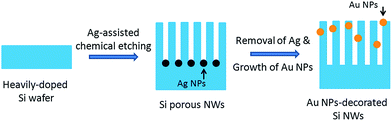
Surface plasmon enhanced photoluminescence from porous silicon nanowires decorated with gold nanoparticles†
Haiping Tang*a,
Chao Liub and
Haiping Heb
aInstitute of Mechanical Engineering, Baoji University of Arts and Sciences, Baoji 721007, China. E-mail: thp315@163.com
bSchool of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
First published on 6th June 2016
Abstract
We demonstrate ∼8-fold photoluminescence enhancement in porous Si nanowires via coupling of the surface plasmon of Au nanoparticles. We find that the formation of an insulating SiOx layer during the Au nanoparticle growth is essential to the photoluminescence enhancement. A schematic energy-level diagram is proposed to interpret the experimental results.
Introduction
Luminescent silicon materials have attracted great attention owing to their unique properties and promising applications in high performance optoelectronic devices.1–4 In the past decade, various novel Si nanostructures such as Si nanocrystals5–9 and Si nanowires10–17 have been synthesized, which show tunable photoluminescence (PL). In particular, porous Si nanowires13–17 can be prepared in a large scale simply by metal-assisted chemical etching of a Si wafer. Such nanowires may open up new opportunities for nanoscale optoelectronic devices taking advantages of their one-dimensional geometry and excellent combination of electrical and luminescence properties. Unfortunately, the improvement of luminescence efficiency is to some extend hindered by the very complex surface chemistry as well as the controversial PL origin of such nanostructures.4 Therefore, efficient approaches to the PL improvement are highly desired.Recently, surface plasmon (SP) coupling has emerged as a promising method for significant luminescence enhancement.18,19 It has been well established that the coupling between PL and the SP of metals (usually noble metals Au, Ag, Pt) can greatly enhance the luminescence internal quantum efficiency.18 So far it has been successfully applied to a lot of semiconductor films and nanostructures.18–22 Although there are many reported work23,24 on the SP-enhanced PL from Si quantum dots or nanoparticles, the work on porous Si nanowires is much less.25,26 In this work, we demonstrate that significant PL enhancement (∼8 folds) can be achieved via decorating gold nanoparticles onto the surface of porous Si nanowires. We find that the match between the PL wavelength of Si nanowires and the SP resonance of gold nanoparticles, and a separation layer of silicon oxide are essential to the PL enhancement. The role of silicon oxide formed during the growth of Au nanoparticles is also discussed.
Material and methods
Our experimental strategy is illustrated in Fig. 1. Firstly, porous Si nanowires are obtained by two-step Ag-assisted chemical etching of Si wafers. Secondly, the porous Si nanowires are decorated with Au nanoparticles by solution-growth. After growth, the samples are ready for PL measurements.Porous Si nanowire arrays were prepared by metal-assisted chemical etching of heavily-doped p-type silicon wafers. Detailed experimental procedures can be found in a previous work by Gan et al.17 In brief, the cleaned Si wafers with fresh H-terminated surface were first immersed into an aqueous solution of HF + AgNO3 for 1 min to deposit a layer of Ag nanoparticles on the Si surface. The wafers were then immersed into an aqueous solution of HF + H2O2 for etching to produce porous Si nanowire arrays. Finally, the samples were washed by HNO3 solution to remove the residual Ag nanoparticles. The length, orientation, porosity, and luminescence can be tailored by tuning the wafer type, concentration of etching solution, etching duration, etc.
The porous Si nanowire arrays were then transferred into an aqueous solution for Au nanoparticle growth. The solution was composed of 0.01 M HAuCl4, 0.1 M Na3C6H5O7, and 1.5 × 10−2 M cetyltrimethyl ammonium bromide (CTAB). The solution containing Si nanowires were then sealed and heated to 110 °C to trigger the Au nanoparticle growth for 4–48 h. Afterward, the Si nanowires were washed by deionized water and dried by nitrogen flow.
The morphology microstructure and of the samples were examined by field emission scanning electron microscope (FE-SEM, Hitachi S-4800) and transmission electron microscopy (TEM, Tecnai G2 F30 S-Twin). Optical absorption of Au nanoparticles was measured by a UV-vis spectrometer (UNICO-UV2100). PL measurements were performed on a FLS920 fluorescence spectrometer (Edinburgh Instruments) using 325 nm light from a He–Cd laser as the excitation source. All the measurements were taken at room temperature.
