Cu–Al mixed oxide catalysts for azide–alkyne 1,3-cycloaddition in ethanol–water†
R. González-Olveraa,
C. I. Urquiza-Castrob,
G. E. Negrón-Silva*a,
D. Ángeles-Beltrána,
L. Lomas-Romero*b,
A. Gutiérrez-Carrillob,
V. H. Larab,
R. Santillanc and
J. A. Morales-Sernab
aDepartamento de Ciencias Básicas, Universidad Autónoma Metropolitana-Azcapotzalco, Av. San Pablo No. 180, Ciudad de México, C.P. 02200, Mexico. E-mail: gns@correo.azc.uam.mx; Fax: +52-55-5318-9000 ext. 2169; Tel: +52-55-5318-9593
bDepartamento de Química, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco No. 186, Ciudad de México, C.P. 09340, Mexico. E-mail: llr@xanum.uam.mx; Fax: +52-55-5804-4666; Tel: +52-55-5804-4913
cDepartamento de Química, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Apartado Postal 14-740, Ciudad de México, 07000, Mexico
First published on 29th June 2016
Abstract
Cu(Al)O mixed oxides, which are obtained by the calcination of Cu–Al layered double hydroxide (LDH), promote the formation of 1,2,3-triazoles from an alkyne–azide cycloaddition reaction (Huisgen-type reaction) with excellent yields using an EtOH–H2O mixture as the solvent under microwave heating. The yield of the reaction is the result of both heterogeneous and homogeneous catalytic processes, as a consequence of capturing Cu(II) from the material by sodium ascorbate. Although the appropriate reaction conditions were employed (EtOH–H2O, 80 °C, MW, 10 min), the reconstruction of LDH by the so-called memory effect was not observed.
1. Introduction
Layered double hydroxides (LDHs) are a class of materials that are considered hydrotalcite-like compounds1 because the brucite-type octahedral layer in their structure is similar to the naturally occurring Mg–Al hydrotalcite mineral.2 In both cases, the general formula is [M1−x2+Mx3+(OH)2]x+[An−x/n·mH2O]x−, where the excess of positive charges from the substitution of M2+ (Mg, Cu) by M3+ (Al, Fe) is compensated by carbonate anions in the interlayer space.3 As in brucite [Mg(OH)2], the layers are built up by the condensation of octahedral MO6 units (M2+ or M3+) to form anionic clays in which the OH groups point towards the interlayer region and are shared by three octahedral cations. Heating the material to 500–600 °C yields a homogeneous mixed oxide with very small crystals, a large surface area and good thermal stability.4 Exposing the mixed oxide to aqueous solutions or humid atmospheres can lead to the reconstruction of the original LDH by the so-called memory effect (Scheme 1).5 The reconstructed layered material exhibits different morphologies and textural properties depending on whether the reconstruction process has been carried out in the presence of CO2 or carbonates.6 Absence of those compounds in the rehydration reaction generates Brønsted base sites7 between the new layers where carbonate anions are substituted by hydroxyl anions.8Layered double hydroxides have been increasingly explored as heterogeneous catalysts, exploiting their unique properties to create more efficient, and ultimately more eco-friendly, processes.9 Recently, Cu–Al LDHs have been synthesized and used in alkyne–azide cycloaddition reactions (Huisgen-type reaction)10–12 with different substrates, demonstrating that the Cu(II) (M2+) present in the material is able to participate in the organic reaction.13 Those studies were carried out in the presence of the as-synthesized Cu–Al LDH but not in the presence of the calcined material. In this context, as part of our research into the use of LDHs in organic reactions,14 we focus on the structural effect of as-synthesized15 and calcined (mixed oxide)16 Cu–Al LDHs on the same alkyne–azide cycloaddition reaction to obtain 1,2,3-triazoles, considering that the different morphologies and textural properties could affect the development of the reaction.
