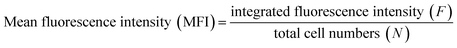Dual peptides modified fluorescence-SERS dual mode imaging nanoprobes with improved cancer cell targeting efficiency†
Yizhi Zhang,
Zhuyuan Wang*,
Lei Wu,
Shenfei Zong,
Binfeng Yun and
Yiping Cui*
Advanced Photonics Center, Southeast University, Nanjing 210096, Jiangsu, China. E-mail: cyp@seu.edu.cn; wangzy@seu.edu.cn; Tel: +86-25-83792470
First published on 11th August 2016
Abstract
The field of cancer theragnostics has long been starving for imaging agents with an improved targeting efficiency. Herein, a dual receptor targeting nanoprobe with fluorescence-SERS dual mode imaging capacity has been demonstrated for the specific targeting of cervical cancer cells. First, silver nanoparticles were modified with Raman reporters and silica-protected fluorescence dyes to incorporate fluorescence-SERS dual mode imaging capacity. Then, two targeting peptides, transferrin receptor-specific peptide T7 and integrin ανβ3 bonding peptide RGD, were functionalized onto the outer surfaces of the nanoparticles, endowing the nanoprobes with enhanced recognition towards HeLa cells. Finally, to investigate the targeting capability of this synergetic probe, the cellular internalization was compared between single and dual targeting nanoprobes. The results from fluorescence-SERS dual-mode imaging demonstrated that the dual peptide functionalized nanoprobes not only promoted an elevated uptake by cancer cells, but were also responsible for an enhanced targeting ability towards cancer cells. This type of nanoprobe with dual-mode imaging and a dual-targeting ability would give a new prospect for the diagnosis and therapeutics of cancer.
Introduction
During the past decades, targeted diagnoses and therapies based on nanotechnology have been widely evaluated in cancer.1 As for a nanoparticle system, the active targeting of specific cell populations is an important capability in biomedical science, particularly in cancer imaging and theragnostics. The main mechanism behind active targeting is using ligands on the surface of nanoparticles to enhance the specific uptake by cancer cells.2,3 One of the on-going challenges for targeted nanosystems is how to improve their specific accumulation at the disease site and reduce uptake or impairment to healthy cells.Cervical cancer is the fourth-most common cause of death in females worldwide, affecting more than half a million women in the world.4,5 The HeLa cell is one of the most important cervical cancer cell lines extensively researched for theragnostics of cervical cancer. Many cell surface receptors that pathologically overexpress HeLa, such as folate receptors, transferrin receptors, nucleolin and integrin receptors, have been investigated as targets for cervical cancer imaging and diagnosis.6–8 In general, to realize selective identification of HeLa cells, a ligand that is capable of binding to the upregulated receptors was engineered onto nanoparticles such as small molecules9 (e.g. folate), proteins10 (e.g. transferrin or antibodies), aptamers8,11 (e.g. AS1411) and peptides.1 Traditionally, only one single type of molecules conjugated onto the nanoparticles served as the targeting ligand. However, due to the complex pathologies of cancer, multiple types of potential targeted receptors often exist simultaneously and heterogeneously on the surfaces of cancer cells, which is a little intractable for single targeting strategy nanosystems to lead to optimal outcomes.3
A rational design that may help to improve the targeting effects is to simultaneously target more than one related molecular epitope on the cell membrane.12 It can be speculated that this synergetic targeting strategy may enhance the binding affinity to a specific disease, improve the probability of specific cell internalization and elevate the selectivity of targeting nanosystems.3 The transferrin receptor (TfR) is one of the major pathologically overexpressed receptors on cervical cancer cells. Transferrin conjugated nanomedicines have been widely employed in TfR overexpressed cancer theragnostics.10 Compared with proteins, peptides have become one of the best candidates for targeting ligands in recent years owning to their relatively small molecular weight, high avidity and improved biocompatibility.1 Peptide T7 (HAIYPRH), which is of interest recently, possess a high affinity for TfR (Kd of ∼10 nM) but a different binding site than Tf.13 A T7 conjugated drug delivery system has shown favorable uptake and an efficient therapeutic effect on TfR overexpressed cancer cells,14 which could be an alternative for TfR targeting. In addition, the RGD peptide is a type of cell adhesion molecule specifically binding to integrin ανβ3, which has been extensively investigated as a therapeutic target against cancer.12,15 As integrin ανβ3 is also high expressed on cervical cancer cells,16 the union of these two peptides may be able to elevate their targeting capability.