Results and discussion
In order to evaluate the properties of Au nanoparticles, we first synthesized bare Au nanoparticles in the absence of Si nanowires. TEM measurements reveal that the Au nanoparticles are mainly cubic in shape, with a few triangles and nanorods. The nanoparticle shape is almost independent on the growth time. Fig. 2a is a typical image for Au nanoparticles grown for 48 h. However, we find the yield of Au nanoparticles greatly depends on the growth time. This is evidenced by the intensity of Au SP resonance around 530 nm.27 As shown in Fig. 2b, for the sample grown for 4 h, no SP can be detected due to the very low concentration of Au nanoparticles. For 8 h-growth, the SP resonance can be observed but still very weak. For 24 h- and 48 h-growth, the SP resonance becomes much stronger, indicating a reasonable yield of Au nanoparticles. In this regard, we set the growth time of 24 and 48 h for the decoration of Au nanoparticles onto the Si nanowires. | ||
| Fig. 2 (a) Typical TEM image of Au nanoparticles grown for 48 h. (b) Optical absorption spectra of Au nanoparticles grown for different time. The surface plasmon resonance of Au is about 530 nm. | ||
Fig. 3 shows the morphology of the Si nanowires without and with Au nanoparticle decoration. SEM images show that the nanowire arrays are maintained after Au decoration. However, morphology change can still be clearly seen. As shown in Fig. 3b, the Si nanowires aggregate on the top. This is reasonable because our nanowires have length of more than 10 μm and diameter of ∼200 nm. They can easily form bundles due to the electrostatic charges on the surface during the deposition of Au nanoparticles. TEM measurements were carried out to check the decoration of Au nanoparticles. As shown in Fig. 3c, the as-prepared Si nanowires are very clean, with obvious porous surface. After Au growth, Au nanoparticles are attached onto the nanowires surface, indicated by the black dots in Fig. 3d. It can be seen that the Au nanoparticles distribute on the entire nanowire, with much nanoparticles on the top. These results suggest that Au nanoparticles have been successfully decorated onto the Si porous nanowires. Unfortunately, it is hard to determine the amount of Au nanoparticles on the nanowires because the mass of remaining Si wafer is much larger than that of the Si nanowires.
As have been reported in many literatures, porous Si nanowires prepared by metal-assisted chemical etching show orange-red luminescence. To realize good PL-SP coupling, tuning the PL wavelength of Si to match the SP resonance of Au nanoparticles is desired. For this sake, we try to tailor the PL of porous Si nanowires via tuning the etching conditions. According to our recent work,28 the PL of porous Si nanowires can be attributed to donor–acceptor pair (DAP) recombination. We note that such assignment is reasonable because the bandgap of the small Si nanocrystals in our nanowires may reach ∼2.5 eV according to the optical absorption spectra (Fig. S1 in ESI†). This is obviously larger than the emission peak energy, ∼1.8–2.0 eV. Therefore, it is possible to tune the PL via changing the doping concentration of the starting Si wafer, because the DAP energy depends on the pair distance29
 | (1) |
Fig. 5 shows the PL spectra of Si porous nanowires decorated with Au nanoparticles with 24 and 48 hours growth. Interestingly, for Au nanoparticles growth with 24 h the PL is greatly quenched (∼25-fold), while for the 48 h sample the PL is enhanced by about 8-fold. The enhancement ratio is higher than the reported 3–4-fold enhancement due to SP effect25,26 in Si nanowires. We note that although up to 94-fold PL enhancement has been reported in porous Si nanowires using AuAg bilayer as the etching catalyst, the authors suggested that the contribution of SP effect is only ∼5-fold.30 To study the quenching mechanism, we carried out control experiments to evaluate the influence of the immersion of Si nanowires in the precursor solution of Na3C6H5O7 + CTAB. We find the immersion results in ∼5-fold quenching of PL. The results indicate that the PL enhancement of the 48 h sample is due to SP coupling.