2. Results and discussion
2.1. Material synthesis
The LDH investigated in the present study Cu2+/Al3+ in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were synthesized by the co-precipitation technique and microwave-hydrothermal crystallization.17 The rapid synthesis method used in this work facilitated a homogeneous and crystalline material, which was characterized by X-ray powder diffraction (XRD), Brunauer–Emmett–Teller (BET) method, scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The obtained XRD patterns are shown in Fig. 1. The as-synthesized LDHs exhibited Cu–Al reflections associated with the layered double hydroxide crystal structure. The maxima correspond to typical diffraction by planes (1 1 0), (0 0 2), (1 1 1), (1 1 2), (0 2 0), (1 0 1) and (1 1 3) (Fig. 1, black line). These planes are similar to those shown by malachite [Cu2CO3(OH)2] because of the presence of Cu in the material.18 Calcining the material yields a Cu(Al)O mixed oxide19 with a periclase-like structure with (110), (111), (202), (022), (113), (311) and (220) plane reflections, which are typical of CuO (Fig. 1, green line).
1 were synthesized by the co-precipitation technique and microwave-hydrothermal crystallization.17 The rapid synthesis method used in this work facilitated a homogeneous and crystalline material, which was characterized by X-ray powder diffraction (XRD), Brunauer–Emmett–Teller (BET) method, scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The obtained XRD patterns are shown in Fig. 1. The as-synthesized LDHs exhibited Cu–Al reflections associated with the layered double hydroxide crystal structure. The maxima correspond to typical diffraction by planes (1 1 0), (0 0 2), (1 1 1), (1 1 2), (0 2 0), (1 0 1) and (1 1 3) (Fig. 1, black line). These planes are similar to those shown by malachite [Cu2CO3(OH)2] because of the presence of Cu in the material.18 Calcining the material yields a Cu(Al)O mixed oxide19 with a periclase-like structure with (110), (111), (202), (022), (113), (311) and (220) plane reflections, which are typical of CuO (Fig. 1, green line).
The adsorption of N2 (the BET method) was used to quantify the surface area and pore size of materials (Table 1). The as-synthesized LDH sample revealed lower porosity and a higher surface area than the calcined material [Cu(Al)O mixed oxide].
| Entry | Parameters | As-synthesized LDH | Cu(Al)O mixed oxide |
|---|---|---|---|
| 1 | SBET (m2 g−1) | 74 | 63 |
| 2 | Pore volume (cm3 g−1) | 0.44 | 0.42 |
| 3 | Pore size (Å) | 161 | 174 |
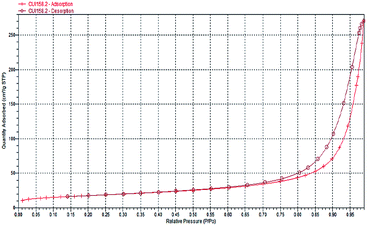
The isotherms of both materials closely resemble a type II isotherm. Nitrogen uptake monotonically increases with p/po values due to sorption in the LDH mesoporous, and the hysteresis loops belong to type H III, which are due to a different adsorption and desorption behaviour and are usually found in solids consisting of agglomerates or aggregates of slit-shaped particles (Fig. 2 and 3).
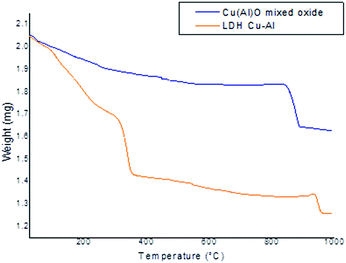
The thermal decomposition behaviour of the as-synthesized and calcined hydrotalcite was observed by TGA in a nitrogen atmosphere. The profiles in Fig. 4 correspond to the as-synthesized (yellow) and calcined (blue) samples. The as synthesized sample showed three steps. The first one at approximately 100 °C is assigned to the loss of physically adsorbed and interlayer water molecules, the second at 337 °C is attributed to the dehydroxylation and decarbonization of the brucite-like material,18 and the last at 945 °C is the loss associated with the collapse of the structure. The calcined sample exhibited one significant peak after 800 °C due to structural collapse.
The FTIR spectra of as-synthesized LDH and the Cu(Al)O mixed oxide are shown in Fig. 5. The bands in the wave-number range of 500–800 cm−1 correspond to the vibration of metal oxygen bonds in both materials. The absence of the band at 1321 and 1397 cm−1 (CO32−) in the Cu(Al)O mixed oxide FTIR spectrum confirms that the calcination evolved favourably. The wide bands in the wave region of 1625 and 3200 cm−1 (O–H) demonstrates both the presence of water molecules in the interlayer space of as-synthesized LDH and the presence of the hydroxyl vibration from the hydrogen-bonded metal hydroxide sheets in both materials (Fig. 5).
