sodium chloride solution.
c is −4.29 in 3 mol L−1 sodium chloride solution and the calculated Γmax is 1.7 ± 0.1 μmol m−2. The polarity of the sodium chloride solution is higher than that of distilled water; therefore, the DAGA-ES trends to adsorb onto the air/water interface due to hydrophobic interactions. The concentration of DAGA-ES on the air/water interface is higher than that in the solution with electrolyte present, so the Γmax is more obvious in 3 mol L−1 sodium chloride solution.
 | (3) |
According to the value of Γmax, the Amin in distilled water and solution with 3 mol L−1 NaCl are 3.3 ± 0.1 nm2 and 0.9 ± 0.1 nm2, respectively. It is indicated that the DAGA-ES molecules are arranged more closely at the air/water interface in the presence of electrolyte that weakens the repulsion between molecules. The results are very consistent with CMC and γCMC in distilled water and 3 mol L−1 NaCl solution.
Oil–water interfacial tension results
The effects of surfactant concentration and NaCl concentration on the IFT were investigated, respectively, by a TX500C rotating drop ultra-low oil/water interfacial tension instrument. The results of IFT were calculated automatically by software based on the length and width of the oil droplet and are summarized in Fig. 8.It can be concluded from Fig. 8(a) that, when the surfactant concentration is invariable (0.30 wt%), IFT decreases with the increase of mineralization degree. The reduction could be due to sodium ions combining with sulfonate ions, which weaken the repulsion between the hydrophilic head and make the surfactant molecules arrange more closely. While interfacial tension increases slightly when the concentration of NaCl surpasses 3 mol L−1. The excessive sodium ions destroy the hydration film around the hydrophilic head, promoting the surfactants on the oil/water interface to transfer to the oil phase and the surface tension increases with a reduction of aggregation degree.44,45 This change trend can also be found in Fig. 8(b), when the NaCl concentration is invariable at 3 mol L−1, with the surfactant concentration ranging from 0.2 wt% to 0.3 wt%, IFT is reduced to its lowest point and the minimum value is 0.05 mN m−1.
However, it slightly increases when the surfactant concentration exceeds 0.3 wt%. When a part of the surfactant molecules have been adsorbed on the oil/water interface, the molecules in solution still exist in the form of micelles which influences regular arrangement of surfactants on oil/water interface, therefore, the equilibrium oil/water interfacial tension is higher than that of optimum concentration (0.3 wt%).46,47
Fig. 8(c) shows the varieties of IFT in 0.30 wt% surfactant solutions (DAGA-ES and AES) diluted using 3 mol L−1 NaCl. The DAGA-ES can reduce the equilibrium IFT to 0.05 mN m−1 while AES just reduces it to 0.33 mN m−1. Photographs of the crude oil drops at equilibrium IFT are shown in Fig. 8(d). In summary, compared to AES, DAGA-ES possesses the more excellent ability to reduce IFT.
The wettability on hydrophilic and hydrophobic surfaces
The HARKE-SPCA contact angle measuring instrument was used to determine the wettability. Each of the contact angles was determined 3 times by imaging software. From Fig. 9(a) and (b), the wetting angle is recorded as θ and the oil drops shrink on the hydrophilic surface while they spread on the hydrophobic surface without surfactant.The contact angles on the hydrophilic and hydrophobic surfaces against concentration of DAGA-ES are shown in Fig. 9(c). The contact angles decrease gradually from 79.9 °to 47.6 °and 137.5° to 80.9° with the increasing of surfactant concentration on the hydrophilic and hydrophobic surfaces, respectively. The wettability reversal phenomenon only occurs on the hydrophobic surface, which is different from conventional surfactants.48 The strengthened hydrophilicity on the hydrophilic surface might be due to two main aspects: firstly, the water-wet surface is negatively charged because the glass is made of silica, which has [Si(OH)] groups in an aqueous medium.49 Therefore, there is a repulsive interaction between [HSO3−] and [Si(OH)]. A portion of [HSO3−] groups, as the hydrophilic group of DAGA-ES, will get away from the air/water interface rather than adsorb on the hydrophilic surface. Secondly, the volume of the sugar ring hydrophilic ionic head is huge, so some of the hydrophilic atoms, such as oxygen atom, will also be exposed to the surface. For the above two reasons, the hydrophilicity of the hydrophilic surface is gradually strengthened. On the contrary, the strengthened hydrophilicity on the hydrophobic surface derives from the long-chain alkanes of DAGA-ES that adsorb onto the oil-wet surface through hydrogen bonds and hydrophobic interactions.50 Two hydrophilic ions extend outside the interface covering the surface, and finally, the wettability reversal is realized on the hydrophobic surface (Fig. 10).