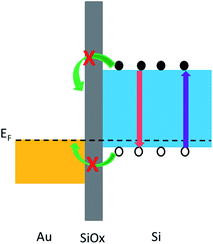
The control experiment also indicates that the great quenching of the 24 h sample is not merely due to the immersion. We suggest the main quenching mechanism is the charge transfer between Si and Au. Since we use heavily doped p-type Si wafer for etching (our previous results indicate that n-type Si wafer can hardly produce luminescent nanowires), charge transfer between Au and Si is possible because the work function of Au (∼5.0 eV) is close to the Si Fermi level, as illustrated in Fig. 6. Such charge transfer-induced PL quenching has been reported in other semiconductors.31,32 However, for the 48 h sample, the charge transfer can be blocked by the formation of a thin layer of insulating SiOx. The formation of SiOx is reasonable because the nanowires are kept in oxygen-containing solution at 110 °C for such long time, and can be evidenced either by the PL peak around 470 nm (ref. 33) as shown in Fig. 5, or by the XPS core level spectrum of Si. As shown in Fig. S2,† the dominant peak around 103.1 eV is characteristic for Si with +4 valence, indicating the formation of SiOx on the nanowire surface. We note that the presence of thin SiOx layer would not obviously affect the PL-SP coupling provided the SiOx thickness is less than 10 nm.32 For the 24 h sample, the SiOx should be too thin to effectively prevent the charge transfer. This is supported by the extremely weak PL around 470 nm in this sample. Unfortunately, in our cases it is hard to evaluate the thickness of SiOx layer due to the rather rough surface of the nanowires (Fig. S2†).
It was reported34 that the formation of SiO2 could increase the PL emission from porous Si nanowires. However, in our case we found that the formation of SiOx does not increase the PL. On contrary, the PL decreases dramatically. As shown in Fig. S3,† the PL emission of our Si nanowires decreases remarkably after oxidation with increasing temperature. At this moment we do not clearly know why our results are different from ref. 34. We hypothesize that the PL mechanism of the etched Si nanowires is rather complicated. Better understanding and elaborate control are thus needed for the future studies and possible applications.
Conclusions
In summary, porous Si nanowires array decorated with Au nanoparticles is prepared by two-step metal-assisted chemical etching of p-type Si wafers followed by aqueous solution growth of Au. The photoluminescence of Si nanowires can be tuned to ∼600 nm to match the surface plasmon resonance of Au nanoparticles. Both enhancement and quenching of PL can be observed for such samples depending on the growth time of Au nanoparticles. The surface plasmon-mediated PL enhancement is as high as ∼8-fold. We suggest that the formation of insulating SiOx layer during the Au nanoparticle growth is essential to the PL enhancement, which is rarely reported before. The role of SiOx is suggested to prevent the charge transfer between Au and Si nanowires according to the proposed energy level diagram. The results suggest that surface plasmon enhanced PL may find applications in nanoscale optoelectronic devices based on porous Si nanowires such as light-emitting devices and PL-based sensors.Acknowledgements
This work was supported by the Research Program in Baoji University of Arts and Sciences under Grant No. ZK072, and the National Natural Science Foundation of China under Grant No. 51372223.References
- A. G. Cullis and L. T. Canham, Nature, 1991, 353, 335 CrossRef CAS
.
- M. J. Sailor and E. C. Wu, Adv. Funct. Mater., 2009, 19, 3195 CrossRef CAS
.
- Z. H. Kang, Y. Liu and S. T. Lee, Nanoscale, 2011, 3, 777 RSC
.
- A. Sa'ar, J. Nanophotonics, 2009, 3, 032501 CrossRef
.
- W. D. A. M. de Boer, D. Timmerman, K. Dohnalova, I. N. Yassievich, H. Zhang, W. J. Buma and T. Gregorkiewicz, Nat. Nanotechnol., 2010, 5, 878 CrossRef CAS PubMed
.
- Z. H. Kang, Y. Liu, C. H. A. Tsang, D. D. D. Ma, X. Fan, N. B. Wong and S. T. Lee, Adv. Mater., 2009, 21, 661 CrossRef CAS
.
- M. Dasog, G. B. De los Reyes, L. V. Titova, F. A. Hegmann and J. G. C. Veinot, ACS Nano, 2014, 8, 9636 CrossRef CAS PubMed
.
- J. R. Chen, D. C. Wang, H. C. Hao and M. Lu, Appl. Phys. Lett., 2014, 104, 061105 CrossRef
.
- Y. L. Li, B. Qian, Z. P. Sui and C. P. Jiang, Appl. Phys. Lett., 2013, 103, 161908 CrossRef
.
- H. Haick, P. T. Hurley, A. I. Hochbaum, P. D. Yang and N. S. Lewis, J. Am. Chem. Soc., 2006, 128, 8990 CrossRef CAS PubMed
.
- J. Valenta, B. Bruhn and J. Linnros, Nano Lett., 2011, 11, 3003 CrossRef CAS PubMed
.