The SEM images of the an as-synthesized LDH sample (Fig. 6A) and the Cu(Al)O mixed oxide (Fig. 6B) showed micro-particles similar to flakes and agglomerates.20
2.2. Catalytic activity
To study the catalytic activity of as-synthesized and calcined (mixed oxides) Cu–Al LDH in the alkyne–azide cycloaddition reaction to obtain 1,2,3-triazoles, we chose benzyl chloride, phenylacetylene and sodium azide as a model system. In our initial experiment, the multicomponent reaction (MCR)21–23 was conducted in the presence of as-synthesized or calcined Cu–Al LDH, with sodium ascorbate and ethanol–water as the solvent (Table 2, entries 1 and 4). The highest yield was obtained when the reaction was carried out with the Cu(Al)O mixed oxide and sodium ascorbate (98%, Table 2, entry 4). In contrast, a poor yield (30%) was obtained in the absence of sodium ascorbate (Table 2, entry 3). The use of as-synthesized Cu–Al LDH allowed obtaining the desired product with a 75% yield. That yield decreased in the absence of sodium ascorbate (Table 2, entries 1 and 2). The mixture of CuO and sodium ascorbate catalysed the reaction with a 40% yield, and only trace amounts of the triazole was isolated when the reaction was performed with only CuO. It is noteworthy, that after a long reaction time (8 h), the yield does not increase in all cases.| Entry | Catalyst | Yieldb (%) |
|---|---|---|
a Reagents: alkyne 1a (1 mmol), benzyl chloride 2a (1.2 mmol), NaN3 (1.2 mmol), catalyst (10 mg) and EtOH–H20 (2 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).b Yield of isolated product after purification by crystallization.c The reaction was performed in the presence of sodium ascorbate (10 mg).d CuO 10 mg. 1).b Yield of isolated product after purification by crystallization.c The reaction was performed in the presence of sodium ascorbate (10 mg).d CuO 10 mg. |
||
| 1 | As-synthesized Cu–Al LDH | 65 |
| 2c | As-synthesized Cu–Al LDH in the presence of sodium ascorbate | 75 |
| 3 | Cu–Al mixed oxide | 30 |
| 4c | Cu–Al mixed oxide in the presence of sodium ascorbate | 98 |
| 5d | CuO | 5 |
| 6c,d | CuO in the presence of sodium ascorbate | 45 |
The scope of the multicomponent reaction was studied with benzyl halides 2b–2e, and in all cases the yield was excellent when the reaction was performed in the presence of both the Cu(Al)O mixed oxide and sodium ascorbate (Table 3). Then, the reaction was tested with other alkynes to confirm these results. In this case, the pyrimidine derivatives uracil 1b and thymine 1c were used as the alkyne substrates.24 In both cases, the reaction evolved with an excellent yield if the calcined LDH and sodium ascorbate were used as the catalytic system (Scheme 2).
| Entry | Benzyl halide | X | R | Product | Yieldb (%) |
|---|---|---|---|---|---|
a Reagents: alkyne 1a (1 mmol), benzyl halide 2a–e (1.2 mmol), NaN3 (1.2 mmol), catalyst (10 mg), sodium ascorbate (10 mg) and EtOH–H20 (2 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).b Yield of the isolated product after purification by crystallization. 1).b Yield of the isolated product after purification by crystallization. |
|||||
| 1 | 2b | Cl | F | 3b | 90 |
| 2 | 2c | Cl | Cl | 3c | 95 |
| 3 | 2d | Br | Br | 3d | 85 |
| 4 | 2e | Br | I | 3e | 90 |
Finally, the catalytic system was tested with steroidal azide derivatives of bile acids: lithocholic 4b, deoxycholic 4d, chenodeoxycholic acids 4f and cholic 4h. The reactions were carried out in the presence of uracil 1d and thymine 1e25 to generate the pyrimidine–steroid conjugate systems 5a–5f (Table 4). In general, the reactions evolved with excellent yields; however, when the steroidal azide derivatives had a free hydroxyl group (4d), the yield decreased notably. This loss in the yield can be avoided by using an acetyl protective group (4f and 4h), which may be added and removed with near quantitative yield. Attempts to use mesylate derivatives of the same bile acids (4a, 4c, 4e, and 4g) and NaN3 in the multicomponent reaction resulted products 5a–5f with moderate yields (Table 4). In those cases, the long reaction time and high temperatures did not change the yield. Analysis of the supernatant of the reactions by 1H NMR demonstrated the presence of a free hydroxyl group at C-3 on the steroids as a consequence of the hydrolysis of mesylate derivatives.