Fluorescence is a commonly used imaging modality in cancer targeting studies due to its high speed acquirement, intuitive observation and ease of use.17 However, fluorophore molecules may encounter photobleaching or quenching during the process of sample preparation and imaging. Moreover, the auto fluorescence of biological samples could interfere with the specific fluorescent signal. These limitations may complicate and hinder fluorescence imaging. In these aspects, surface enhanced Raman scattering (SERS), as an alternative imaging method to fluorescence, may make up for the deficiency because of its high photostability, ultra sensitivity and ample spectroscopic information.18 Accordingly, the union of fluorescence and SERS could extend the functionality of imaging probes and improve the imaging quality.9 In recent years, fluorescence and SERS joint imaging nanoprobes have successfully shown its potential in the field of cancer diagnostic imaging in vitro and in vivo.19,20 As a multimode imaging modality, the potential application of these probes may be improved when combined with an enhanced targeting capability.
Herein, we designed a dual-peptide functionalized dual-mode imaging nanoprobe simultaneously targeting two receptors on the cell membrane, transferrin receptors (TfR) and integrin ανβ3, in order to explore the feasibility of a synergistic targeting strategy and increase the cellular internalization and targeting effects of the nanoprobes. Two peptides, a heptapeptide, T7, and a cyclic pentapeptide, c(RGDyC), were chosen as targeting ligands, which specifically bind to TfR and integrin ανβ3. In preparation, a bifunctional crosslinking agent NHS-PEG2000-MAL was functioned as a bridge to link up these cysteine-bearing peptides with aminated dual mode imaging nanoprobes. Peptide T7 and RGD were assembled onto the nanoprobes at the same mole ratio to obtain dual-targeting probes. To elucidate the synergetic targeting ability, the cellular internalization was compared between single and dual targeting nanoprobes. The uptake contrast between cervical cancer cells and normal cells was also examined.
Experimental
Materials
Silver nitrate (AgNO3), tetraethoxysilane (TEOS), ammonia water, absolute ethanol and (3-aminopropyl) triethoxysilane (APTES) were purchased from Alfa Aesar. Trisodium citrate dehydrate was purchased from Shanghai Heiwei Co., Ltd. N-Hydroxysulfo succinimide polyoxyethylene maleimide (NHS-PEG2000-MAL, MW = 2000) and methoxy PEG succinimidylcarboxymethyl ester (NHS-mPEG, MW = 2000) were purchased from Ponsure Shanghai Biotech. Co., Ltd. 4-Mercaptobenzoic acid (4MBA), rhodamine B isothiocyanate (RBITC) and transferrin were obtained from Sigma Aldrich. Peptide T7 (cysteine–histidine–alanine–isoleucine–tyrosine–proline–arginine–histidine, T7 for short) and c(RGDyC) (cyclo (Arg–Gly–Asp–D-Tyr–Cys), RGD for short) employed in our experiments were synthesized by BankPeptide Inc. Human cervical cancer (HeLa) cells and human embryonic lung fibroblasts (MRC5) were purchased from Cell resource center of Shanghai Life Science Research Institute. Distilled water with a resistivity of 18.0 MΩ cm−1 was used in all the experiments.Preparation of fluorescence-SERS core–shell nanoparticles (denoted as FSNs)
The silver nanoparticles (NPs) were prepared using citrate reduction as described by Lee and Meisel.21 Then, 10 mL of the resulting silver colloid was centrifuged and dispersed in 10 mL of an ethanol solution. 10 μL of a 4-mercaptobenzoic acid (4 MBA) ethanol solution (10 mM) was added and stirred using a magnetic bar for 2 h at room temperature. The number of reporters immobilized onto each SERS probe is estimated to be 2.0 × 104.Next, these SERS-labelled NPs were coated with a layer of silica by the Stöber method22 to prevent aggregation and fluorescence quenching. Briefly, 200 μL of NH4OH (28%) was added to the abovementioned solution, followed by the addition of TEOS (5 μL). The mixture was vigorously stirred for over 4 h at room temperature. Then, the abovementioned SERS nanoprobes were encapsulated by a fluorescence dye-doped silica shell as described below. 1 mg mL−1 of RBITC in ethanol and 4.2 μL of APTES were mixed (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) overnight to form the conjugates of APTES and the fluorescent dye. NH4OH and TEOS were added to the silica-coated SERS NPs solution with the same amounts as before, followed by the addition of 5 μL of the RBITC–APTES ethanol solution. The mixture was stirred for 4 h under a light-sealed condition. The final FSNs were centrifuged and rinsed with ethanol several times and then re-dispersed in 10 mL of ethanol. The number of dye molecules in each one of the FSNs is approximately 1430.