- X. T. Lu, C. M. Hessel, Y. X. Yu, T. D. Bogart and B. A. Korgel, Nano Lett., 2013, 13, 3101 CrossRef CAS PubMed
.
- A. I. Hochbaum, D. Gargas, Y. J. Hwang and P. D. Yang, Nano Lett., 2009, 9, 3550 CrossRef CAS PubMed
.
- Y. Q. Qu, L. Liao, Y. J. Li, H. Zhang, Y. Huang and X. F. Duan, Nano Lett., 2009, 9, 4539 CrossRef CAS PubMed
.
- E. Mulazimoglu, G. Nogay, R. Turan and H. E. Unalan, Appl. Phys. Lett., 2013, 103, 143124 CrossRef
.
- H. P. He, C. Liu, L. W. Sun and Z. Z. Ye, Appl. Phys. Lett., 2011, 99, 123106 CrossRef
.
- L. Gan, L. W. Sun, H. P. He and Z. Z. Ye, J. Mater. Chem. C, 2014, 2, 2668 RSC
.
- K. Okamoto, I. Niki, A. Shvartser, Y. Narukawa, T. Mukai and A. Scherer, Nat. Mater., 2004, 3, 601 CrossRef CAS PubMed
.
- P. P. Pompa, L. Martiradonna, A. D. Torre, F. D. Sala, L. Manna, M. de Vittorio, F. Calabi, R. Cingolani and R. Rinaldi, Nat. Nanotechnol., 2006, 1, 126 CrossRef CAS PubMed
.
- Y. D. Jin and X. H. Gao, Nat. Nanotechnol., 2009, 4, 571 CrossRef CAS PubMed
.
- X. J. Zhang, H. Tang, J. A. Huang, L. B. Luo, J. A. Zapien and S. T. Lee, Nano Lett., 2011, 11, 4626 CrossRef CAS PubMed
.
- Y. J. Wang, H. P. He, Y. L. Zhang, L. W. Sun, L. Hu, K. W. Wu, J. Y. Huang and Z. Z. Ye, Appl. Phys. Lett., 2012, 100, 112103 CrossRef
.
- N. A. Harun, B. R. Horrocks and D. A. Fulton, Chem. Commun., 2014, 50, 12389 RSC
.
- W. Li, S. L. Wang, M. Y. Hu, S. F. He, P. P. Ge, J. Wang, Y. Y. Guo and Z. W. Liu, Sci. Rep., 2015, 5, 11881 CrossRef PubMed
.
- W. Chern, K. Hsu, I. S. Chun, B. P. D. Azeredo, N. Ahmed, K. H. Kim, J.-M. Zuo, N. Fang, P. Ferreira and X. Li, Nano Lett., 2010, 10, 1582 CrossRef CAS PubMed
.
- M. Bassu, M. L. Strambini, G. Barillaro and F. Fuso, Appl. Phys. Lett., 2010, 97, 143113 CrossRef
.
- E. Hutter, J. H. Fendler and D. Roy, J. Phys. Chem. B, 2001, 105, 11159 CrossRef CAS
.
- Q. Q. Yu, H. P. He, L. Gan and Z. Z. Ye, RSC Adv., 2015, 5, 80526 RSC
.
- D. G. Thomas, J. J. Hopfield and W. M. Augustyniak, Phys. Rev., 1965, 140, A202 CrossRef
.
- R. Ghosh, K. Imakita, M. Fujii and P. K. Giri, Phys. Chem. Chem. Phys., 2016, 18, 7715 RSC
.
- B. Nikoobakht, C. Burda, M. Braun, M. Hun and M. A. El-Sayed, Photochem. Photobiol., 2002, 75, 591 CrossRef CAS PubMed
.
- Y. H. Chan, J. X. Chen, S. E. Wark, S. L. Skiles, D. H. Son and J. D. Batteas, ACS Nano, 2009, 3, 1735 CrossRef CAS PubMed
.
- H. Tamura, M. Ruckschloss, T. Wirschem and S. Veprek, Appl. Phys. Lett., 1994, 65, 1537 CrossRef CAS
.
- Y.-R. Choi, M. R. Zheng, F. Bai, J. J. Liu, E.-S. Tok, Z. F. Huang and C.-H. Sow, Sci. Rep, 2014, 4, 4940 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Optical absorption, XPS core level spectra, and additional PL spectra of the porous Si nanowires. See DOI: 10.1039/c6ra06019f |
| This journal is © The Royal Society of Chemistry 2016 |