Synthesis, characterization and properties of amino acid ionic liquids derived from the triaminocyclopropenium cation†
Abstract
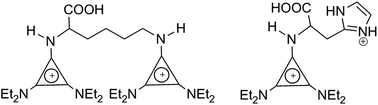
Amino acid ionic liquids (AAILs) [C3(NEt2)2(NHR)]X (X = MeSO4 and NTf2) based on the triaminocyclopropenium (TAC) cation were synthesized where NHR is derived from an amino acid. Reaction of the alkoxycyclopropenium salt [C3(NEt2)2(OCH3)]MeSO4 with an amino acid in the presence of Et3N gives the corresponding AAILs; the methylsulfate anion was readily exchanged for bistriflamide. Monocations were obtained with L-alanine, L-proline, L-valine, L-threonine, L-serine, L-methionine, L-leucine, L-isoleucine, L-tryptophan, L-tyrosine and L-phenylalanine, whereas dications were obtained with L-lysine (two TAC cations), L-histidine (one TAC and one imidazolium cation) and L-arginine (one TAC and one guanidinium cation). L-Cysteine generated a mixture of the monocationic IL as well as a dication containing one TAC cation and one thiodiaminocyclopropenium cation. L-Asparagine and L-glutamine appear to produce mixtures of amino-linked and amido-linked TAC cations. These materials were obtained as a mixture of the IL and its zwitterion which was initially indicated by the 1H NMR giving a reduced integral for the methylsulfate anion. The ratios of these IL/zwitterion mixtures were determined by pH titration and microanalysis. The chloride contents, decomposition temperatures, DSC data, viscosity, specific rotations, and pKa values were determined and solubility studies were also carried out.

 Please wait while we load your content...
Please wait while we load your content...