1) overnight to form the conjugates of APTES and the fluorescent dye. NH4OH and TEOS were added to the silica-coated SERS NPs solution with the same amounts as before, followed by the addition of 5 μL of the RBITC–APTES ethanol solution. The mixture was stirred for 4 h under a light-sealed condition. The final FSNs were centrifuged and rinsed with ethanol several times and then re-dispersed in 10 mL of ethanol. The number of dye molecules in each one of the FSNs is approximately 1430.
Synthesis of peptide-conjugated FSNs
The surface of the FSNs was functionalized with amine groups by treatment with APTES as described below. 10 mL of the as-prepared NPs ethanol solution was mixed with distilled water (20 μL) and APTES (20 μL) with a volume ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The dispersion was then reacted in a water bath at 40 °C for 2 h with ultrasonication. After centrifugation and rinsing with ethanol and water, the amine-functionalized FSNs were dispersed in 10 mL of distilled water (DI).
1. The dispersion was then reacted in a water bath at 40 °C for 2 h with ultrasonication. After centrifugation and rinsing with ethanol and water, the amine-functionalized FSNs were dispersed in 10 mL of distilled water (DI).
In our experiment, peptides were covalently conjugated onto the amine-functionalized FSNs through the thiol groups of cysteine using the bifunctional crosslinking agent NHS-PEG2000-MAL. In brief, 20 μL of NHS-PEG2000-MAL (1 mg mL−1) was added into 1 mL of FSNs-NH2 in DI (pH = 7.0). The mixture was stirred for 1 h at room temperature. Unreacted reagents were discarded by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 8 min. The obtained FSNs-PEG-MAL were redispersed in 1 mL of DI.
000 rpm for 8 min. The obtained FSNs-PEG-MAL were redispersed in 1 mL of DI.
NHS-mPEG (MW = 2000) functionalized F-SERS nanoparticles (FSN-mPEG) were applied as a none-targeting control to evaluate the targeting effect of peptide-modified nanoprobes. The synthesis process was the same as that described above. 30 μL of NHS-mPEG (MW = 2000, 1 mg mL−1) was added to a milliliter of amine-functionalized nanoparticles. After one hour of reaction at room temperature, FSNs-mPEG were harvested by centrifugation.
As for the single-targeting FSNs, peptide T7 (HAIYPRH), in about 104 times molar excess, was added into an as-prepared FSNs-PEG-MAL suspension. After two hours of reaction, FSNs-T7 was obtained. The synthesis of peptide c(RGDyC) modified FSNs (FSN-RGD) was done in a similar way. The number of peptides that were immobilized on the FSNs-T7 and FSNs-RGD are estimated to be around 3 × 103.
For preparing the two peptide co-functionalized FSNs (FSNs-T7&RGD), T7 and c(RGDyC) were both added into the FSNs-PEG-MAL solution. The total quantity of the two peptides used in conjugation was the same as that of the single-targeting nanoparticles. The molar ratio of two peptides was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The amount of T7 and RGD that conjugated on the FSNs-T7&RGD is estimated to be around 1.5 × 103, for each. Two hours later, the resultant nanoparticles were collected by centrifugation at 10
1. The amount of T7 and RGD that conjugated on the FSNs-T7&RGD is estimated to be around 1.5 × 103, for each. Two hours later, the resultant nanoparticles were collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 4 °C. The procedure is illustrated in Scheme 1. The final nanoparticle concentration used in our experiments was approximately 0.2 nM.
