An AIE based tetraphenylethylene derivative for highly selective and light-up sensing of fluoride ions in aqueous solution and in living cells†
Guoyu Jianga,
Xiang Liua,
Yongquan Wua,
Jianguo Wang*a,
Xiaobiao Dongb,
Guanxin Zhang*b,
Yongdong Li*a and
Xiaolin Fanac
aKey Laboratory of Organo-Pharmaceutical Chemistry, Gannan Normal University, Ganzhou 341000, P. R. China. E-mail: wangjg@iccas.ac.cn; Tel: +86 797 8393536
bOrganic Solids Laboratory, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China
cMaterial and Chemical Engineering Department, Pingxiang University, Pingxiang 337055, P. R. China
First published on 15th June 2016
Abstract
An AIE based tetraphenylethylene derivative (MOTIPS-TPE) with a pyridinium pendant was synthesized for light-up sensing of F− in aqueous solution without additives. The fluoride ion promoted cleavage reaction gave MOTIPS-TPE high selectivity toward F− over other common anions. Moreover, MOTIPS-TPE can be used to monitor F− in living HeLa cells.
The development of methods to sense fluoride ions has emerged as a topical target in the field of chemical sensors,1 not only because of the preventive and protective roles it plays in dental health and in the treatment of osteoporosis,2 but also because of the risks of overexposure to high levels of fluoride, which may result in dental and skeletal fluorosis, neurological and metabolic dysfunctions, osteosarcoma, lower IQ, and more recently also cancers.3 Among the well-established fluoride sensing techniques, including ion chromatography,4 19F NMR,5 electrochemical method,6 colorimetric and fluorescent sensing, HPLC7 and fluorimetry,8 fluorescent sensors are of great interest for their convenience, high sensitivity, simple handling procedure, and inexpensive instruments. Up to now, scientists have developed various fluoride fluorescent sensors or sensing systems based on the interactions between fluoride and Lewis acids, using the hydrogen-bond interactions, and also reaction based sensors.1 Reaction based sensors for fluoride, usually making use of fluoride promoted cleavage reaction, is characterized by their irreversibility and high selectivity.1 Unfortunately, most of these reported fluorescent sensors can only detect fluoride ion in a tetrabutylammonium fluoride (TBAF) salt in organic solvents or aqueous solutions with the presence of a certain amount of surfactant, which will limit their applications in the living cells. Only a few reported fluorescent sensors can detect inorganic fluoride in fully aqueous systems or biological systems without any additives, probably because of the small size, high electronegativity, and high hydration enthalpy of fluoride ion.9 Thus, to make sensors applicable in aqueous solution or biological samples remains one of the most challenging problems in the field of fluoride sensing.
Most reported fluorescent sensors for fluoride ion are designed using traditional fluorophores whose emission is often quenched at high concentration, which is well known as the aggregation caused quenching (ACQ). Researchers are forced to use dilute solutions of sensors to avoid the quenching of emission at high concentrations, which significantly reduced the label-to-analyte ratio.10 In recent years, tetraphenylethylene (TPE) compounds are attracting increasing interest because of their unique emission properties opposite to ACQ molecules. In contrast to traditional fluorophores which show aggregation-induced quenching of fluorescence, TPE derivatives are weakly or non-emissive in the solution state, while highly emissive in the aggregation state. This phenomenon is called aggregation induced emission (AIE) effect by Tang and co-workers.11 By manipulating aggregation/disaggregation, various fluorescent turn-on sensors based on TPE derivatives were fabricated for sensitively detection of metal ions, sugars, proteins or anions.12
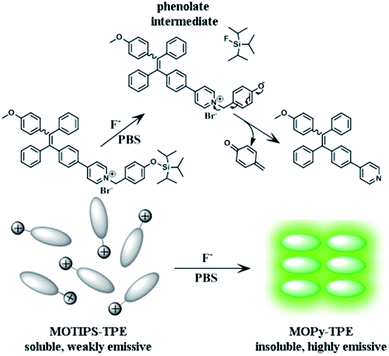
Herein, we report a tetraphenylethylene based AIE light-up sensor for fluoride ion detection in aqueous samples and in living cells. As shown in Scheme 1, MOTIPS-TPE is designed to bear a positively charged pyridinium pendant, which rendered the whole fluorophore solubility in water. A triisopropylsily group, which can be cleaved specifically by fluoride ion, was conjugated to the terminal of the pyridinium pendant. Fluoride ion promoted cleavage of the triisopropylsily group resulted in phenolate intermediate, which can spontaneously undergo 1,6-elimination of p-quinone-methide to generate the water insoluble MOPy-TPE. As a result, aggregation will occur and turn on the fluorescence of the TPE moiety based on the AIE effect.
