Structural, compositional and functional properties of Sb-doped Mg2Si synthesized in Al2O3-crucibles†
Abstract
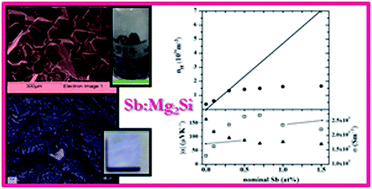
Magnesium silicide (Mg2Si) is a promising candidate for thermoelectric energy conversion due to its low toxicity, the abundance of its raw constituents and its low density, allowing manufacturing of light, sustainable and relatively cheap devices. Mg2Si needs to be doped in order to increase its efficiency, making this material competitive among materials operating in the intermediate temperature range. In this work, a synthesis procedure based on melting of the raw elements in easily available and cheap Al2O3 crucibles was developed to obtain polycrystalline Sb doped Mg2Si materials in a wide range of compositions. Powders from the crushed lumps were consolidated via spark plasma sintering and then thermally annealed to obtain dense pellets of Sb:Mg2Si with Sb = 0.0, 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5 at%. The effects of Sb doping and of the synthesis and sintering technique on composition, morphology and stability of n-type Mg2Si are discussed. Transport properties (Seebeck coefficient, electrical and thermal conductivity, charge carrier density) were evaluated in order to elucidate the composition–property relationship within this material system and find the optimal doping amount to optimize its thermoelectric properties.

 Please wait while we load your content...
Please wait while we load your content...