The results above demonstrate that the presence of sodium ascorbate was important for very high yields. Therefore, understanding the role of sodium ascorbate in the catalytic process is evidently necessary. The difference between the yields when the reactions were carried out in the presence or absence of this salt suggests that a redox process occurred during the azide–alkyne 1,3-cycloaddition. Cu2+ is known to catalyse Huisgen-type reactions, and Cu2+ can be reduced to Cu1+ in the presence of sodium ascorbate to make the cycloaddition more efficient. However, in this case, how can Cu2+ into Cu1+ be reduced into a rigid structure?
Sodium ascorbate is able to capture different metals, and in this case, it is a chelating agent that degrades the as-synthesized LDH and Cu(Al)O mixed oxide. The degradation is minimal,19 but it is enough to decisively increase the reaction yield (Table 1, entries 2 and 4). This hypothesis is supported by the post-reaction identification and quantification by UV of Cu2+ dissolved in the mixture EtOH–H2O. In the case of as-synthesized LDH, the material lost 4% Cu. On the other hand, 0.4% Cu was quantified from the reaction carried out with the Cu(Al)O mixed oxide.
Once the in situ degradation of material occurs, the redox process then occurs in the solution, and Cu1+ catalyses the azide–alkyne 1,3-cycloaddition. Thus, we assume that both heterogeneous and homogeneous processes take place during the organic reaction. The homogeneous process was demonstrated when the reaction of cycloaddition between 1a, 2a and NaN3 was carried out using the recovered sodium ascorbate–Cu2+/EtOH–H2O mixture (40% yield). Finally, the difference in the yield between as-synthesized LDH and the Cu(Al)O mixed oxide could be attributed to the major catalytic activity shown for Cu(Al)O mixed oxide, as expected in this type of materials.2a,26 Mixed oxide resistance to rehydration.
An XRD analysis of the material after the organic reaction demonstrates that the structure of the Cu(Al)O mixed oxide was not modified, which means that under the organic reaction conditions (EtOH–H2O, 80 °C, MW, 10 min), the original LDH was not obtained by the reconstruction process (Fig. 7). We tried to reconstruct the original structure in using various conditions described in the literature.27 However, the reconstruction in the presence of H2O or an EtOH–H2O mixture was not possible even at high temperatures (150 °C) and long reconstruction times (48 h). In all cases, Cu(Al)O mixed oxide was identified by XRD as the only structure.
This behaviour demonstrates the stability of the Cu(Al)O mixed oxide under conditions that are not anhydrous, which is an advantage from material view point, given that this type of mixed oxide, which is highly catalytically active, cannot be stored for a long time. However, in this case the material has been stored for one year without the need for special conditions and without a loss of catalytic activity.
2.3. Recyclability
To screen the recyclability of the Cu(Al)O mixed oxide catalyst, two reactivation processes were applied to the material. In the first one, after separating the material from the cycloaddition reaction mixture between 1a, 2a and NaN3, the material was dried at 100 °C for 24 h. The second process includes calcination at 500 °C for 5 h in the ambient atmosphere, without the need for N2. Thus, if the used catalyst was reactivated at high temperature (500 °C), the reactions still proceeded well, and high yields were obtained at the end of the reaction (Fig. 8). The Cu(Al)O mixed oxide can be recycled ten times without significant losses in its catalytic activity. In all cases, the crystalline structure of the material was monitored by XRD. That analysis revealed that the structure of the catalyst remained unchanged, even after ten cycles. The full characterization of material after ten catalytic cycles is showed in the ESI.†This result contrasts with the results using the Cu–Al dry material at 100 °C, which demonstrated a loss of catalytic activity through reuse, despite maintaining the structure of a mixed oxide. The reduced activity of the Cu(Al)O mixed oxide is probably due to the presence of organic material on the surface, which is eliminated after calcination at 500 °C, thus recovering catalytic properties of the material (Fig. 8, red column, yield 80%).