000 rpm for 5 min at 4 °C. The procedure is illustrated in Scheme 1. The final nanoparticle concentration used in our experiments was approximately 0.2 nM.
Characterization
Zeta potential and hydrodynamic diameter of the as-synthesized nanoparticles were determined by dynamic light scattering (DLS) using a Zetasizer Nano ZS90 (Malvern Instruments Ltd UK). All samples for DLS testing were diluted ten times using distilled water. The morphology of the nanoparticles was observed by transmission electron microscopy (TEM, Phillips Tecnai 12 microscope operated at 100 kV). Extinction spectra were obtained by a Shimadzu UV-3600 PC spectrophotometer with quartz cuvettes of 1 cm path lengths.Cell culture
Human cervical cancer (HeLa) cells and human embryonic lung fibroblasts (MRC5) were cultured in the minimum essential medium (MEM, Nanjing KeyGen Biotech. Co., Ltd) containing 10% fetal bovine serum (GIBCO, New York) and 1% penicillin–streptomycin (Nanjing KeyGen Biotech. Co., Ltd). All cells were maintained under the standard cell culture conditions (5% CO2, 37 °C).Competition experiments for single targeting FSNs
In order to demonstrate that the uptake of single-targeting FSNs is through the recognition of specific receptors, some competition experiments were performed. HeLa cells were seeded at the same density in two chambers and incubated for 24 h, one of which was treated with 50 μL of c(RGDyC) peptide molecules (1 mg mL−1) before the addition of the FSNs-RGD (∼0.03 nM), whereas the other was only treated with FSNs-RGD (∼0.03 nM). Similarly, before incubation with FSNs-T7, 50 μL of T7 peptides (1 mg mL−1) and 50 μL of transferrin molecules (10 mg mL−1) were added into two HeLa cell culture chambers. The third chamber was only cultured with FSNs-T7 (∼0.03 nM) as a control. After two hours of incubation, all cells were washed with PBS and fixed for imaging.In vitro experiments for dual-targeting FSNs
To quantitatively investigate the internalization of FSNs, HeLa cells were seeded at 5 × 103 cells per chamber into an 8-chamber slide (Lab-Tek® Chamber Slide™) and cultured for 24 h in a cell incubator. Then, cells were treated with FSNs-mPEG, FSNs-T7, FSNs-RGD, and FSNs-T7&RGD in specified chambers. Each type of FSN was at a final concentration of ∼0.03 nM. Two hours later, the culture media was removed and the cells were washed with PBS three times. Subsequently, 4% paraformaldehyde was added for fixation. After 15 min, the cells were washed with PBS again and reserved for imaging.To examine the targeting capability of the dual-targeting FSNs, HeLa and MRC5 were seeded into cell culture dishes. After 24 hours, the same amount of dual-targeting probes was added into the culture dishes and incubated for two hours. Then, the supernatant medium was discarded and the cells were washed 3 times by PBS before fixation for imaging.
Fluorescent imaging and SERS mapping by confocal laser scanning microscopy
500 μL of PBS was added to the as-prepared fixed cells and these samples were observed under a confocal laser scanning microscope (CLSM, FluoView FV1000, Olympus). The fluorescence images and SERS mapping were both taken under a 60× oil-immersion objective. The fluorescence spectra for the RBITC dyes in FSNs were obtained using an excitation wavelength of 543 nm and received from 560 to 590 nm. For SERS mapping, the excitation wavelength was 633 nm, whereas the emission wavelengths of SERS signals ranged from 670 to 710 nm.Statistics analysis
To quantitatively assess the amount of internalized nanoprobes, we collected more than five fluorescence images from each sample group under the same imaging conditions, and there are over 20 cells per image (some confocal images for analysis are shown in Fig. S1–S3†). For every cell image, the integrated fluorescence intensity was measured directly and the total cell numbers were counted manually. The mean fluorescence intensity per cell on a cell image was obtained according to the equation below:Finally, more than 50 cells were analysed for each sample group and their fluorescence average and standard deviation were calculated. As for SERS analysis of probe-treated cells, the SERS signal from one single cell was collected using CLSM and the SERS spectrum for one sample group was taken from the average of ten cells measurements. Moreover, the peak intensities at 1578 cm−1 were utilized to form a histogram.