Oil displacement experiment
Oil displacement experiments were conducted to evaluate EOR using DAGA-ES and AES. Core parameters are shown in Table 3. The varieties of injection pressure and EOR rate with injected volume are shown in Fig. 11. In the three stages (water flooding, surfactant flooding and subsequent water flooding), the injection pressure increases firstly and then decreases to be constant. It can be concluded that both the DAGA-ES and AES play a role in reducing pressure and increasing injection.51,52 The oil recovery gathers with increasing injected volume and finally increases by 16.29% using DAGA-ES, versus 12.82% using AES, when subsequent water flooding reaches the economic limit (the water content is greater than 98%).| Number | Diameter (cm) | Length (cm) | Voidage (%) | Permeability (%) | Dry weight (g) | Wet weight (g) | Void volume (mL) | Irreducible water saturation (%) |
|---|---|---|---|---|---|---|---|---|
| 1# | 3.84 | 7.52 | 19.03 | 65.33 | 152.62 | 169.19 | 16.57 | 39.3% |
| 2# | 3.83 | 7.64 | 18.98 | 68.06 | 152.94 | 169.65 | 16.71 | 34.2% |
Conclusions
(1) As a type of eco-friendly surfactant, DAGA-ES was synthesized with anhydrous glucose, dodecylamine, and 2-ethyl chloride sulfonic acid sodium as raw materials based on two steps and its chemistry structure was confirmed by ESI-TOFMS, 1H NMR, and FT-IR, sequentially. (2) Its adsorption morphology was reticular and the diameter of the micelle was 197.2 nm in distilled water. The CMC and γCMC of DAGA-ES in 3 mol L−1 NaCl solution were 1 mmol L−1 and 22.3 mN m−1, which were lower than that in distilled water. The excellent water solubility was verified by the value of HLB (11.72). The value of Γmax and Amin indicated that more DAGA-ES molecules adsorbed on the air/water interface and arranged closely when electrolyte was added.(3) The IFT could be reduced significantly when DAGA-ES interacted with NaCl and the minimum value was 0.05 mN m−1. Furthermore, DAGA-ES enhanced the hydrophilicity of both the hydrophilic and lipophilic surfaces because of its special structure. Finally, the hydrophobic surface was converted to a hydrophilic surface and this property was conducive to the displacement of crude oil.
(4) Displacement efficiency was studied in this paper through the core flow experiment. The results showed that DAGA-ES possessed even more excellent properties in EOR process than the conventional anion-nonionic surfactant (AES).
Acknowledgements
This study was supported by College of Chemistry and Chemical Engineering and State Key Laboratory of Southwest Petroleum University. All authors contributed at various stages of planning, experiment and write-up of this manuscript.References
- N. Ahmad, R. Ramsch, M. Llinàs, C. Solans, R. Hashim and H. Tajuddin, Colloids Surf., B, 2014, 115, 267 CrossRef CAS PubMed.
- N. N. Al-Mohammed, R. S. D. Hussen, Y. Alias and Z. Abdullah, RSC Adv., 2015, 4, 2869 RSC.
- L.-liang Dong, C.-fang Zhang, Y.-yao Zhang, Y.-xiang Bai, J. Gu, Yu-ping Sun and M.-qing Chen, RSC Adv., 2015, 7, 4947 RSC.