The synthesis of MOTIPS-TPE started with the preparation of monobromo-, monomethoxy-substituted tetraphenylethylene derivative (compound 1) according to reported method.13 Palladium-catalysed Suzuki coupling reaction of 1 and 4-pyridinylboronic acid yielded MOPy-TPE (compound 2), which was allowed to further react with (4-(bromomethyl)phenoxy)triisopropylsilane (compound 3) in toluene to afford MOTIPS-TPE (Scheme 2). The chemical structure of MOTIPS-TPE was confirmed by 1H NMR, 13C NMR, and HRMS.
As expected, the solution of MOTIPS-TPE showed very weak emission in PBS buffered solution (Fig. 1a, curve i). The fluorescence intensity gradually enhanced as the incubation time of MOTIPS-TPE with F− increased and levelled off after 15 min, accompanied by the emission peak progressively blue-shifted from 536 nm to 504 nm (Fig. S1†). As anticipated, the fluoride ion promoted cleavage of the triisopropylsily group in MOTIPS-TPE would generate MOPy-TPE. To verify this, absorption spectra of MOTIPS-TPE before and after incubation with fluoride ion were compared to those of MOPy-TPE. As shown in Fig. S2,† the spectra of MOTIPS-TPE and MOPy-TPE exhibited absorption peaks at 384 nm and 343 nm respectively; while after incubation with F− at 37 °C for 15 min in PBS, the absorption peak of MOTIPS-TPE shifted to 341 nm, which is in accordance with that of MOPy-TPE. High resolution MS also exhibited the [M + H]+ peak of MOPy-TPE after 15 min incubation of MOTIPS-TPE with F− (Fig. S3†).
When MOTIPS-TPE was incubated with increasing concentrations of fluoride ion in PBS, the emission intensity at 504 nm, which is the characteristic emission peak of MOPy-TPE, was gradually enhanced. The plot of fluorescence intensity ratio (I − I0)/I0 at 504 nm versus the fluoride ion concentration displayed a good linear relationship (R2 = 0.996) with a fluoride ion concentration range from 2.5 to 5 μM (Fig. 1b and the inset), resulting the detection limit of fluoride ion in PBS low to 90 nM (n = 12, S/N = 3). Obviously, MOTIPS-TPE is a very promising probe for detection of fluoride ion in environmental and biological systems since exposure to high concentration of fluoride ion is quite dangerous (0.1 mM for drinking water and over 3 mM for biological systems).14
We then evaluated the selectivity of MOTIPS-TPE toward other common anions under the same experimental condition. As shown in Fig. 2, addition of various anions such as Cl−, Br−, I−, Ac−, H2PO4−, HPO42−, BrO3−, NO2−, NO3−, OH−, and SO42− had negligible effect on the fluorescence spectra of MOTIPS-TPE, indicating the high selectivity of MOTIPS-TPE for fluoride anion.
 | ||
| Fig. 2 The fluorescence responses of MOTIPS-TPE (4 μM in 5 mM PBS) in PBS toward various anions (20 μM in 5 mM PBS) (Eex = 344 nm, Eex/Eem slit = 2.0/5.0 nm). | ||
As shown in Scheme 1, we have expected that the fluorescence turn on sensing of fluoride ion in aqueous solution results from the fluoride ion promoted cleavage of triisopropylsily group in MOTIPS-TPE and the subsequent generation of the water insoluble MOPy-TPE, which can form fluorescent aggregates in aqueous solution. To verify this, both fluorescence imaging and dynamic light scattering (DLS) experiments were carried out. As illustrated in Fig. S4,† the solution of MOTIPS-TPE exhibited negligible emission before incubation with fluoride ion. However, after incubation, green emissive aggregates can be clearly observed from the fluorescence images. DLS data also indicated the formation of 400–1100 nm aggregates for the solution of MOTIPS-TPE incubated 15 min with fluoride ion (Fig. S5†).