Results and discussion
Characterization of FSNs
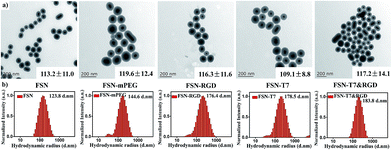
The synthesis procedure for the dual-targeting fluorescence-SERS co-imaging nanoprobes is illustrated in Scheme 1. Briefly, to obtain imaging nanoprobes simultaneously possessing fluorescence and SERS signals, SERS reporter-labelled silver nanospheres were first coated with a layer of silica and then encapsulated with an outer layer of a fluorescence dye-doped silica shell. The sandwiched silica shell was used to avoid silver nanoparticle aggregation and fluorescence quenching of the dyes. It can be clearly seen in the transmission electron microscopy (TEM) image (Fig. 1a) that the uniform silica layer was wrapped around the silver nanoparticles. The average diameter of the FSNs is 113.2 ± 11.0 nm and the thickness of the overall silica shell is 32.9 ± 1.9 nm. The data from dynamic light scattering (Fig. 1) indicate that FSNs have a good dispersibility and a quite uniform morphology. As shown in Fig. 2c, the extinction band of the prepared F-SERS probes (peaked at 444 nm) underwent an obvious red-shift and a slight broadening compared with that of the silver colloids (extinction band at 414 nm), which indicated the successful silica coating. It could be interpreted that the increase of the refractive index is caused by the silica shell coating.9For generating SERS signals, 4-mercaptobenzoic acid (4MBA) was conjugated on the surfaces of the silver nanospheres through the binding of its thiol groups to silver. Moreover, RBITC molecules doped in the silica shell functioned as fluorophores. The SERS spectrum of the FSNs is shown in Fig. 2a, the peaks of which are consistent with the previously reported results.9,23 The two main characteristic peaks of 4MBA are at 1068 and 1578 cm−1, attributed to the ring-breathing modes. Due to the high intensity and position stability of these two peaks, they are considered to be the ‘fingerprints’ of our FSNs. Fig. 2b displays the fluorescence signal of the dual mode imaging nanoprobes. The doping by the organic dyes barely influence further modifications on the probe surface.
Characterization of FSN-peptides complexes
Peptide T7 and c(RGDyC) were conjugated onto the aminated surfaces of the FSN probes through the bifunctional crosslinker agent NHS-PEG-MAL, the procedure of which is shown in Scheme 1. Specifically, the amine groups on the surfaces of the FSNs first reacted with the NHS ester parts of the NHS-PEG2000-MAL to form amide bonds, while the maleimide parts then reacted with the thiol groups of the peptides to form stable thioether bonds. The synthetic process was monitored by surface zeta potentials (Fig. 2d) and hydrodynamic diameters (Fig. 1). Zeta potential results indicate that the as-prepared FSNs are negatively charged (−21.2 mV) because of the outer silica shell. After the amination process, the zeta potential increased to +14.7 mV due to the cationic nature of the amino groups. Then, the grafting of NHS-PEG-MAL onto the FSN-NH2 renders the probes negatively charged (−17.9 mV) owing to the consumption of the amino groups and the slightly negative charge of PEG. With the further step of peptide functionalization, the zeta potential value slightly increased but still remained negative. The hydrodynamic diameters of the nanoparticles continuingly increased during this conjugation procedure. As shown in Fig. 1a, the morphology of the nanoprobes remained good even after continuous surface modifications. Because of the disturbance caused by PEG and peptides on the surface properties, the extinction band shows a slight blue-shift compared to that of FSN (Fig. 2c), which might further confirm the conjugation. Finally, the hydrodynamic radiuses of FSN-T7, FSN-RGD and FSN-T7&RGD are around 180 nm and their zeta potentials are negative. NHS-mPEG modified FSNs (FSN-mPEG) acted as control probes in our experiments.Single-targeting FSNs for dual-mode cell imaging
