DOI:
10.1039/C6RA11123H
(Paper)
RSC Adv., 2016,
6, 62602-62611
Covalent surface modification of α-MnO2 nanorods with L-valine amino acid by solvothermal strategy, preparation of PVA/α-MnO2-L-valine nanocomposite films and study of their morphology, thermal, mechanical, Pb(II) and Cd(II) adsorption properties
Received
29th April 2016
, Accepted 21st June 2016
First published on 22nd June 2016
Abstract
The surface of α-manganese dioxide (α-MnO2) nanorods was modified chemically with L-valine amino acid by a solvothermal strategy. The α-MnO2 nanorods were prepared by a hydrothermal method. Then poly(vinyl alcohol)/α-MnO2-L-valine nanocomposites (NCs) containing 1, 3 and 5 wt% of modified α-MnO2 nanorods were prepared through an ultrasound-assisted technique. Fourier transform infrared spectroscopy, X-ray diffraction, field emission scanning electron microscopy, transmission electron microscopy and UV-visible spectroscopy were used to investigate and characterize nanostructures and NCs. Following this, the effects of α-MnO2-L-valine nanorods on the properties of NCs, such as the mechanical and thermal properties, were studied. The Brunauer–Emmett–Teller (BET) results showed that NC 3 wt% had higher surface area, pore volume and pore size than pure PVA with mesoporous structure. Finally, NC 3 wt% was investigated as an adsorbent for sorption of Pb(II) and Cd(II) ions. It showed good adsorption potential for the removal of Pb(II) and Cd(II) in aqueous solution.
Introduction
Polymer nanocomposites (NCs) are the result of the combination of polymers as matrix and inorganic/organic fillers at the nanoscale as a dispersed phase. The interaction between the dispersed phase components of NCs at the nanoscale enables them to act as molecular bridges in the polymer matrix.1,2 Poly(vinyl alcohol) (PVA) is a biodegradable, biocompatible, nontoxic and synthetic thermoplastic polymer that is used as a matrix. It exhibits good thermostability, chemical resistance and film forming ability.3,4 PVA is water-soluble, because of the polar hydroxyl groups on alternating carbon atoms, and can react with various organic and inorganic materials by physical and chemical interactions. It is used for water soluble packaging films, removal of heavy metal ions, paper adhesives, biosensors, drug delivery system, membranes with selective permittivity and biomaterials.5–9 The hydroxyl groups of the PVA can act as chelating sites for the adsorption of heavy metal ions by donation of the lone-pair electrons of oxygen to heavy metal ions to form coordinate bonds.
One type of the inorganic nanofillers is metal oxides. Manganese dioxide (MnO2) is a metal oxide that has attracted intensive interest because of its low cost, environmental friendliness, natural abundance, high surface area, strong oxidizing/adsorptive abilities and high theoretical specific capacitance.10–12 The MnO2 has wide applications in removal of heavy metal ion,13–17 catalysts,18,19 energy storage systems including batteries and supercapacitors.20–23 It exists in several different crystalline structures such as α, β, γ and δ types, which are involve different linking of the basic unit [MnO6] octahedral.24,25 The α-MnO2 with 2 × 2 tunnel structure with wide tunnel size (0.46 nm) and large surface area26 is suitable for adsorption heavy metal ions. The MnO2 nanostructures with a high ratio of surface area to volume tend to agglomerate, which severely impedes their application. Thus chemical and physical surface modification can be utilized to effectively prevent agglomeration and increase the dispersibility of them. L-Valine is a branched-chain amino acid with suitable biodegradability, biocompatibility, nontoxic and ecofriendly properties, that obtained by hydrolysis of proteins.7,27,28 L-Valine can use as modifier, with a coordination of carboxylic group (COOH) to surface Mn atoms.
The removal of heavy metals from aqueous solution is urgent environmental problem because the heavy metals like lead, nickel, cadmium and etc. are non-biodegradable and toxic even at low concentrations.8 Hence recent researches have focused on the property of novel materials for adsorption of heavy metal ions from aqueous solution. Recently, the use of carbon nanotubes,29 ZrO kaolinite30 and β-MnO2 (ref. 31) as adsorbent for Pb(II) removal, crosslinked chitosan/polyvinyl alcohol32 and MnO2/o-MWCNTs33 as adsorbent for Cd(II) have been reported. As mentioned, PVA and MnO2 have potential adsorption of heavy metal ions.
In this study, the α-MnO2 nanorods were prepared through hydrothermal method. L-Valine was used as biocompatible modifier for the chemically surface modification of α-MnO2 nanorods by solvothermal approach. The binding forms of a carboxylic group on α-MnO2 may be in several ways, including by simple adsorption (electrostatic attraction and hydrogen bonding) and chemical adsorption (ester linkage, bridging and chelating) as illustrated by Scheme 1.
 |
| | Scheme 1 Possible binding modes of COOH group on MnO2 nanorods: (i) electrostatic attraction, (ii and iii) H-bonding, (iv) monodentate (ester-like linkage), (v) bidentate bridging, and (vi) bidentate chelating. | |
Then, modified MnO2 nanorods were introduced into the PVA matrix for the preparation of PVA/α-MnO2-L-valine NCs. Finally, the α-MnO2 nanorods, modified α-MnO2 nanorods and NCs were characterized. Mechanical and thermal properties of NCs were investigated. Also, the adsorption properties of NC 3 wt% for Pb(II) and Cd(II) ions sorption was investigated by flame atomic absorption spectrometer (FAAS).