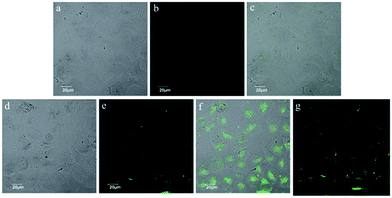
Based on the abovementioned results, we try to test the applicability of MOTIPS-TPE for sensing fluoride ion in living cells. Firstly, we evaluated the cytotoxicity of MOTIPS-TPE for HeLa cells using MTT method. As displayed in Fig. S6,† cell viability was barely affected by 5 μM of MOTIPS-TPE, and the IC50 value was estimated to be 15 μM, much higher than those used in our detection experiments. Based on the relatively low cytotoxicity of MOTIPS-TPE, we tested the applicability of MOTIPS-TPE in monitoring fluoride ion in living HeLa cells using confocal fluorescence microscopy. As we can see from Fig. 3b, MOTIPS-TPE showed little green emission in the absence of fluoride ion. While after incubation with 20 μM of fluoride ion, intensive emission can be observed within HeLa cells (Fig. 3e). Overlay of the confocal fluorescence and bright-filed images demonstrated that the bright emission was evident in the cytoplasm (Fig. 3f), which was further confirmed by Z-scan confocal microscopy (Fig. 3g). All these results demonstrated that MOTIPS-TPE had good cell membrane permeability and can easily enter the living cells, indicating that MOTIPS-TPE can be used to monitor fluoride ion in living cells.
Furthermore, we also used MOTIPS-TPE for the detection of fluoride ion in tap water. Tap water was diluted properly and analysed with MOTIPS-TPE method. The results were shown in Table 1, which agree well with those obtained by fluoride ion selective electrode (ISE) standard method.
Conclusions
A tetraphenylethylene derivative with a pyridinium pendant has been synthesized. The pyridinium moiety rendered MOTIPS-TPE some solubility in water, and as a result, the solution of MOTIPS-TPE exhibited weak emission in PBS. The fluoride ion promoted cleavage of the triisopropylsily group in MOTIPS-TPE accompanied by the subsequent elimination of p-quinone-methide generated MOPy-TPE with poor water solubility. Thus, aggregation occurred and turned on the emission of the solution. The fluorescence turn-on detection of fluoride possesses the following features: (1) the probe MOTIPS-TPE can be easily prepared; (2) the detection of fluoride ion can be applied in aqueous solution without any surfactant additives; (3) 90 nM of fluoride ion (in NaF salt) can be detected and the interference of other common anion can be avoided; (4) MOTIPS-TPE can also be used to detect fluoride ion in living HeLa cells. All these results indicated high application potential of MOTIPS-TPE for fluoride ion sensing in environmental and biological systems.Acknowledgements
We thank National Natural Science Foundation of China (21301182, 21502022), the Natural Science Foundation of Jiangxi Province (20151BAB213012), Science and Technology Project of Jiangxi Provincial Department of Education (GJJ150987), Beijing National Laboratory for Molecular Sciences (20140117), the Key Laboratory of Photochemical Conversion and Optoelectronic Materials, TIPC, CAS, the 2015 Bidding Project of Gannan Normal University for the financial support.Notes and references
- (a) M. Cametti and K. Rissanen, Chem. Commun., 2009, 45, 2809 RSC; (b) M. Cametti and K. Rissanen, Chem. Soc. Rev., 2013, 42, 2016 RSC; (c) H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79 RSC; (d) P. A. Gale, S. E. García-Garrido and J. Garric, Chem. Soc. Rev., 2008, 37, 151 RSC; (e) Y. Zhou, J. F. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511 CrossRef CAS PubMed; (f) T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094 CrossRef CAS; (g) C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520 RSC; (h) S. Kubik, Chem. Soc. Rev., 2009, 38, 585 RSC; (i) R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419 CrossRef PubMed; (j) P. A. Gale and C. Caltagirone, Chem. Soc. Rev., 2015, 44, 4212 RSC; (k) T. D. Ashton, K. A. Jolliffe and F. M. Pfeffer, Chem. Soc. Rev., 2015, 44, 4547 RSC; (l) C. Ribeiro, P. Brogueira, G. Lavareda, C. N. Carvalho, A. Amaral, L. Santos, J. Morgado, U. Scherf and V. D. B. Bonifácio, Biosens. Bioelectron., 2010, 26, 1662 CrossRef CAS PubMed; (m) V. D. B. Bonifácio, J. Morgado and U. Scherf, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2878 CrossRef.
- (a) L. K. Kirk, Biochemistry of the Halogens and Inorganic Halides, Plenum Press, New York, 1991 CrossRef; (b) M. Kleerekoper, Endocrinol. Metab. Clin. North Am., 1998, 27, 441 CrossRef CAS PubMed; (c) D. Briancon, Rev. Rhum. Mal. Osteo-Articulaires, 1997, 64, 78 CAS.