In our experiments, the targeting effects of single-peptide conjugated dual-mode probes have been investigated through competition assays. Peptide T7 is a heptapeptide obtained through phage display and has exhibited a high affinity to TfR (Kd of ∼10 nM), which is one of the overexpressed receptors on the cervical cancer cell membrane.24 To demonstrate the uptake of T7-functionalized FSNs through a TfR-mediated process, HeLa cells were incubated with the probes under three different conditions: (1) incubated with the probes for 2 h; (2) treated with excess free T7 peptide prior to the addition of the probes and then incubated for 2 h; (3) treated with excess free Tf before the probes were added and then incubated for 2 h. The fluorescence images and SERS mapping of a single cell under these conditions were collected using CLSM with a 60× oil-immersed objective and shown in Fig. 3a. It can be observed that much stronger fluorescence and SERS signals were detected in the cells incubated with only FSN-T7 than that in the cells with free T7 and Tf.In order to quantitatively evaluate their uptake levels, we analysed more than fifty cells to calculate the mean fluorescence intensity per cell. As shown in Fig. 3b, the uptake level is in the order of FSN-T7 only > FSN-T7 with free Tf > FSN-T7 with free peptide T7. Furthermore, we acquired the SERS spectra from ten different cells for each group. The average SERS signals are shown in Fig. 3c, where the predominant characteristic peaks of the probes at 1068 and 1578 cm−1 are clearly displayed. The intensities of the characteristic peak at 1578 cm−1 are plotted in Fig. 3d, revealing the same trends as the fluorescence does. These results indicate that the internalization of FSN-T7 prominently decreased in the presence of free peptide T7, whereas the addition of free Tf slightly weakened but did not completely blocked the uptake of the probes. It has been reported that the binding site of peptide T7 to TfRs differs from that of Tf,25 which means the combination of free Tf and the receptors perhaps could not competitively inhibit the endocytosis of FSN-T7 but consumed the receptors on the cytomembrane. As a result, the bonding possibility of FSN-T7 to the receptors decreased. The effects that free peptide T7 and Tf had on the experiment demonstrate that the uptake of FSN-T7 is through a TfR-specific process.
Integrin ανβ3 is also an important membrane receptor of HeLa cells,16,26 which has been extensively investigated as a therapeutic target against cancer and angiogenesis. The RGD sequence is well-known to preferentially bind to integrin ανβ3.15 A similar competition assay was conducted to validate the targeting capability of FSN-RGD. RGD modified dual-mode nanoprobes were co-cultured with HeLa cells in the presence or absence of free RGD peptides. After two hours of incubation, cells were fixed and imaged in dual fluorescence and SERS modes. As shown in Fig. 4a, the existence of free RGD partly impeded the internalization of the targeting probes according to the brightness of the fluorescence and SERS images. This is probably because free RGD in the medium and RGD bound to the nanoprobes competed for the integrin ανβ3. The average intensities of SERS and fluorescence acquired from HeLa cells obviously show the distinct uptake amount of the probes (Fig. 4b and d).