- A. A. Salman, M. Tabandeh, T. Heidelberg, R. S. D. Hussen and H. M. Ali, Carbohydr. Res., 2015, 412, 28 CrossRef CAS PubMed.
- X. F. Zheng and Q. Lian, J. Dispersion Sci. Technol., 2015, 36, 2451 Search PubMed.
- L.-c. Wang, H.-y. Wang, Y.-w. Zhu, X.-w. Song, S.-j. Liu, X. Liu and S.-x. Jiang, J. Dispersion Sci. Technol., 2012, 33, 374 CrossRef CAS.
- B. Song, X. Hu, X. Shui, Z. Cui and Z. Wang, Colloids Surf., A, 2016, 489, 433 CrossRef CAS.
- P. J. Liyanage, J. Lu, G. W. P. Arachchilage, U. P. Weerasooriya and G. A. Pope, J. Pet. Sci. Eng., 2015, 128, 73 CrossRef CAS.
- S. M. Tawfik and N. A. Negm, J. Mol. Liq., 2016, 215, 18 CrossRef.
- J. Łuczak, A. Latowska and J. Hupka, Colloids Surf., A, 2015, 471, 26 CrossRef.
- X. Zeng, Z. Lu and Y. Liu, J. Surfactants Deterg., 2013, 16, 131 CrossRef CAS.
- A. Al-Sabagh, E. Azzam, S. Mahmoud and N. Saleh, J. Surfactants Deterg., 2007, 10, 3 CrossRef CAS.
- H. Li, J. Wang, C. Liu, J. Han, C. Li and M. Ning, Open J. Appl. Sci., 2012, 2, 93 CrossRef CAS.
- L. Zhang, J. Shi, A. Xu, B. Geng and S. Zhang, J. Surfactants Deterg., 2013, 16, 183 CrossRef CAS.
- Z. Hricovíniová and M. Hricovíni, Tetrahedron: Asymmetry, 2014, 25, 1008 CrossRef.
- C. Gan, H. Wang, Z. Zhao and B. Yin, J. Surfactants Deterg., 2014, 17, 465 CrossRef CAS.
- H. S. Ewan, C. S. Muli, S. Touba, A. T. Bellinghiere, A. M. Veitschegger, T. B. Smith, W. L. Pistel, W. T. Jewell, R. K. Rowe and J. P. Hagen, Tetrahedron Lett., 2014, 55, 4962 CrossRef CAS.
- T. H. Ali, H. A. B. Tajuddin, R. S. D. Hussen and T. Heidelberg, J. Surfactants Deterg., 2015, 18, 881 CrossRef CAS.
- M. R. Borges and R. de Carvalho Balaban, J. Biotechnol., 2014, 192, 42 CrossRef CAS PubMed.
- M. H. Mondal, S. Malik, A. Roy, R. Saha and B. Saha, RSC Adv., 2015, 112, 92707 RSC.
- D. Zhu, K. N. Baryal, S. Adhikari and J. Zhu, J. Am. Chem. Soc., 2014, 136, 3172 CrossRef CAS PubMed.
- R. C. Bazito and O. A. El Seoud, Carbohydr. Res., 2001, 332, 95 CrossRef CAS PubMed.
- L. Zhang and P. Somasundaran, J. Colloid Interface Sci., 2006, 302, 25 CrossRef CAS PubMed.
- A. Behler, H. Hensen and W. Seipel, Proceedings 6th World Surfactant Congress, Cesio, 2004, pp. 20–23 Search PubMed.
- M. Roussel, T. Benvegnu and V. Lognoné, Eur. J. Org. Chem., 2005, 14, 3085 CrossRef.
- Z.-d. Liu, P.-l. Liang, X. b. Chen, and J. Yu, Fine Chemicals, Dalian, 2007, vol. 24, p. 870 Search PubMed.
- Z. Xin, S. Yan, J. Ding, Z. Yang, B. Du and S. Du, Appl. Surf. Sci., 2014, 300, 8 CrossRef CAS.