Experimental
Materials
Potassium permanganate (KMnO4) was purchased from Sigma Aldrich (Seelze, Germany). PVA (Mw = 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 99% hydrolysis), L-valine and hydrate manganese sulfate (MnSO4·H2O) were obtained from Merck Chemical Co. (Germany). Lead(II) nitrate was purchased from Cica-Reagent (Tokyo, Japan). Cadmium-nitrate-4-hydrate (Cd(NO3)2·4H2O) was purchased from Riedel-de Haën (Seelze, Germany).
000 g mol−1 and 99% hydrolysis), L-valine and hydrate manganese sulfate (MnSO4·H2O) were obtained from Merck Chemical Co. (Germany). Lead(II) nitrate was purchased from Cica-Reagent (Tokyo, Japan). Cadmium-nitrate-4-hydrate (Cd(NO3)2·4H2O) was purchased from Riedel-de Haën (Seelze, Germany).
Equipments
Fourier transform infrared spectroscopy (FT-IR) spectra were recorded at room temperature in the range of 400–4000 cm−1 using a Jasco-680 (Japan) spectrophotometer. The solid spectra were obtained using KBr pellets. The vibrational frequencies are accounted in wave numbers (cm−1). X-ray diffraction (XRD) patterns of the samples were recorded on Philips X'Pert MPD X-ray diffractometer (Germany) by a copper target operating at a voltage of 40 kV and Cu Kα radiation (λ = 1.54 Å) over a range of 10–80° with the scanning rate of 0.05 °C min−1. Thermal stability of samples was studied by recording TGA thermograms and performed with a STA503 TA thermal analyzer (Bahr-Thermoanalyse GmbH, Hüllhorst, Germany) under argon atmosphere at a heating rate of 20 °C min−1 from 25 °C to 800 °C. The morphology of nanostructures and NC films were investigated by field emission scanning electron microscopy (FE-SEM) Hitachi S-4160 (Japan). Transmission electron microscopy (TEM) images were taken using a Philips CM 120 operated (Netherlands) at voltage of 100 kV. The BET were characterized surface area, pore volume and pore size using nitrogen adsorption and desorption at liquid nitrogen temperatures (BELSRB mini). The UV-vis spectra of solid sample of nanostructures and NC films were measured on a JASCO V-570 (Japan) spectrometer in the wavelength scan range from 200 to 800 nm. Tensile testing of NC films was performed on a testometric universal testing machine M350/500 (United Kingdom) according to ASTM D 882 at room temperature. Tests were carried out with a crosshead speed of 5 mm min−1. The bone-shaped of the test specimens were 40 × 20 mm (length × width). Fabrication of NC films was performed with the probe of the ultrasonic horn being immersed directly into the mixture solution system with the frequency of 20 KHz and power of 100 W (TOPSONICS, Tehran, Iran). The Pb(II) and Cd(II) ion concentrations were determined by the Perkin-Elmer 2380-Waltham FAAS.
α-MnO2 nanorods preparation
1 mmol of hydrate manganese sulfate (MnSO4·H2O, 0.2 g) and 2.5 mmol of potassium permanganate(VII) (KMnO4, 0.5 g), which were dissolved in 10 mL of deionized water, were mixed for 30 min at room temperature. Then, the mixed solution was transferred in a sealed Teflon-lined autoclave and hydrothermal synthesis was performed at 140 °C for 12 h. After the reaction was completed, the autoclave was naturally cooled at room temperature. The resulting brownish black solid product was collected by centrifugation at 4000 rpm and washed with deionized water several times to remove ions possible remaining in the final product and then dried at 60 °C in air.34
Modification of α-MnO2 nanorods
Typically, 0.035 g of α-MnO2 nanorods in 5 mL ethanol was sonicated for 15 min and was added to 0.2 g L-valine amino acid (4 mmol), which was dissolved in deionized water/ethanol solvents (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture was transferred to sealed Teflon-lined autoclave, followed by a solvothermal reaction at 100 °C for 24 h. After the autoclave was naturally cooled to room temperature, modified α-MnO2 nanorods were isolated by centrifugation and washed with deionized water and ethanol. Then, the product dried at 60 °C in vacuum.
1). The mixture was transferred to sealed Teflon-lined autoclave, followed by a solvothermal reaction at 100 °C for 24 h. After the autoclave was naturally cooled to room temperature, modified α-MnO2 nanorods were isolated by centrifugation and washed with deionized water and ethanol. Then, the product dried at 60 °C in vacuum.
Fabrication of PVA/α-MnO2-L-valine amino acid NC films
Different amounts of chemically surface modified α-MnO2 nanorods (1, 3 and 5%) were dispersed in deionized water and sonicated for 20 min. Then, 0.1 g of PVA was dissolved in 10 mL deionized water at 80 °C and mixed with α-MnO2 nanorods suspension. The mixture was stirred for 24 h and ultrasonicated for 2 h. Finally, the obtained suspension was poured into Petri dishes, followed by solvent evaporation at room temperature to form NC films (Scheme 2).
 |
| | Scheme 2 Preparation of PVA/α-MnO2-L-valine NCs. | |
Adsorption experiment
The various amounts of the adsorbent PVA/α-MnO2-L-valine NC 3 wt% from 20 mg to 80 mg in 10 mL solution containing 100 ppm of Pb(II) and Cd(II) ions at a constant temperature (25 °C) for 12 h placed to a shaker table (150 rpm). Then solutions filtered and their adsorption determined by FAAS. The adsorption capacity Qe (mg g−1) and the removal percentage of Pb(II) and Cd(II) ions were calculated according to eqn (1) and (2).| | |
Removal (%) = 100 × (C0 − Ce)/C0
| (2) |
where C0 (mg L−1) is the Pb(II) and Cd(II) ions concentration in the initial solution, Ce (mg L−1) is the equilibrium concentration of Pb(II) and Cd(II) ions in the supernatant, V (L) is the volume of the testing solution and m is the weight of sorbent (g).