3. Conclusions
In summary, the present study demonstrates the capacity of as-synthesized LDH and Cu–Al mixed oxides to produce 1,2,3-triazoles via an alkyne–azide cycloaddition reaction (Huisgen-type reaction) using an EtOH–H2O mixture as the solvent. The yield of the reactions depends primarily on the surface area of the material, which is large in the case of Cu(Al)O mixed oxide. However, the formation of 1,2,3-triazoles notably increased when the reaction was performed in the presence of sodium ascorbate, as a consequence of both heterogeneous and homogeneous processes. In addition, this study demonstrated that the reconstruction of Cu–Al LDH from Cu(Al)O mixed oxides by the so-called memory effect was not possible, despite the use of severe reaction conditions.Acknowledgements
The authors would like to thank Consejo Nacional de Ciencia y Tecnología, CONACyT (project 181448) for financial support. RGO, GENS, DAB, LLR, AGC, LVH, RS and JAMS wish to acknowledge the SNI (Sistema Nacional de Investigadores) for the distinction of their membership and their stipend. CIUC is grateful to CONACyT for her student fellowship. We also wish to thank Maria Eugenia Ochoa, Rebeca Yépez and Geiser Cuellar for the technical assistance. Thanks to Laboratorio Divisional de Microscopía Electrónica UAM-A for the SEM images.Notes and references
- (a) J. Qu, Q. Zhang, X. Li, X. He and S. Song, Appl. Clay Sci., 2016, 119, 185 CrossRef CAS; (b) C. Li, M. Wei, D. G. Evans and X. Duan, Catal. Today, 2015, 247, 163 CrossRef CAS; (c) Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124 CrossRef CAS PubMed.
- For recent reviews about LDHs, see: (a) T. Baskaran, J. Christopher and A. Sakthivel, RSC Adv., 2015, 5, 98853 RSC; (b) S. N. Basahel, S. A. Al-Thabaiti, K. Narasimharao, N. S. Ahmed and M. Mokhtar, J. Nanosci. Nanotechnol., 2014, 14, 1931 CrossRef CAS PubMed; (c) S. Nishimura, A. Takagaki and K. Ebitani, Green Chem., 2013, 15, 2026 RSC.
- (a) G. Abellán, C. Martí-Gastaldo, A. Ribera and E. Coronado, Acc. Chem. Res., 2015, 48, 1601 CrossRef PubMed; (b) M. Shao, R. Zhang, Z. Li, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2015, 51, 15880 RSC.
- (a) J. Perez, S. Abello and N. M. van der Pers, Chem.–Eur. J., 2007, 13, 870 CrossRef PubMed; (b) S. Abelló and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2006, 96, 102 CrossRef.
- J. A. van Bokhoven, J. Roelofs, K. P. de Jong and D. C. Koningsberger, Chem.–Eur. J., 2001, 7, 1258 CrossRef CAS.
- (a) J. S. Valente, E. Lima, J. A. Toledo-Antonio, M. A. Cortes-Jacome, L. Lartundo-Rojas, R. Montiel and J. Prince, J. Phys. Chem., 2010, 114, 2089 CAS; (b) H. Pfeiffer, E. Lima, V. Lara and J. S. Valente, Langmuir, 2010, 26, 4074 CrossRef CAS PubMed; (c) J. Peréz-Ramírez, S. Abelló and N. M. van der Pers, J. Phys. Chem., 2007, 111, 3642 CrossRef PubMed; (d) T. Hibino and A. Tsunashima, Chem. Mater., 1998, 10, 4055 CrossRef CAS.
- (a) M. G. Alvarez, R. J. Chimentão, F. Figueras and F. Medina, Appl. Clay Sci., 2012, 58, 16 CrossRef CAS; (b) J. S. Valente, H. Pfeiffer, E. Lima, J. Prince and J. Flores, J. Catal., 2011, 279, 196 CrossRef CAS; (c) Y. Xi and R. J. Davis, J. Catal., 2008, 254, 190 CrossRef CAS.