Targeting performance of dual-targeting FSNs in vitro
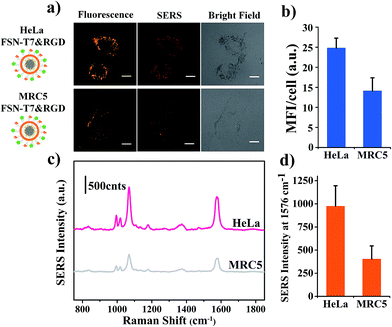
Malignant tumors usually show an increased growth rate for cancer cells and a highly elevated metabolic activity, which makes them eager to uptake nutrients for proliferating. Thus multiple types of membrane receptors are often upregulated simultaneously on malignant cells.5 Transferrin receptors and integrin ανβ3 both have relatively high expression levels on HeLa cells. Each one of them has been employed as targeting receptors to assist nano systems in preferentially entering cancer cells. It is natural to speculate that the union of peptides separately targeting these two receptors will elevate the internalization by cancer cells and strengthen their targeting efficiency. Consequently, to test the assumption, some comparative experiments (illustrated in Scheme 1) were performed, evaluating the cellular uptake levels of FSN-mPEG, FSN-T7, FSN-RGD and FSN-T7&RGD. As expected, HeLa cells incubated with FSN-T7&RGD showed stronger fluorescence and SERS intensities and a more spreadout distribution in cells according to the obtained CLSM images (Fig. 5a). A quantitative assessment between these different targeting strategies was conducted on the basis of mean fluorescence intensity and SERS peak intensity per cell (Fig. 5b and c). Clear SERS spectra are shown in Fig. 5d. The highest values of these two parameters were achieved in HeLa cells incubated with FSN-T7&RGD, indicating the synergistic target efficiency of the dual-peptide modified nanoprobes. The results indicate that recognition by two receptors on the cancer cell membrane may more greatly improve the cellular uptake of nanoparticles than the traditional one receptor recognition targeting strategy.Moreover, to explore the performance of dual-targeting FSNs in the identification of HeLa from normal cells, HeLa (cervical cancer cells) and MRC5 (normal cells) were both treated with the same amount of FSN-T7&RGD for 2 hours. MRC5 is the human normal embryonic lung fibroblasts, which have a low expression of TfR and integrin ανβ3.27 Then, the cells were subjected to fluorescence and SERS imaging under the same conditions. The results are shown in Fig. 6. Both fluorescence imaging and SERS mapping reveal the remarkable uptake difference between cancerous and normal cells. The mean fluorescence intensity displayed in Fig. 6b shows that the fluorescence intensity of HeLa is nearly twice as that of MRC5. Fig. 6c exhibits the average SERS spectrum obtained from ten randomly selected HeLa and MRC5. The intensity of the characteristic peak at 1578 cm−1 shown in Fig. 6d presents the difference between cancerous and normal cells. Furthermore, the selectivity performance of FSN-T7&RGD was compared with that of single-targeting FSNs and control probes of FSN-mPEG. The fluorescence and SERS performances of single targeting FSNs in MRC5 cells, and the mean fluorescence intensity ratio obtained from HeLa over MRC5 is shown in Fig. S4.† These results indicate that dual-targeting probes have a good performance in recognition of cervical cancer cells and have a higher selectivity than single-targeting and control probes. This synergetic targeting strategy of T7 and RGD may give a new prospect for cancer targeted imaging and therapeutics.
Conclusion
In summary, we successfully presented a dual-peptide functionalized dual-mode imaging nanoprobe, which can simultaneously target TfR and integrin ανβ3 on the cervical cell membrane. Owning to the combined action of peptide T7 and c(RGDyC), the dual-targeting imaging probes exhibited an elevated cancerous cellular uptake and an enhanced recognition of HeLa from normal cells. Fluorescence-SERS dual mode imaging intuitively and quantitatively demonstrated the results of cellular uptake of the probes and may provide an alternative analytical method in targeting research. This synergetic targeting strategy is expected to be employed in nanomedicine or drug delivery systems in the future. Moreover, the two-receptor-targeted methods show a great opportunity for generalizing in the theragnostics of other cancers.Acknowledgements
This study was financially supported by the State Key Program of the National Natural Science of China (Grant No. 61535003), the Excellent Youth Foundation of Jiangsu Province (BK20140023), the National Key Basic Research Program of China (Grant No. 2015CB352002), the National Science Foundation of China (Grant No. 61177033, 61275182 and 11374048), the Scientific Research Foundation of Graduate School of Southeast University (No. YBPY1507), and the Science Foundation for the Excellent Youth Scholars of Southeast University and the Fundamental Research Funds for the Central Universities.Notes and references
- L. D. Field, J. B. Delehanty, Y. Chen and I. L. Medintz, Acc. Chem. Res., 2015, 48, 1380–1390 CrossRef CAS PubMed.
- N. Bertrand, J. Wu, X. Xu, N. Kamaly and O. C. Farokhzad, Adv. Drug Delivery Rev., 2014, 66, 2–25 CrossRef CAS PubMed.
- C. L. Modery-Pawlowski and A. S. Gupta, Biomaterials, 2014, 35, 2568–2579 CrossRef CAS PubMed.
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 87–108 CrossRef PubMed.
- S. M. Crafton and R. Salani, Clin. Ther., 2016, 38, 449–458 CrossRef CAS PubMed.