- S. M. Tawfik and N. A. Negm, J. Mol. Liq., 2016, 215, 185 CrossRef CAS.
- A. M. Hussein and M. M. Khowdiary, J. Surfactants Deterg., 2014, 17, 615 CrossRef CAS.
- A. E. M. Eissa, M. El Hefnawy and S. Bader, J. Surfactants Deterg., 2012, 15, 411 CrossRef.
- M. Zhou, L. Xia and Y. He, J. Surfactants Deterg., 2015, 18, 303 CrossRef CAS.
- Y.-Y. Zhu and P.-P. Shen, Synthesis, properties and application of surfactant for tertiary oil recovery, Petroleum Industry Press, Beijing, 2002 Search PubMed.
- M. Sagisaka, T. Narumi, M. Niwase, S. Narita, A. Ohata, C. James, A. Yoshizawa, E. Taffin de givenchy, F. Guittard and S. Alexander, Langmuir, 2014, 30, 6057 CrossRef CAS PubMed.
- H. Azira and A. Tazerouti, J. Surfactants Deterg., 2007, 10, 185 CrossRef CAS.
- B. Gao, Y. Yu and L. Jiang, Colloids Surf., A, 2007, 293, 210 CrossRef CAS.
- S. Chauhan, M. Kaur, K. Kumar and M. S. Chauhan, J. Chem. Thermodyn., 2014, 78, 175 CrossRef CAS.
- Z. Jiahua and C. Yingde, Spec. Petrochem., 2001, 2, 4 Search PubMed.
- M. Ao, P. Huang, G. Xu, X. Yang and Y. Wang, Colloid Polym. Sci., 2009, 287, 395 CAS.
- S. M. Shaban, I. Aiad, M. M. El-Sukkary, E. Soliman and M. Y. El-Awady, J. Mol. Liq., 2015, 207, 256 CrossRef CAS.
- T. Fan, C. Chen, T. Fan, F. Liu and Q. Peng, J. Hazard. Mater., 2015, 297, 340 CrossRef CAS PubMed.
- X.-P. Liu, J. Feng, L. Zhang, Q.-T. Gong, S. Zhao and J. Y. Yu, Colloids Surf., A, 2010, 362, 39 CrossRef CAS.
- M. Abe, K. Tsubone, T. Koike, K. Tsuchiya, T. Ohkubo and H. Sakai, Langmuir, 2006, 22, 8293 CrossRef CAS PubMed.
- M. Ao and D. Kim, J. Chem. Eng. Data, 2013, 58, 1529 CrossRef CAS.
- L. Zhang, L. Luo, S. Zhao and J.-Y. Yu, Acta Phys.-Chim. Sin., 2001, 17, 62 CAS.
- A. Bera, A. Mandal and B. Guha, J. Chem. Eng. Data, 2013, 59, 89 CrossRef.
- M. Aoudia, Z. Al-Harthi, R. S. Almaamari, C. Lee and P. Berger, J. Surfactants Deterg., 2010, 13, 233 CrossRef CAS.
- R. Granet, R. Khadirian and S. Piekarski, Colloids Surf., 1990, 49, 199 CrossRef CAS.
- R. G. Chaudhuri and S. Paria, J. Colloid Interface Sci., 2014, 413, 24 CrossRef PubMed.
- S. Takeda, K. Yamamoto, Y. Hayasaka and K. Masumoto, J. Non-Cryst. Solids, 1999, 258, 244 CrossRef.
- B.-f. Hou, Y.-f. Wang and Y. Huang, Appl. Surf. Sci., 2015, 330, 56 CrossRef CAS.
- S. M. Szlendak, N. M. Nguyen and Q. P. Nguyen, J. Pet. Sci. Eng., 2016, 142, 36 CrossRef CAS.
- S. Shaddel and S. A. Tabatabae-Nejad, Transp. Porous Media, 2015, 106, 621 CrossRef CAS.
- S. Das, S. Maiti and S. Ghosh, RSC Adv., 2014, 4, 12275 RSC.
| This journal is © The Royal Society of Chemistry 2016 |