- (a) A. Wiseman, Handbook of Experimental Pharmacology XX/2, Springer-Verlag, Berlin, 1970, Part 2, pp. 48–97 Search PubMed; (b) J. A. Weatherall, Pharmacology of Fluorides, in Handbook of Experimental Pharmacology XX/1, Springer-Verlag, Berlin, 1969, Part 1, pp. 141–172 Search PubMed; (c) R. H. Dreisbuch, Handbook of Poisoning, Lange Medical Publishers, Los Altos, CA, 1980 Search PubMed; (d) M. L. Cittanova, B. Lelongt and M. C. Verpont, Anesthesiology, 1996, 84, 428 CrossRef CAS PubMed; (e) P. P. Singh, M. K. Barjatiya, S. Dhing, R. Bhatnagar, S. Kothari and V. Dhar, Urol. Res., 2001, 29, 238 CrossRef CAS PubMed; (f) T.-J. Chen, T.-M. Chen, C.-H. Chen and Y.-K. Lai, J. Cell. Biochem., 1998, 69, 221 CrossRef; (g) M. H. Arhima, O. P. Gulati and S. C. Sharma, Phytother. Res., 2004, 18, 244 CrossRef CAS PubMed; (h) S. Ayoob and A. K. Gupta, Crit. Rev. Environ. Sci. Technol., 2006, 36, 433 CrossRef CAS; (i) E. B. Bassin, D. Wypij and R. B. Davis, Cancer, Causes Control, Pap. Symp., 2006, 17, 421 CrossRef PubMed; (j) S.-X. Wang, Z.-H. Wang, X.-T. Cheng, J. Li, Z.-P. Sang, X.-D. Zhang, L.-L. Han, X. Y. Qiao, Z.-M. Wu and Z.-Q. Wang, Environ. Health Perspect., 2006, 115, 643 CrossRef PubMed; (k) Y. Yu, W. Yang, Z. Dong, C. Wan, J. Zhang, J. Liu, K. Xiao, Y. Huang and B. Lu, Fluoride, 2008, 41, 134 CAS.
- (a) M. T. S. D. Vasconcelos, C. A. R. Gomes and A. A. S. C. Machado, J. Chromatogr. A, 1994, 685, 53 CrossRef CAS; (b) O. P. Kalyakina and A. M. Dolgonosov, J. Anal. Chem., 2003, 58, 951 CrossRef CAS; (c) S. Thangavel, K. Dash, S. M. Dhavile, S. C. Chaurasia and T. Mukherjee, J. Chromatogr. A, 2005, 1074, 299 CrossRef; (d) E. N. Kapinus, I. A. Revelsky, V. O. Ulogov and A. Yu, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 800, 321 CrossRef CAS.
- P. Konieczka, B. Zygmunt and J. Namiesnik, Bull. Environ. Contam. Toxicol., 2000, 64, 794 CrossRef CAS PubMed.
- (a) P. Cosentino, B. Grossman, C. Shieh, S. Doi, H. Xi and P. Erbland, J. Geotech. Eng., 1995, 121, 610 CrossRef; (b) A. Ruiz-Payan, M. Ortiz and M. Duarte-Gardea, Microchem. J., 2005, 1, 19 CrossRef; (c) V. Capka, C. P. Bowers, J. N. Narvesen and R. E. Rossi, Talanta, 2004, 64, 869 CrossRef CAS PubMed; (d) H. Hirokazu, Y. Keiko, H. Mayumi and O. Masahiro, Anal. Chim. Acta, 1998, 364, 117 CrossRef.
- X. R. Xu, H. B. Li, J. D. Gu and K. J. Paeng, Chromatographia, 2004, 59, 745 CrossRef CAS.
- (a) M. Garrido, A. G. Lista, M. Palomeque and B. S. F. Band, Talanta, 2002, 58, 849 CrossRef CAS PubMed; (b) J. Nishimoto, T. Yamada and M. Tabata, Anal. Chim. Acta, 2001, 428, 201 CrossRef CAS; (c) G. Sivaraman and D. Chellappa, J. Mater. Chem. B, 2013, 1, 5768 RSC.