Results and discussions
FT-IR spectra investigation
Fig. 1 shows the FT-IR spectra of the α-MnO2 nanorods and α-MnO2/L-valine nanorods. The α-MnO2 nanorods showed absorption peaks at 460, 520, 1110, 1404, 1634 and 3402 cm−1. The peaks at 460 and 520 cm−1 could be attributed to the Mn–O bending vibration. The peaks observed at 1110, 1404 and 1634 cm−1 were ascribed to the bending vibrations of –OH group attached to the manganese atoms. The broad peak at 3402 cm−1 could be assigned to stretching vibration of the –OH group in adsorbed water by the α-MnO2 nanorods.35 The FT-IR spectrum of α-MnO2/L-valine nanorods showed a new peak at 2923 cm−1, which is attributed to C–H stretching band of L-valine amino acid. The peak at 1721 cm−1 is ascribed to ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. Two absorption peaks at 1628 and 1457 cm−1 are attributed to the bending vibration of N–H group and stretching vibration of C–N group, respectively and also can be attributed to carboxylate anion (COO−) asymmetric and symmetric stretching bonds. The peaks at 490 and 445 cm−1 could be attributed to the Mn–O bending vibration and the peak at 595 cm−1 is attributed to bending vibration of N–H (–NH3+) group. The FT-IR spectrum of α-MnO2/L-valine nanorods suggests that L-valine molecules were chemically bound on the MnO2 nanorods surfaces rather than simply adsorbed. The FT-IR spectra of pure PVA and PVA/α-MnO2-L-valine amino acid NCs are demonstrated in Fig. 1. In spectrum of pure PVA, the broad band peak at 3400 cm−1 showed the –OH stretching vibration of hydroxyl groups in PVA. Also, this spectrum represented peaks at 2941, 1420 and 1093 cm−1 that are attributed to C–H stretching, C–H bending and C–O stretching, respectively. The peak observed at 1716 cm−1 is due to the carbonyl functional groups of the remaining acetate groups during the fabricating of PVA from hydrolysis of poly(vinyl acetate).36 In spectrum of PVA/α-MnO2-L-valine amino acid NCs absorption band around 480 and 590 cm−1 could be attributed to the vibration of Mn–O group and N–H bending (–NH3+) which increase with α-MnO2/L-valine nanorods percent. The peak around 1710 cm−1 is attributed to the vibration of C
O stretching vibration. Two absorption peaks at 1628 and 1457 cm−1 are attributed to the bending vibration of N–H group and stretching vibration of C–N group, respectively and also can be attributed to carboxylate anion (COO−) asymmetric and symmetric stretching bonds. The peaks at 490 and 445 cm−1 could be attributed to the Mn–O bending vibration and the peak at 595 cm−1 is attributed to bending vibration of N–H (–NH3+) group. The FT-IR spectrum of α-MnO2/L-valine nanorods suggests that L-valine molecules were chemically bound on the MnO2 nanorods surfaces rather than simply adsorbed. The FT-IR spectra of pure PVA and PVA/α-MnO2-L-valine amino acid NCs are demonstrated in Fig. 1. In spectrum of pure PVA, the broad band peak at 3400 cm−1 showed the –OH stretching vibration of hydroxyl groups in PVA. Also, this spectrum represented peaks at 2941, 1420 and 1093 cm−1 that are attributed to C–H stretching, C–H bending and C–O stretching, respectively. The peak observed at 1716 cm−1 is due to the carbonyl functional groups of the remaining acetate groups during the fabricating of PVA from hydrolysis of poly(vinyl acetate).36 In spectrum of PVA/α-MnO2-L-valine amino acid NCs absorption band around 480 and 590 cm−1 could be attributed to the vibration of Mn–O group and N–H bending (–NH3+) which increase with α-MnO2/L-valine nanorods percent. The peak around 1710 cm−1 is attributed to the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. The peak around 1650 cm−1 could be ascribed to banding vibration of N–H group and the bending vibrations of –OH group attached to the Mn atoms.
O group. The peak around 1650 cm−1 could be ascribed to banding vibration of N–H group and the bending vibrations of –OH group attached to the Mn atoms.
 |
| | Fig. 1 FT-IR spectra of (a) α-MnO2, (b) α-MnO2-L-valine, (c) pure PVA, (d) PVA/α-MnO2-L-valine NC film 1 wt%, (e) PVA/α-MnO2-L-valine NC film 3 wt% and (f) PVA/α-MnO2-L-valine NC film 5 wt%. | |
XRD diffraction pattern
XRD method permits to study the crystallinity and determine crystal phase. The XRD patterns for the α-MnO2, surface modified α-MnO2, PVA and PVA/α-MnO2-L-valine NCs with different loadings (1, 3 and 5 wt%) are shown in Fig. 2. All diffraction peaks in the XRD pattern are well matched with JCPDS data (44-0141); indicating pure tetragonal crystalline α-MnO2 is successfully synthesized. The XRD pattern of α-MnO2-L-valine indicated diffraction peaks related to α-MnO2 and L-valine. The diffraction peaks at 2θ = 7.4, 14.8, 22, around 29.5–34 and 37.3° are related to L-valine37 and other diffraction peaks are related to α-MnO2 (JCPDS 44-0141). It confirmed that α-MnO2 is modified surface with L-valine and crystalline phase structure did not change.
 |
| | Fig. 2 XRD patterns of (a) α-MnO2, (b) α-MnO2-L-valine, (c) pure PVA, (d) PVA/α-MnO2-L-valine NC film 1 wt%, (e) PVA/α-MnO2-L-valine NC film 3 wt% and (f) PVA/α-MnO2-L-valine NC film 5 wt%. | |
Pure PVA exhibited a semicrystalline structure due to the strong interaction hydrogen bonding at the chain polymer and showed two peaks at about 2θ = 19.5° (main crystalline peak) and 2θ = 41° with low intensity.38,39 The XRD patterns of PVA/α-MnO2-L-valine NC films showed both peak of PVA and the characteristic peak related to the α-MnO2-L-valine. The intensity of peak related to the α-MnO2-L-valine is enhanced with increasing content of the modified α-MnO2 in the PVA matrix that indicated NCs prepared.