- (a) C. Xu, Y. Gaso, X. Liu, R. Xin and Z. Wang, RSC Adv., 2013, 3, 793 RSC; (b) G. Lee, Y. Jeong, A. Takagaky and J. C. Jung, J. Mol. Catal. A: Chem., 2014, 393, 289 CrossRef CAS.
- (a) G. Fan, F. Li, D. G. Evans and X. Duan, Chem. Soc. Rev., 2014, 43, 7040 RSC; (b) Z. P. Xu, J. Zhang, M. O. Adebajo, H. Zhang and C. Zhou, Appl. Clay Sci., 2011, 53, 139 CrossRef CAS.
- (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; (b) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 113, 2056 CrossRef; (c) R. Huisgen, Angew. Chem., Int. Ed., 1963, 75, 604 CrossRef CAS; (d) R. Huisgen, Angew. Chem., Int. Ed., 1963, 2, 565 CrossRef; (e) R. Huisgen, Proc. Chem. Soc., London, 1961, 15, 357 Search PubMed.
- For recent review about Huisgen reaction, see: (a) C. Wang, D. Ikhlef, S. Kahlal and J.-Y. Saillard, Coord. Chem. Rev., 2016, 316, 1 CrossRef CAS; (b) V. Castro, H. Rodríguez and F. Albericio, ACS Comb. Sci., 2016, 18, 1 CrossRef CAS PubMed; (c) S. Chassaing, V. Bénéteau and P. Pale, Catal. Sci. Technol., 2016, 6, 923 RSC; (d) S. B. Ötvös and F. Fülöp, Catal. Sci. Technol., 2015, 5, 4926 RSC; (e) E. Haldón, M. C. Nicasio and P. J. Pérez, Org. Biomol. Chem., 2015, 13, 9528 RSC; (f) K. A. Kumar, Int. J. ChemTech Res., 2013, 5, 3032 CAS.
- For recent examples about Huisgen reaction, see: (a) H. Koganei, S. Tachikawa, M. E. El-Zaria and H. Nakamura, New J. Chem., 2015, 39, 6388 RSC; (b) A. Shaygan Nia, S. Rana, D. Döhler, F. Jirsa, A. Meister, L. Guadagno, E. Koslowski, M. Bron and W. Binder, Chem.–Eur. J., 2015, 21, 10763 CrossRef CAS PubMed; (c) R. Slimi, S. Kalhor-Monfared, B. Plancq and C. Girard, Tetrahedron Lett., 2015, 56, 4339 CrossRef CAS; (d) W. Zhang, X. He, B. Ren, Y. Jiang and Z. Hu, Tetrahedron Lett., 2015, 56, 2472 CrossRef CAS; (e) P. Diz, P. Pernas, A. El Maatougui, C. R. Tubio, J. Azuaje, E. Sotelo, F. Guitian, A. Gil and A. Coelho, Appl. Catal., A, 2015, 502, 86 CrossRef CAS; (f) K. Jayaramulu, V. M. Suresh and T. K. Maji, Dalton Trans., 2015, 44, 83 RSC; (g) N. Saravanan, M. Arthanareeswari, P. Kamaraj and B. Sivakumar, Res. Chem. Intermed., 2015, 41, 5379 CrossRef CAS; (h) M. E. M. Mekhzoum, H. Benzeid, A. E. K. Qaiss, E. M. Essassi and R. Bouhfid, Catal. Lett., 2016, 146, 136 CrossRef CAS; (i) A. N. Semakin, D. P. Agababyan, S. Kim, S. Lee, A. Y. Sukhorukov, K. G. Fedina, J. Oh and S. L. Loffe, Tetrahedron Lett., 2015, 56, 6335 CrossRef CAS; (j) R. F. Schumacher, P. B. Von Laer, E. S. Betin, R. Cargnelutti, G. Perin and D. Alves, J. Braz. Chem. Soc., 2015, 26, 2298 CAS; (k) T. M. A. Barlow, M. Jida, D. Tourwé and S. Ballet, Org. Biomol. Chem., 2014, 12, 6986 RSC; (l) Y. Jiang, D. Kong, J. Zhao, Q. Qi, W. Li and G. Xu, RSC Adv., 2014, 4, 1010 RSC; (m) Y. Jiang, D. Kong, J. Zhao, W. Zhang, W. Xu, W. Li and G. Xu, Tetrahedron Lett., 2014, 55, 2410 CrossRef CAS; (n) W. Chaouni, M. Aadilb, A. Djellal and G. Kirsch, Zeitschrift für Naturforschung B, 2014, 69, 509 CrossRef CAS; (o) X. Han, S. Bian, Y. Liang, K. N. Houk and A. B. Braunschweig, J. Am. Chem. Soc., 2014, 136, 10553 CrossRef CAS PubMed.