- (a) Y.-C. Huang, C.-P. Chen, P.-J. Wu, S.-Y. Kuo and Y.-H. Chan, J. Mater. Chem. B, 2014, 2, 6188 RSC; (b) B. Zhu, F. Yuan, R. Li, Y. Li, Q. Wei, Z. Ma, B. Du and X. Zhang, Chem. Commun., 2011, 47, 7098 RSC; (c) S. Y. Kim, J. Park, M. Koh, S. B. Park and J. I. Hong, Chem. Commun., 2009, 45, 4735 RSC; (d) X. Wu, X.-X. Chen, B.-N. Song, Y.-J. Huang, W.-J. Ouyang, Z. Li, T. D. James and Y. B. Jiang, Chem. Commun., 2014, 50, 13987 RSC; (e) G. Wei, J. Yin, X. Ma, S. Yu, D. Wei and Y. Du, Anal. Chim. Acta, 2011, 703, 219 CrossRef CAS PubMed; (f) R. Badugu, J. R. Lakowicz and C. D. Geddes, Sens. Actuators, B, 2005, 104, 103 CrossRef CAS; (g) P. Sokkalingam and C. H. Lee, J. Org. Chem., 2011, 76, 3820 CrossRef CAS PubMed; (h) B. Sui, B. Kim, Y. Zhang, A. Frazer and K. D. Belfield, ACS Appl. Mater. Interfaces, 2013, 5, 2920 CrossRef CAS PubMed; (i) A. Roy, D. Kand, T. Saha and P. Talukdar, RSC Adv., 2014, 4, 33890 RSC.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed.
- (a) R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228 RSC; (b) K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570Z RSC; (c) J. Zhao, S. M. Chen, J. W. Y. Lam, P. Lu, Y. C. Zhong, K. S. Wong, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 2221 RSC; (d) V. S. Vyas and R. Rathore, Chem. Commun., 2010, 46, 1065 RSC; (e) Z. J. Zhao, C. M. Deng, S. M. Chen, J. W. Y. Lam, W. Q. P. Lu, Z. M. Wang, H. S. Kwok, Y. G. Ma, H. Y. Qiu and B. Z. Tang, Chem. Commun., 2011, 47, 8847 RSC; (f) B. J. Xu, Z. G. Chi, X. Q. Zhang, H. Y. Li, C. J. Chen, S. W. Liu, Y. Zhang and J. R. Xu, Chem. Commun., 2011, 47, 11080 RSC; (g) N. B. Shustova, B. D. McCarthy and M. Dinc, J. Am. Chem. Soc., 2011, 133, 20126 CrossRef CAS PubMed; (h) Z. J. Zhao, P. Lu, J. W. Y. Lam, Z. M. Wang, C. Y. K. Chan, H. H. Y. Sung, I. D. Williams, Y. G. Ma and B. Z. Tang, Chem. Sci., 2011, 2, 672 RSC; (i) L. Liu, G. X. Zhang, J. F. Xiang, D. Q. Zhang and D. B. Zhu, Org. Lett., 2008, 10, 4581 CrossRef CAS PubMed; (j) L. H. Peng, G. X. Zhang, D. Q. Zhang, J. F. Xiang, R. Zhao, Y. L. Wang and D. B. Zhu, Org. Lett., 2009, 11, 4014 CrossRef CAS PubMed; (k) M. Wang, X. G. Gu, G. X. Zhang, D. Q. Zhang and D. B. Zhu, Anal. Chem., 2009, 81, 4444 CrossRef CAS PubMed; (l) W. Xue, G. Zhang, D. Zhang and D. Zhu, Org. Lett., 2010, 12, 2274 CrossRef CAS PubMed; (m) M. Wang, G. X. Zhang, D. Q. Zhang, D. B. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858 RSC; (n) Y. N. Hong, H. Xiong, J. W. Y. Lam, M. Haussler, J. Z. Liu, Y. Yu, Y. C. Zhong, H. H. Y. Sung, I. D. Williams, K. S. Wong and B. Z. Tang, Chem.–Eur. J., 2010, 16, 1232 CrossRef CAS PubMed; (o) M. Faisal, Y. N. Hong, J. Z. Liu, Y. Yu, J. W. Y. Lam, A. J. Qin, P. Lu and B. Z. Tang, Chem.–Eur. J., 2010, 16, 4266 CrossRef CAS PubMed; (p) Y. Liu, C. M. Deng, L. Tang, A. J. Qin, R. R. Hu, J. Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2011, 133, 660 CrossRef CAS PubMed; (q) Y. N. Hong, S. J. Chen, C. W. T. Leung, J. W. Y. Lam, J. Z. Liu, N. W. Tseng, R. T. K. Kwok, Y. Yu, Z. K. Wang and B. Z. Tang, ACS Appl. Mater. Interfaces, 2011, 3, 3411 CrossRef CAS PubMed.