Thermal degradation characteristics
The thermal stability of the α-MnO2 nanorods and α-MnO2/L-valine nanorods were investigated by TGA analysis (Fig. 3A). Around 5% weight loss in α-MnO2 nanorods is observed up to the temperature 250 °C, which can be attributed to the removal of physically adsorbed water by the α-MnO2 nanorods. After which decrease in mass can be corresponded to a phase transition form α-MnO2 to Mn2O3 with the release of oxygen.40,41 The α-MnO2/L-valine nanorods exhibited two steps, weight loss in the temperature ranges of below 150 °C is due to physically adsorbed water and weight loss in 150–500 °C is reasonably associated with desorption of chemically bound L-valine in the α-MnO2/L-valine nanorods sample. The amount of the L-valine attached on the α-MnO2 surface was determined based on α-MnO2 and α-MnO2/L-valine char yield differences at 800 °C. The calculated L-valine content was found to be about 32 wt%. Thermal decomposition behavior of the PVA and PVA/α-MnO2-L-valine NCs is depicted in Fig. 3B. Also, the resulting TGA data for thermal degradation are summarized in Table 1. TGA curve of the pure PVA polymer revealed three weight loss regions. The weight loss at 100–200 °C can be due to the evaporation of adsorbed water in PVA chains. At the second stage at 200–400 °C, the weight loss could be related to the polymer dehydration and formation of a polyacetylene-like structure. In the last stage, at 400–550 °C the decomposition of PVA main chain is leaded to release of CO2 gas and formation of oxides.42,43 TGA curve of the PVA/α-MnO2-L-valine NCs showed three weight loss regions. The first region at temperature of 100–220 °C is due to the evaporation of weakly physical bound water and the evaporation of residual solvents. At the second stage, the weight loss region of 200–400 °C is corresponded to the decomposition of L-valine amino acid, the polymer dehydration and formation of a polyacetylene-like structure. The third degradation stage at 400–550 °C corresponded to the decomposition of main chain of PVA. The pure PVA film showed 7% residue at 800 °C, while the weight percent remaining after major degradation at 800 °C for NCs (1, 3 and 5 wt%) is higher than the pure PVA. PVA/α-MnO2-L-valine NC 1 wt%, PVA/α-MnO2-L-valine NC 3 wt% and PVA/α-MnO2-L-valine NC 5 wt% had 11%, 13% and 17% residue at 800 °C. According to this result PVA/α-MnO2-L-valine NCs have higher thermal stability than the pure PVA and NC 5 wt% could be optimized amount.
 |
| | Fig. 3 TGA thermograms of (A) α-MnO2, α-MnO2-L-valine, (B) pure PVA (a), PVA/α-MnO2-L-valine NC film 1 wt% (b), PVA/α-MnO2-L-valine NC film 3 wt% (c) and PVA/α-MnO2-L-valine NC film 5 wt% (d). | |
Table 1 Thermal properties of pure PVA and PVA/α-MnO2-L-valine NCs
| Sample name |
T10a (°C) |
Char yieldb [%] |
| Temperature at which 10% weight loss was recorded by TGA at a heating rate of 20 °C min−1 under argon atmosphere. Weight percentage of material left undecomposed after TGA at a temperature of 800 °C under argon atmosphere. |
| Pure PVA |
256 |
7 |
| NCS 1 wt% |
261 |
11 |
| NCS 3 wt% |
258 |
13 |
| NCS 5 wt% |
259 |
17 |
Optical properties
The UV-vis absorption spectra of the α-MnO2 nanorods and α-MnO2/L-valine nanorods are illustrated in Fig. 4A. The α-MnO2 nanorods exhibited an absorption band attributable to the d–d transition of Mn ions in the MnO6 octahedra of the α-MnO2 nanorods around 380 nm.44 The absorption band around 250 nm can be largely attributed to the electron transition from the valence band to the conduction band. The α-MnO2/L-valine nanorods exhibited blue shifted edge at 320 nm compared to the bulk MnO2 (380 nm) which can be attributed to the effect of modifier. Fig. 4B shows the UV-visible absorption spectra of PVA and PVA/α-MnO2-L-valine NC films. The spectra of PVA/α-MnO2-L-valine NC films showed absorption peaks related to the α-MnO2/L-valine nanorods. The absorbance of PVA/α-MnO2-L-valine NC films increase with increasing content of α-MnO2/L-valine nanorods in PVA matrix. This increase in absorbance could be related to successive interaction of α-MnO2/L-valine nanorods with PVA matrix. By increasing the amount of α-MnO2/L-valine nanorods the absorption edges is moved towards lower wavelengths (blue shift) which is due to the quantum confinement effects.
 |
| | Fig. 4 UV-visible absorbance spectra of (A) α-MnO2, α-MnO2-L-valine nanorods, (B) pure PVA (a), PVA/α-MnO2-L-valine NC film 1 wt% (b), PVA/α-MnO2-L-valine NC film 3 wt% (c) and PVA/α-MnO2-L-valine NC film 5 wt% (d). | |
Mechanical properties
The mechanical tests, including the E-modulus, tensile strength and elongation at break, were performed to study the effect of modified α-MnO2 nanorods on the mechanical properties of PVA. The tensile stress–strain curves of pure PVA and NC films containing 1, 3 and 5 wt% of α-MnO2/L-valine nanorods are shown in Fig. 5 and the results are summarized in Table 2. Several factors can be affected on the mechanical properties of NCs such as particle size and morphology, particle loading and distribution, the polymer matrix and interfacial adhesion between modified α-MnO2 and polymer. The tensile strength and E-modulus are increased in comparison with pure PVA which can be due to good interfacial adhesion between α-MnO2/L-valine nanorods and the PVA matrix and homogenous dispersion of modified α-MnO2 into the PVA matrix.