- (a) S. B. Ötvös, A. Georgiádes, M. Ádok-Sipiczki, R. Mészáros, I. Pálinkó, P. Sipos and F. Fülöp, Appl. Catal., A, 2015, 501, 63 CrossRef; (b) A. N. Prasad, B. Thirupathi, G. Raju and B. M. Reddy, Catal. Sci. Technol., 2012, 2, 1264 RSC; (c) S. Kale, S. Kahandal, S. Disale and R. Jayaram, Curr. Chem. Lett., 2012, 1, 69–80 CrossRef CAS; (d) K. Pitchumani, Chem.–Eur. J., 2009, 15, 2755 CrossRef PubMed.
- (a) J. A. Morales-Serna, M. Á. Jaime-Vasconcelos, E. García-Ríos, A. Cruz, D. Angeles-Beltrán, L. Lomas-Romero, G. E. Negrón-Silva and J. Cárdenas, RSC Adv., 2013, 3, 23046 RSC; (b) J. A. Morales-Serna, E. Sánchez, R. Velázquez, J. Bernal, E. García-Ríos, R. Gaviño, G. Negrón-Silva and J. Cárdenas, Org. Biomol. Chem., 2010, 8, 4940 RSC; (c) J. Cárdenas, J. A. Morales-Serna, E. Sánchez, R. Gaviño, L. Lomas, N. Guerra and G. Negrón, ARKIVOC, 2005, 6, 428 Search PubMed.
- (a) T. Kameda, T. Uchiyama and T. Yoshioka, New J. Chem., 2015, 39, 6315 RSC; (b) D. Damodara, R. Arundhathi and P. R. Likhar, Adv. Synth. Catal., 2014, 356, 189 CrossRef CAS; (c) T. Kameda, K. Hoshi and T. Yoshioka, Solid State Sci., 2013, 17, 28 CrossRef CAS; (d) R. Arundhathi, D. Damodara, K. V. Mohan, M. L. Kantam and P. R. Likhar, Adv. Synth. Catal., 2013, 355, 751 CrossRef CAS.
- R. Valencia, J. A. Tirado, R. Sotelo, F. Trejo and L. Lartundo, React. Kinet., Mech. Catal., 2015, 116, 205 CrossRef CAS.
- (a) C. Chen, K. Zhang and B. Li, Adv. Mater. Res., 2012, 554–556, 532 CAS; (b) G. Fetter, F. Hernández, A. M. Maubert, V. H. Lara and P. Bosch, J. Porous Mater., 1997, 4, 27 CrossRef CAS.
- Y. Lewin, M. A. Yarmob, Z. Yaakoba, A. B. Mohamada, W. Ramli and W. Dauda, Mater. Res. Bull., 2001, 36, 193 CrossRef.
- S. Britto and P. V. Kamath, J. Solid State Chem., 2009, 182, 1193 CrossRef CAS.
- S. Chakraborty, I. Sarkar, K. Haldar, S. Kanta Pal and S. Chakraborty, Appl. Clay Sci., 2015, 107, 98 CrossRef CAS.
- For recent review about multicomponent reaction, see: (a) J. Totobenazara and A. J. Burke, Tetrahedron Lett., 2015, 56, 2853 CrossRef CAS; (b) T. Hosseinnejad, B. Fattahi and M. M. Heravi, J. Mol. Model., 2015, 21, 264 CrossRef PubMed; (c) B. Schulze and U. S. Schubert, Chem. Soc. Rev., 2014, 43, 2522 RSC; (d) Y. Jiang and C. Kuang, Mini-Rev. Med. Chem., 2013, 13, 713 CrossRef CAS PubMed; (e) K. C. Majumdar and K. Ray, Synthesis, 2011, 23, 3767 CrossRef; (f) V. P. Krivopalov and O. P. Shkurko, Russ. Chem. Rev., 2005, 74, 339 CrossRef CAS.