- (a) Y. L. Wu, S. L. Huang, F. Zeng, J. Wang, C. M. Yu, J. Huang, H. T. Xie and S. Z. Wu, Chem. Commun., 2015, 51, 12791 RSC; (b) C. M. Yu, Y. L. Wu, F. Zeng, X. Z. Li, J. B. Shi and S. Z. Wu, Biomacromolecules, 2013, 14, 4507 CrossRef CAS PubMed; (c) W. Shen, J. J. Yu, J. Y. Ge, R. Y. Zhang, F. Cheng, X. F. Li, Y. Fan, S. A. Yu, B. Liu and Q. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 927 CrossRef CAS PubMed; (d) Y. Y. Yuan, C.-J. Zhang, S. D. Xu and B. Liu, Chem. Sci., 2016, 7, 1862 RSC; (e) G. Huang, G. Zhang and D. Zhang, Chem. Commun., 2012, 48, 7504 RSC; (f) F. Sun, G. Zhang, D. Zhang, L. Xue and H. Jiang, Org. Lett., 2011, 13, 6378 CrossRef CAS PubMed; (g) D. Li and Y. Zheng, Chem. Commun., 2011, 47, 10139 RSC; (h) Y. Liu, C. Deng, L. Tang, A. Qin, R. Hu, J. Sun and B. Tang, J. Am. Chem. Soc., 2011, 133, 660 CrossRef CAS PubMed; (i) Q. Chen, N. Bian, C. Cao, X. Qiu, A. Qi and B. Han, Chem. Commun., 2010, 46, 4067 RSC; (j) X. Xu, J. Huang, J. Li, J. Yan, J. Qin and Z. Li, Chem. Commun., 2011, 47, 12385 RSC; (k) R. Zhang, M. Gao, S. Bai and B. Liu, J. Mater. Chem. B, 2015, 3, 1590 RSC; (l) Y. Yuan, S. Xu, C.-J. Zhang, R. Zhang and B. Liu, J. Mater. Chem. B, 2016, 4, 169–176 RSC; (m) H. Wang, G. Liu, S. Dong, J. Xiong, Z. Du and X. Cheng, J. Mater. Chem. B, 2015, 3, 7401 RSC; (n) G. Jiang, J. Wang, Y. Yang, G. Zhang, Y. Liu, H. Lin, G. Zhang, Y. Li and X. Fan, Biosens. Bioelectron., 2016, 85, 62 CrossRef CAS PubMed; (o) S. Samanta, U. Manna, T. Ray and G. Das, Dalton Trans., 2015, 44, 18902 RSC; (p) B. H. Choi, J. H. Lee, H. Hwang, K. M. Lee and M. H. Park, Organometallics, 2016, 35, 1771 CrossRef CAS; (q) A. Chatterjee, D. G. Khandare, P. Saini, A. Chattopadhyay, M. S. Majik and M. Banerjee, RSC Adv., 2015, 5, 31479 RSC; (r) H. Zhou, F. Liu, X. Wang, H. Yan, J. Song, Q. Ye, B. Z. Tang and J. Xu, J. Mater. Chem. C, 2015, 3, 5490 RSC; (s) W. Zhang, J. Kang, P. Li, H. Wang and B. Tang, Anal. Chem., 2015, 87, 8964 CrossRef CAS PubMed; (t) D. G. Khandare, H. Joshi, M. Banerjee, M. S. Majik and A. Chatterjee, Anal. Chem., 2015, 87, 10871 CrossRef CAS PubMed; (u) V. Mahendran, K. Pasumpon, S. Thimmarayaperumal, P. Thilagar and S. Shanmugam, J. Org. Chem., 2016, 81, 3597 CrossRef CAS PubMed.
- (a) X. Gu, J. Yao, G. Zhang, C. Zhang, Y. Yan, Y. Zhao and D. Zhang, Chem.–Asian J., 2013, 8, 2362 CrossRef CAS PubMed; (b) F. Hu, G. Zhang, C. Zhan, W. Zhang, Y. Yan, Y. Zhao, H. Fu and D. Zhang, Small, 2015, 11, 1335 CrossRef CAS PubMed.
- (a) H. Matsui, M. Morimoto, K. Horimoto and Y. Nishimura, Toxicol. In Vitro, 2007, 21, 1113 CrossRef CAS PubMed; (b) R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedure, characterization and supporting figures. See DOI: 10.1039/c6ra10878d |
| This journal is © The Royal Society of Chemistry 2016 |