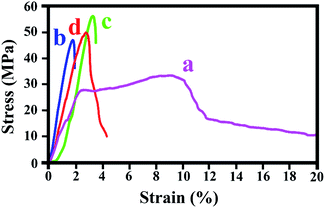 |
| | Fig. 5 Mechanical testing of pure PVA (a), PVA/α-MnO2-L-valine NC film 1 wt% (b), PVA/α-MnO2-L-valine NC film 3 wt% (c) and PVA/α-MnO2-L-valine NC film 5 wt% (d). | |
Table 2 Mechanical properties of pure PVA and PVA/α-MnO2-L-valine NC films
| Sample name |
Tensile strength (MPa) |
Strain (%) |
Elongation (mm) |
E-modulus (MPa) |
| Pure PVA |
33.4 |
9.0 |
3.6 |
1259.9 |
| NC 1 wt% |
46.9 |
1.7 |
0.7 |
3071.3 |
| NC 3 wt% |
56.1 |
3.3 |
1.3 |
1342.1 |
| NC 5 wt% |
49.8 |
2.9 |
1.1 |
1491.0 |
Morphological, pore structure and surface area studies
The morphology of the α-MnO2 and α-MnO2/L-valine was studied by FE-SEM and TEM techniques. The FE-SEM images of α-MnO2 and α-MnO2/L-valine are presented in Fig. 6. The FE-SEM images of α-MnO2 indicated that the α-MnO2 consist of a large number of aggregated nanorods with diameters 20–80 nm, as shown in Fig. 6(a and b). The modified α-MnO2 nanorods with L-valine showed no aggregation and diameters of nanorods were 30–70 nm, as shown in Fig. 6(c and d). A similar observation can be detected in TEM images of the MnO2/α-L-valine nanorods shown in Fig. 7, suggesting consistent results. These images displayed homogenous dispersion of nanorods with size of 30–70 nm. The FE-SEM photographs of PVA/α-MnO2-L-valine NC films demonstrated that modified α-MnO2 nanorods are uniformly distributed into the polymer matrix without conglomeration (Fig. 8). TEM images of PVA/α-MnO2-L-valine NC 3 wt% as a model were studied to investigate the modified nanorods effects on their dispersion state in the PVA matrix (Fig. 9). TEM images of PVA/α-MnO2-L-valine NC 3 wt% demonstrated that the α-MnO2-L-valine nanorods are well-dispersed in PVA matrix based on hydrogen bonding. Diameter of nanorods was in the range of 25–50 nm in these images.
 |
| | Fig. 6 FE-SEM micrographs of α-MnO2 (a and b) and α-MnO2-L-valine (c and d). | |
 |
| | Fig. 7 TEM images of α-MnO2-L-valine with different magnifications. | |
 |
| | Fig. 8 FE-SEM micrographs of PVA/α-MnO2-L-valine NC film 1 wt% (a and b), PVA/α-MnO2-L-valine NC film 3 wt% (c and d) and PVA/α-MnO2-L-valine NC film 5 wt% (e and f). | |
 |
| | Fig. 9 TEM images of PVA/α-MnO2-L-valine NC 3 wt% with different magnification. | |
The nitrogen adsorption–desorption isotherms of the pure PVA and NC 3 wt% are shown in Fig. 10. The isotherms are identified as type IV with hysteresis loop, which are the characteristic isotherm of meso-porous materials. Table 3 shows the BET results for the pure PVA and NC 3 wt%. The BET surface areas for the pure PVA and NC 3 wt% are found to be 0.0017 and 0.0063 m2 g−1, respectively, which indicate an increasing in the surface area for NC 3 wt% compare to pure PVA. The pore volume and pore size values for pure PVA obtained 0.0038 cm3 g−1 and 3.09 nm, respectively, while their values for NC 3 wt% were 0.0138 cm3 g−1 and 3.52 nm, respectively. These values are higher for NC 3 wt% than pure PVA. These results indicate that NC 3 wt% has a meso-porous structure, which is benefit for the adsorption performance.
 |
| | Fig. 10 Nitrogen adsorption–desorption isotherms of the pure PVA and NC 3 wt%. | |
Table 3 BET surface areas, pore volume and pore diameter for pure PVA and NC 3 wt%
| Sample |
BET surface areas (m2 g−1) |
Pore volume (m3 g−1) |
Pore size (nm) |
| Pure PVA |
0.0017 |
0.0038 |
3.09 |
| NC 3 wt% |
0.0063 |
0.0138 |
3.52 |
Pb(II) and Cd(II) adsorption property
Adsorbent dose is one of the parameters that influence the adsorption equilibrium. To examine the effect of adsorbent dose on Pb(II) and Cd(II) ions removal, various amounts of PVA/α-MnO2-L-valine NC 3 wt% from 0.02 g to 0.08 g is studied. Fig. 11 shows the effect of adsorbent dose on removal efficiency of Pb(II) and Cd(II) ions. The removal efficiency of Pb(II) and Cd(II) is increased with the increasing dosage of adsorbent. This is due to the available sites on NC base surface increase with an increasing NC base content and provide more sorption sites to adsorb Pb(II) and Cd(II) ions.
