The impact of MOF feasibility to improve the desalination performance and antifouling properties of FO membranes†
Abstract
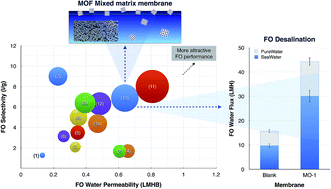
In this study, a hydrophilic metal–organic framework (MOF) was applied to improve the performance of a cellulosic membrane for forward osmosis (FO) desalination application. The characterization results confirmed that the MOF particles existed within the matrix of the modified membrane. The MOF loading led to adjustment of the membranes in terms of overall porosity, pore inter-connectivity and hydrophilicity. These features caused an improvement in the pure water permeability (72%) and reduce the structural parameter of the modified membrane to 136 μm. The FO water flux of the modified membrane enhanced by about 180% compared to an unmodified membrane, without decreasing its selectivity. FO fouling experiments were performed with a feed solution comprising a model organic foulant. The results demonstrated that the modification considerably improved the membrane antifouling properties when compared to the unmodified membranes. Water flux was also more easily recovered through physical cleaning for the modified membrane. The modified membrane was continuously tested under FO seawater desalination to investigate the performance stability. The modified membrane demonstrated a noteworthy water flux of above 30 LMH, using a 2 M NaCl draw solution. The modified membrane developed in this study exhibited outstanding permselectivity compared to ones reported in the literature.

 Please wait while we load your content...
Please wait while we load your content...