pH- and light-regulated ion transport in hourglass shaped Al2O3 nanochannels patterned with N719 and APTES†
Abstract
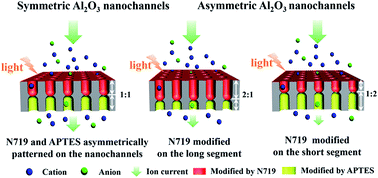
Artificial nanochannel systems regulated by diverse stimuli can provide rich ion transport, and are frequently used to mimic intelligent ion channels in biological membranes. Combining shape control and chemical modification of artificial nanochannels is an effective approach in the construction of nanochannel systems with ion transport regulation by multiple stimuli. In this work, symmetric and asymmetric hourglass shaped Al2O3 nanochannels were fabricated and the nanochannels were chemically patterned with N719 and APTES at expectant positions. By regulating the pH value, the ionization status of the coated molecules was changed and the charges in the nanochannels were redistributed, which resulted in pH-responsive ion rectification characteristics. When irradiated with light, the charges on the surfaces modified with N719 molecules were increased and new charge distributions in the nanochannels were formed, which led to light-responsive ion transportation behaviour. In the symmetric Al2O3 nanochannels, an ion current rectification ratio of about 4.3 was obtained. In the asymmetric Al2O3 nanochannels, the light induced current change ratio reached about 1.1. The study of symmetric and asymmetric nanochannels combined with different responsive molecules at expectant positions may provide an innovative approach for the design and fabrication of smart nanochannel systems to simulate biological ion channels.

 Please wait while we load your content...
Please wait while we load your content...