 |
| | Fig. 11 The effect of PVA/α-MnO2-L-valine NC 3% dose on Pb(II) and Cd(II) removal efficiency. | |
In general, the adsorption isotherms show the distribution of adsorbed molecules between the solid and liquid when the adsorption reaches equilibrium.45 Langmuir adsorption and Freundlich adsorption isotherm models are used to describe the Pb(II) and Cd(II) adsorption mechanism of the PVA/α-MnO2-L-valine NC (Fig. 12). The Langmuir isotherm assumes that a site can only occupied by one pollutant molecule, and the adsorbent surface is homogeneous, (eqn (3)).15
| | |
Ce/qe = 1/bqm + Ce/qm
| (3) |
where
qe is the adsorption capacity at equilibrium (mg g
−1),
Ce is the equilibrium concentration (mg L
−1),
qm is the maximum adsorption capacity (mg g
−1) and
b is Langmuir coefficient related to the affinity of the binding site (L mg
−1).
 |
| | Fig. 12 The Freundlich adsorption isotherm (A) and Langmuir adsorption isotherm (B) of PVA/α-MnO2-L-valine NC 3%. | |
The Freundlich model is often applicable to describe the models of multilayer adsorption onto the surface of heterogeneous site with different bond energy, (eqn (4)).14
where
k and
n are the Freundlich constants related to the sorption capacity and sorption intensity, respectively. The corresponding Langmuir and Freundlich parameters, along with the correlation coefficients, are reported in
Table 4. According to
Table 4, the Freundlich model appears to be the best fitting model for Pb(
II) and Cd(
II) sorption, indicating the heterogeneous nature of the multilayer adsorption of Pb(
II) and Cd(
II) on the PVA/α-MnO
2-
L-valine NC.
Table 4 Isotherm constants and values of R2 for PVA/α-MnO2-L-valine NC 3 wt%
| Langmuir isotherm |
Freundlich isotherm |
| Metal ion |
qm (mg g−1) |
b (L mg−1) |
R2 |
k (L g−1) |
n |
R2 |
| Pb(II) |
16.45 |
0.034 |
0.7568 |
1.07 |
1.72 |
0.9547 |
| Cd(II) |
46.30 |
0.011 |
0.8053 |
1.099 |
1.45 |
0.9255 |
Table 5 exhibits the comparison of parameters of Freundlich model for adsorption of Pb(II) and Cd(II) on various adsorbents. The mechanism of the adsorption of heavy metal ions in an aqueous solution can be by chelation (donation of the lone-pair electrons of the matrices to metal ions to form coordinate bonds).9 PVA has hydroxyl groups that can serve as chelating sites. For PVA/α-MnO2-L-valine NC 3 wt%, in addition to the hydroxyl groups related to PVA, the surface oxygen-containing functional group including hydroxyl (Mn–OH) and amine group (L-valine) are involved the adsorption process that can serve as chelating sites. So the mechanism of the adsorption of Pb(II) and Cd(II) ions in aqueous solution could be by chelation.
Table 5 The parameters of Freundlich model for adsorption of Pb(II) and Cd(II) on various adsorbents
| Metal ion |
Adsorbent |
k (L g−1) |
n |
Experimental variables |
Reference |
| Pb(II) |
CNTs |
0.18 |
0.940 |
Soaked in HNO3 |
29 |
| Pb(II) |
ZrO-kaolinite |
0.34 |
0.702 |
303 K, pH 5.7 |
30 |
| Pb(II) |
β-MnO2 |
1.78 |
0.635 |
293.15 K |
31 |
| Cd(II) |
Crosslinked chitosan/polyvinyl alcohol |
23.06 |
1.449 |
303 K, pH 6.0 |
32 |
| Cd(II) |
MnO2/o-MWCNTs |
9.616 |
2.045 |
295 K |
33 |
Conclusions
In this study, the solvothermal strategy was used for chemically modifying α-MnO2 nanorods with L-valine amino acid aiming to improve their properties. Different amounts of modified α-MnO2 nanorods (1, 3 and 5 wt%) were introduced into the PVA matrix for the preparation of PVA/α-MnO2-L-valine NC films. The XRD result indicated that tetragonal crystalline α-MnO2 was successfully synthesized. According to the XRD results, FE-SEM and TEM images the modification process had no effect on crystalline phase and morphological structure. TEM and FE-SEM images showed that the α-MnO2 and the modified α-MnO2 presented a rod-like morphology. The surface morphology of NCs was examined using FE-SEM and TEM analysis and images confirmed homogeneous dispersion of modified α-MnO2 in the PVA matrix. The BET results proved that NC 3 wt% had higher surface area, pore volume and pore size than pure PVA with meso-porous structure. In addition, thermal stability and mechanical resistance of the NCs were improved due to high dispersion of nanofiller in the PVA matrix and the existence of hydrogen bonding interaction between nanofiller and polymer. Also, NC 3 wt% as an adsorbent showed good adsorption for removal of Pb(II) and Cd(II) in aqueous solution and the obtained NCs may be have potential of other ion metals removal from water.
Acknowledgements
The authors would like to express their thanks to the Research Affairs Division Isfahan University of Technology (IUT). Also, further financial support from the Iran Nanotechnology Initiative Council (INIC), National Elite Foundation (NEF) is gratefully acknowledged.
References
- R. A. Hule and D. J. Pochan, MRS Bull., 2007, 32, 354–358 CrossRef CAS.
- M. Salavati-niasari and D. Ghanbari, in Advances in Diverse Industrial Applications of Nanocomposites, 2011, pp. 501–521 Search PubMed.
- S. Mallakpour and N. Jarang, J. Plast. Film Sheeting, 2015 DOI:10.1177/8756087915597024.