- For recent review about multicomponent syntheses of triazoles, see: S. Hassan and T. J. J. Müller, Adv. Synth. Catal., 2015, 357, 617 CrossRef CAS.
- For recent examples about multicomponent syntheses of triazoles, see: (a) S. G. Agalave, S. G. Pharande, S. M. Gade and V. S. Pore, Asian J. Org. Chem., 2015, 4, 943 CrossRef CAS; (b) K. R. Senwar, P. Sharma, S. Nekkanti, M. Sathish, A. Kamal, B. Sridhar and N. Shankaraiah, New J. Chem., 2015, 39, 3973 RSC; (c) Z. Zhang, Q. Zhou, F. Ye, Y. Xia, G. Wu, M. L. Hossain, Y. Zhang and J. Wang, Adv. Synth. Catal., 2015, 357, 2277 CrossRef CAS; (d) F. Gang, T. Dong, G. Xu, Y. Fu and Z. Du, Heterocycles, 2015, 91, 1964 CrossRef CAS; (e) A. N. Prasad, B. M. Reddy, E.-Y. Jeong and S.-E. Park, RSC Adv., 2014, 4, 29772 RSC; (f) N. Mukherjee, S. Ahammed, S. Bhadra and B. C. Ranu, Green Chem., 2013, 15, 389 RSC.
- G. E. Negrón-Silva, R. González-Olvera, D. Angeles-Beltrán, N. Maldonado-Carmona, A. Espinoza-Vázquez, M. E. Palomar-Pardavé, M. A. Romero-Romo and R. Santillan, Molecules, 2013, 18, 4613 CrossRef PubMed.
- R. González-Olvera, A. Espinoza-Vázquez, G. E. Negrón-Silva, M. E. Palomar-Pardavé, M. A. Romero-Romo and R. Santillan, Molecules, 2013, 18, 15064 CrossRef PubMed.
- (a) S. Cota, E. Ramírez, F. Medina, G. Layrac, D. Tichit and C. Gérardin, J. Mol. Catal. A: Chem., 2016, 412, 101 CrossRef; (b) D. Bharali, R. Devi, P. Bharali and R. C. Deka, New J. Chem., 2015, 39, 172 RSC; (c) Y. Shao, J. Li, H. Chang, Y. Peng and Y. Deng, Catal. Sci. Technol., 2015, 5, 3536 RSC; (d) S. Paušová, J. Krýsa, J. Jirkovský, C. Forano, G. Mailhot and V. Prevot, Appl. Catal., B, 2015, 170–171, 25 CrossRef; (e) E. Genty, J. Brunet, C. Poupin, S. Casale, S. Capelle, P. Massiani, S. Siffert and R. Cousin, Catalysts, 2015, 5, 851 CrossRef CAS; (f) M. Răciulete, G. Layrac, D. Tichit and I.-C. Marcu, Appl. Catal., A, 2014, 477, 195 CrossRef; (g) S. Tanasoi, N. Tanchoux, A. Urdă, D. Tichit, I. Săndulescu, F. Fajula and I.-C. Marcu, Appl. Catal., A, 2009, 363, 135 CrossRef CAS; (h) L. Dussault, J. C. Dupin, H. Martinez, E. Dumitriu, A. Auroux and C. Guimon, Surf. Interface Anal., 2006, 38, 234 CrossRef CAS; (i) J. Barrault, A. Derouault, G. Courtois, J. M. Maissant, J. C. Dupin, C. Guimon, H. Martinez and E. Dumitriu, Appl. Catal., A, 2004, 262, 43 CrossRef CAS.
- (a) H. R. Prakruthi, B. M. Chandrashekara, B. S. Jai Prakash and Y. S. Bhat, Catal. Sci. Technol., 2015, 5, 3667 RSC; (b) T. Kameda, K. Hoshi and T. Yoshioka, Mater. Res. Bull., 2012, 47, 4216 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and NMR spectra. See DOI: 10.1039/c6ra10097j |
| This journal is © The Royal Society of Chemistry 2016 |