- S. Xu, B. Z. Olenyuk, C. T. Okamoto and S. F. Hamm-Alvarez, Adv. Drug Delivery Rev., 2013, 65, 121–138 CrossRef CAS PubMed.
- Z. Wang, S. Zong, J. Yang, J. Li and Y. Cui, Biosens. Bioelectron., 2011, 26, 2883–2889 CrossRef CAS PubMed.
- P. Zhang, F. Cheng, R. Zhou, J. Cao, J. Li, C. Burda, Q. Min and J. J. Zhu, Angew. Chem., Int. Ed., 2014, 53, 2371–2375 CrossRef CAS PubMed.
- S. Zong, Z. Wang, J. Yang, C. Wang, S. Xu and Y. Cui, Talanta, 2012, 97, 368–375 CrossRef CAS PubMed.
- J. Yang, Z. Wang, X. Tan, J. Li, C. Song, R. Zhang and Y. Cui, Nanotechnology, 2010, 21, 345101 CrossRef PubMed.
- W. Wei, X. He and N. Ma, Angew. Chem., 2014, 53, 5573–5577 CrossRef CAS PubMed.
- E. Kluza, D. W. van der Schaft, P. A. Hautvast, W. J. Mulder, K. H. Mayo, A. W. Griffioen, G. J. Strijkers and K. Nicolay, Nano Lett., 2010, 10, 52–58 CrossRef CAS PubMed.
- W. Du, Y. Fan, B. He, N. Zheng, L. Yuan, W. Dai, H. Zhang, X. Wang, J. Wang, X. Zhang and Q. Zhang, Mol. Pharmaceutics, 2015, 12, 1467–1476 CrossRef CAS PubMed.
- W. Du, Y. Fan, N. Zheng, B. He, L. Yuan, H. Zhang, X. Wang, J. Wang, X. Zhang and Q. Zhang, Biomaterials, 2013, 34, 794–806 CrossRef CAS PubMed.
- F. Danhier, A. Le Breton and V. Preat, Mol. Pharmaceutics, 2012, 9, 2961–2973 CrossRef CAS PubMed.
- M. Z. Zhang, C. Li, B. Y. Fang, M. H. Yao, Q. Q. Ren, L. Zhang and Y. D. Zhao, Nanotechnology, 2014, 25, 255102 CrossRef PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- Y. Wang, B. Yan and L. Chen, Chem. Rev., 2012, 113, 1391–1428 CrossRef PubMed.
- X. Niu, H. Chen, Y. Wang, W. Wang, X. Sun and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 5152–5160 CAS.
- L. Sinha, Y. Wang, C. Yang, A. Khan, J. G. Brankov, J. T. Liu and K. M. Tichauer, Sci. Rep., 2015, 5, 8582 CrossRef PubMed.
- P. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef CAS.
- C. Graf, D. L. Vossen, A. Imhof and A. van Blaaderen, Langmuir, 2003, 19, 6693–6700 CrossRef CAS.
- C. E. Talley, L. Jusinski, C. W. Hollars, S. M. Lane and T. Huser, Anal. Chem., 2004, 76, 7064–7068 CrossRef CAS PubMed.
- Y. Kuang, S. An, Y. Guo, S. Huang, K. Shao, Y. Liu, J. Li, H. Ma and C. Jiang, Int. J. Pharm., 2013, 454, 11–20 CrossRef CAS PubMed.
- S. Oh, B. J. Kim, N. P. Singh, H. Lai and T. Sasaki, Cancer Lett., 2009, 274, 33–39 CrossRef CAS PubMed.
- M. Oba, S. Fukushima, N. Kanayama, K. Aoyagi, N. Nishiyama, H. Koyama and K. Kataoka, Bioconjugate Chem., 2007, 18, 1415–1423 CrossRef CAS PubMed.
- J.-E. Kim, H.-W. Jeong, J.-O. Nam, B.-H. Lee, J.-Y. Choi, R.-W. Park, J. Y. Park and I.-S. Kim, J. Biol. Chem., 2002, 277, 46159–46165 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13802k |
| This journal is © The Royal Society of Chemistry 2016 |