- T. Kida, T. Ohta, K. Kondo and M. Akashi, Polymer, 2014, 55, 2841–2847 CrossRef CAS.
- S. Mallakpour and A. Jarahiyan, J. Iran. Chem. Soc., 2016, 13, 509–518 CrossRef CAS.
- S. Mallakpour and F. Derakhshan, High Perform. Polym., 2015, 27, 458–468 CrossRef CAS.
- S. Mallakpour, M. Dinari and E. Azadi, Int. J. Polym. Anal. Charact., 2015, 20, 82–97 CrossRef CAS.
- B. Hui, Y. Zhang and L. Ye, J. Ind. Eng. Chem., 2015, 21, 868–876 CrossRef CAS.
- S. Wu, F. Li, H. Wang, L. Fu, B. Zhang and G. Li, Polymer, 2010, 51, 6203–6211 CrossRef CAS.
- B. Yin, S. Zhang, Y. Jiao, Y. Liu, F. Qu and X. Wu, CrystEngComm, 2014, 16, 9999–10005 RSC.
- E.-J. Kim, C.-S. Lee, Y.-Y. Chang and Y.-S. Chang, ACS Appl. Mater. Interfaces, 2013, 5, 9628–9634 Search PubMed.
- J. Shen, A. Liu, Y. Tu, H. Wang, R. Jiang, J. Ouyang and Y. Chen, Electrochim. Acta, 2012, 78, 122–132 CrossRef CAS.
- Y. Ren, N. Yan, J. Feng, J. Ma, Q. Wen, N. Lia and Q. Dong, Mater. Chem. Phys., 2012, 136, 538–544 CrossRef CAS.
- L. M. Camacho, R. R. Parra and S. Deng, J. Hazard. Mater., 2011, 189, 286–293 CrossRef CAS PubMed.
- Y. Guo, H. Guo, Y. Wang, L. Liu and W. Chen, RSC Adv., 2014, 4, 14048 RSC.
- H. Xu, Z. Qu, S. Zhao, J. Mei, F. Quan and N. Yan, J. Hazard. Mater., 2015, 299, 86–93 CrossRef CAS PubMed.
- R. Li, L. Liu, Y. Zhanga and F. Yang, RSC Adv., 2016, 6, 20542–20550 RSC.
- J. Mei and L. Zhang, RSC Adv., 2015, 5, 14843–14850 RSC.
- M. M. Kadam, K. B. Dhopte, N. Jha, V. G. Gaikar and P. R. Nemade, New J. Chem., 2016, 40, 1436–1442 RSC.
- Y. Wang, Y. Liu and I. Zhitomirsky, J. Mater. Chem. A, 2013, 1, 12519 RSC.
- Q. Yang, L. Dong, C. Xu and F. Kang, RSC Adv., 2016, 6, 12525–12529 RSC.
- K. Chen, Y. Dong Noh, K. Li, S. Komarneni and D. Xue, J. Phys. Chem. C, 2013, 117, 10770–10779 CrossRef CAS.
- S. Hassan, M. Suzuki and A. A. El-Moneim, J. Power Sources, 2014, 246, 68–73 CrossRef CAS.
- X. Wang and L. Yadong, Chem.–Eur. J., 2003, 9, 300–306 CrossRef CAS PubMed.
- H. J. Kim, J. B. Lee, Y.-M. Kim, M.-H. Jung, Z. Jagličić, P. Umek and J. Dolinšek, Nanoscale Res. Lett., 2007, 2, 81–86 CrossRef CAS.
- X. Su, X. Yang, L. Yu, G. Cheng, H. Zhang, T. Lin and F.-H. Zhao, CrystEngComm, 2015, 17, 5970–5977 RSC.
- S. Mallakpour, K. Banihassan and M. R. Sabzalian, J. Polym. Environ., 2013, 21, 568–574 CrossRef CAS.
- Z. Fereshteh, F. Mallakpour, M. Fathi, S. Mallakpour and A. Bagri, Ceram. Int., 2015, 41, 10079–10086 CrossRef CAS.
- A. Stafiej and K. Pyrzynska, Sep. Purif. Technol., 2007, 58, 49–52 CrossRef CAS.
- S. S. Gupta and K. G. Bhattacharyya, Appl. Clay Sci., 2005, 30, 199–208 CrossRef.
- D. Zhaoa, X. Yanga, H. Zhanga, C. Chena and X. Wang, Chem. Eng. J., 2010, 164, 49–55 CrossRef.
- M. Kumar, B. P. Tripathi and V. K. Shahi, J. Hazard. Mater., 2009, 172, 1041–1048 CrossRef CAS PubMed.
- C. Luo, R. Wei, D. Guo, S. Zhang and S. Yan, Chem. Eng. J., 2013, 225, 406–415 CrossRef CAS.
- X. Wang and Y. Li, Chem. Commun., 2002, 764–765 RSC.
- Y. Du, L. Wang, J. Wang, G. Zheng, J. Wu and H. Dai, J. Environ. Sci., 2015, 29, 71–81 CrossRef PubMed.
- S. Mallakpour, A. Abdolmaleki and S. E. Moosavi, Polym.-Plast. Technol. Eng., 2015, 54, 1448–1456 CrossRef CAS.
- M. K. Sangeetha, M. Mariappan, G. Madhurambal and S. C. Mojumdar, J. Therm. Anal. Calorim., 2015, 119, 907–913 CrossRef CAS.
- S. Mallakpour, A. Abdolmaleki, Z. Khalesi and S. Borandeh, Polymer, 2015, 81, 140–150 CrossRef CAS.
- S. Mallakpour and M. Dinari, J. Macromol. Sci., Part B: Phys., 2013, 52, 1651–1661 CrossRef.
- P. Sen, A. De, A. D. Chowdhury, S. K. Bandyopadhyay, N. Agnihotri and M. Mukherjee, Electrochim. Acta, 2013, 108, 265–273 CrossRef CAS.
- K. J. Bell, P. A. Brooksby, M. I. J. Polson and A. J. Downard, Chem. Commun., 2014, 50, 13687–13690 RSC.
- S. Mallakpour and M. Dinari, J. Reinf. Plast. Compos., 2013, 32, 217–224 CrossRef.
- S. Mallakpour and F. Marefatpour, Prog. Org. Coat., 2015, 85, 60–67 CrossRef CAS.
- K. Kai, Y. Yoshida, H. Kageyama, G. Saito, T. Ishigaki, Y. Furukawa and J. Kawamata, J. Am. Chem. Soc., 2008, 130, 15938–15943 CrossRef CAS PubMed.
- L. Tan, J. Wang, Q. Liu, Y. Sun, X. Jing, L. Liu, J. Liu and D. Song, New J. Chem., 2015, 39, 868–876 RSC.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 99% hydrolysis), L-valine and hydrate manganese sulfate (MnSO4·H2O) were obtained from Merck Chemical Co. (Germany). Lead(II) nitrate was purchased from Cica-Reagent (Tokyo, Japan). Cadmium-nitrate-4-hydrate (Cd(NO3)2·4H2O) was purchased from Riedel-de Haën (Seelze, Germany).
000 g mol−1 and 99% hydrolysis), L-valine and hydrate manganese sulfate (MnSO4·H2O) were obtained from Merck Chemical Co. (Germany). Lead(II) nitrate was purchased from Cica-Reagent (Tokyo, Japan). Cadmium-nitrate-4-hydrate (Cd(NO3)2·4H2O) was purchased from Riedel-de Haën (Seelze, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture was transferred to sealed Teflon-lined autoclave, followed by a solvothermal reaction at 100 °C for 24 h. After the autoclave was naturally cooled to room temperature, modified α-MnO2 nanorods were isolated by centrifugation and washed with deionized water and ethanol. Then, the product dried at 60 °C in vacuum.
1). The mixture was transferred to sealed Teflon-lined autoclave, followed by a solvothermal reaction at 100 °C for 24 h. After the autoclave was naturally cooled to room temperature, modified α-MnO2 nanorods were isolated by centrifugation and washed with deionized water and ethanol. Then, the product dried at 60 °C in vacuum.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. Two absorption peaks at 1628 and 1457 cm−1 are attributed to the bending vibration of N–H group and stretching vibration of C–N group, respectively and also can be attributed to carboxylate anion (COO−) asymmetric and symmetric stretching bonds. The peaks at 490 and 445 cm−1 could be attributed to the Mn–O bending vibration and the peak at 595 cm−1 is attributed to bending vibration of N–H (–NH3+) group. The FT-IR spectrum of α-MnO2/L-valine nanorods suggests that L-valine molecules were chemically bound on the MnO2 nanorods surfaces rather than simply adsorbed. The FT-IR spectra of pure PVA and PVA/α-MnO2-L-valine amino acid NCs are demonstrated in Fig. 1. In spectrum of pure PVA, the broad band peak at 3400 cm−1 showed the –OH stretching vibration of hydroxyl groups in PVA. Also, this spectrum represented peaks at 2941, 1420 and 1093 cm−1 that are attributed to C–H stretching, C–H bending and C–O stretching, respectively. The peak observed at 1716 cm−1 is due to the carbonyl functional groups of the remaining acetate groups during the fabricating of PVA from hydrolysis of poly(vinyl acetate).36 In spectrum of PVA/α-MnO2-L-valine amino acid NCs absorption band around 480 and 590 cm−1 could be attributed to the vibration of Mn–O group and N–H bending (–NH3+) which increase with α-MnO2/L-valine nanorods percent. The peak around 1710 cm−1 is attributed to the vibration of C
O stretching vibration. Two absorption peaks at 1628 and 1457 cm−1 are attributed to the bending vibration of N–H group and stretching vibration of C–N group, respectively and also can be attributed to carboxylate anion (COO−) asymmetric and symmetric stretching bonds. The peaks at 490 and 445 cm−1 could be attributed to the Mn–O bending vibration and the peak at 595 cm−1 is attributed to bending vibration of N–H (–NH3+) group. The FT-IR spectrum of α-MnO2/L-valine nanorods suggests that L-valine molecules were chemically bound on the MnO2 nanorods surfaces rather than simply adsorbed. The FT-IR spectra of pure PVA and PVA/α-MnO2-L-valine amino acid NCs are demonstrated in Fig. 1. In spectrum of pure PVA, the broad band peak at 3400 cm−1 showed the –OH stretching vibration of hydroxyl groups in PVA. Also, this spectrum represented peaks at 2941, 1420 and 1093 cm−1 that are attributed to C–H stretching, C–H bending and C–O stretching, respectively. The peak observed at 1716 cm−1 is due to the carbonyl functional groups of the remaining acetate groups during the fabricating of PVA from hydrolysis of poly(vinyl acetate).36 In spectrum of PVA/α-MnO2-L-valine amino acid NCs absorption band around 480 and 590 cm−1 could be attributed to the vibration of Mn–O group and N–H bending (–NH3+) which increase with α-MnO2/L-valine nanorods percent. The peak around 1710 cm−1 is attributed to the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. The peak around 1650 cm−1 could be ascribed to banding vibration of N–H group and the bending vibrations of –OH group attached to the Mn atoms.
O group. The peak around 1650 cm−1 could be ascribed to banding vibration of N–H group and the bending vibrations of –OH group attached to the Mn atoms.






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln
qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + 1/n
k + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce








