Magnetic-downconversion luminescent bifunctional BaGdF5:Dy3+,Eu3+ nanospheres: energy transfer, multicolor luminescence and paramagnetic properties
Hongxia Guan,
Yanhua Song,
Pingchuan Ma,
Meiqi Chang,
Jie Chen,
Yuexin Wang,
Bo Yuan and
Haifeng Zou*
College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: zouhf@jlu.edu.cn; Fax: +86-431-85155275; Tel: +86-431-85155275
First published on 27th May 2016
Abstract
A series of Dy3+ or/and Eu3+ doped cubic BaGdF5 phosphors were synthesized for the first time by an L-arginine hydrothermal method. The samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), photoluminescence spectroscopies (PL) and luminescence decay. The results indicate that the as-prepared samples are a pure cubic phase of BaGdF5, taking on irregular nanoparticles with an average size of 20 nm. The as-prepared Dy3+ or Eu3+ single doped samples show strong blue and red emissions, originating from the 4F9/2 → 6H15/2 transition of the Dy3+ ions and the 5D1 → 7FJ (J = 1, 2) and 5D0 → 7FJ (J = 1, 2, 4) transition of the Eu3+ ions. Based on the rare earth concentrations and excitation wavelengths, multiple (white, red, blue and green yellow) emissions are obtained by Eu3+ ion co-activated BaGdF5:Dy3+ phosphors. In addition, the energy migration from Dy3+ to Eu3+ has been reported in detail. Furthermore, the obtained samples also exhibit paramagnetic properties at room temperature and low temperature. It is obvious that Dy3+, Eu3+ co-doped BaGdF5 nanomaterials with tunable multicolor emissions may have potential application in the field of full-color displays.
1. Introduction
Luminescent materials based on rare earth ion emission have been extensively investigated for their wide applications in the field of full-color displays,1,2 light emitting diodes (LED),3,4 fluorescence imaging5–7 as well as active laser materials8–10 in modern society. Therefore, an increasing number of researchers have paid attention to studying and developing luminescent nanomaterials, such as phosphates,11–14 lanthanide-doped oxides15,16 and fluorides.17–19 Among these materials, the rare-earth fluoride (such as BaGdF5) nanocrystals are an interesting and highly-focused host material for rare earth ion emission, such as Dy3+, Eu3+, etc. for two reasons: (1) the rare-earth fluoride (such as BaGdF5) nanocrystals possess a low phonon energy and toxicity, large effective Stokes shifts, high refractive index, multicolor emission as well as high resistance to photo-bleaching, blinking, and photochemical degradation.20 (2) For the fluorides containing the Gd3+ ions, the Gd3+ (4f7) has a strong absorption peak at ∼272 nm (S → I transition) and thereby energy transfer is possible to the excited states of the activators (Ln3+);21,22 therefore, BaGdF5 is considered to be a prominent luminescent host material.Nowadays, many red phosphors have been successfully prepared via the Eu3+ doped compounds based on the characteristic emissions resulting from the transitions of 5D0 → 7FJ (J = 1, 2, 3, 4). Furthermore, the emitting color of Dy3+ of 4F9/2 → 6H15/2 and 4F9/2 → 6H13/2 belong to the blue and yellow regions, respectively. Thereby, it is feasible to produce Dy3+ and Eu3+ co-activated white light or color phosphors. Obviously, tunable photoluminescence properties of the Dy3+ and Eu3+ ions have been widely studied in many hosts, such as NaGdF4,23 GdVO4,24 SrY2O4 (ref. 25) and Y2O2S.26 In view of the above studies and take into consideration the characteristic emissions of dysprosium and europium ions, it is expected that multicolor emissions also could be achieved in BaGdF5 system by adjusting different excitation wavelengths and properly designed activator contents. More importantly, up to now, most reports about BaGdF5 nanometer materials have mainly focused on up conversion luminescence in the literature.27–29 However, there are scarcely ever reports about BaGdF5 down conversion luminescence. Meanwhile, BaGdF5 shows relatively excellent chemical and photophysical stability. Hence, it is highly valuable to obtain color-tunable emissions through adjusting the excitation wavelengths and doping Dy3+ along with Eu3+ into BaGdF5 host.
It is known that some of the Ln3+ ions like Gd3+, having seven unpaired electrons, also can show magnetic properties, which could also be used in designing new functional materials. Recently, Ajithkumar reported that Gd2O2S:Yb3+/Er3+ phosphor exhibit strongly magnetic, thus making them suitable as an MRI agent.30 Ju31 has successfully synthesized amine-functionalized lanthanide-doped KGdF4 nanocrystals via a facile one-step solvothermal route by employing polyethylenimine as the surfactant and capping ligand, which have been demonstrated to be potential T1-MRI contrast agents. A simple and fast (7 min) procedure for synthesis of gadolinium phosphate (GdPO4) nanocubes (edge = 75 nm) based on the microwave-assisted heating at 120 °C of gadolinium acetylacetonate and phosphoric acid solutions in buthylene glycol is reported by Ocaña,32 which are demonstrated potential applications as biolabels for in vitro optical imaging and as negative contrast agent for magnetic resonance imaging. Therefore, the BaGdF5 nanomaterial not only has a very good luminescence property, but also has a magnetic property. So it is a multifunctional material, and it can be used in display devices and biological fields.
In this work, we aim to focus our attentions on BaGdF5 as a host, while Dy3+ and Eu3+ ions as activators to synthesize a series of BaGdF5:Dy3+,Eu3+ nanophosphors through a simple hydrothermal method. In addition, we reported the phase, morphology, color tunable luminescence and paramagnetic properties behavior of trivalent rare-earth ions activated BaGdF5 powders. Furthermore, the luminescence and energy transfer properties of Dy3+ and Eu3+ co-doped BaGdF5 phosphors have been discussed in detail.
2. Experimental
2.1 Materials
All chemicals were of analytical grade and utilized as purchased without any further purification. The Gd(NO3)3, Dy(NO3)3, and Eu(NO3)3 stock solutions were prepared by dissolving corresponding appropriate amounts of Gd2O3 (99.99%), Dy2O3 (99.99%), and Eu2O3 (99.99%) in dilute HNO3 (15 mol L−1) under heating with agitation followed by evaporating the excess solvent. The Gd2O3, Dy2O3, and Eu2O3 were purchased from Jiangxi Ganzhou Rare-Earth Limited Corporation.2.2 Preparation
A series of rate-earth doped BaGdF5 phosphors were synthesized by a facile hydrothermal process without further sintering treatment. Firstly, 2 mmol of RE(NO3)3 (RE = Gd, Dy, Eu) were added into a 100 mL flask with 0.5226 g nitrate barium (Ba(NO3)2). Then after vigorous stirring for 20 min, 0.6968 g L-arginine were slowly added into the above solution. Finally, after additional agitation for 20 min, 0.8398 g sodium fluoride (NaF) were poured into the above solution. The as-obtained mixing solution was transferred into a 50 mL Teflon autoclave, which was tightly sealed and maintained at 180 °C for 25.5 h. As the autoclave was cooled to the room temperature naturally, the precipitates were separated by centrifugation washed with deionized water and ethanol in sequence each several times and dried in air at 60 °C for 12 h.2.3 Characterizations
The crystalline nature and phase structure of BaGdF5:Dy3+,Eu3+ were examined by X-ray powder diffraction (XRD) performed on a Rigaku D/max-RA X-ray diffractometer with Cu Kχ radiation, operating at 20 mA, 30 kv, scanning speed, step length and diffraction range were 10° min−1, 0.1° and 20–75°, respectively. The size and morphology of the sample were inspected using a field emission scanning electron microscope (FE-SEM, S-4800, Hitachi, Japan). The transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) micrographs were performed using a FEI Tecnai G2 S-Twin transmission electron microscope with a field emission gun operating at 200 kV. Photoluminescence (PL) excitation and emission spectra were recorded with a Jobin Yvon FluoroMax-4 equipped with a 150 W xenon lamp as the excitation source at room temperature. The luminescence decay curves of the phosphors were measured using a HITACHI F-7000 spectrometer with a 350 W xenon lamp as the excitation source.3. Results and discussion
3.1 Crystallization behavior and structure
The XRD patterns of BaGdF5 phosphors have been shown in Fig. 1. It can be seen that all the diffraction peaks of these samples are basically in agreement with pure cubic phase BaGdF5 crystal in the Fm3m space group (lattice constants a = b = 6.023 Å, c = 6.023 Å, JCPDS card 24-0098) and no other phases or impurities can be detected, which implying that the prepared samples were pure phase BaGdF5. Moreover, the sharp and strong peaks indicate that the products synthesized at low temperatures are still highly crystalline, which is very important for phosphors, since higher crystallinity always means fewer traps and stronger luminescence.28,33 However, it is worth pointing out that compared with the corresponding standard card, the main peaks slightly shift towards to the higher degree by the introduction of Dy3+ ions, but for the co-doping of Dy3+ and Eu3+ ions, the main peaks shifting towards to the higher degree slightly decreases, which is in line with the literature.24,28,29,34 The reason is that the ionic radii of the Gd3+ ions (0.938 Å) are larger than that of Dy3+ ions (0.912 Å) and smaller than that of Eu3+ ions (0.947 Å).24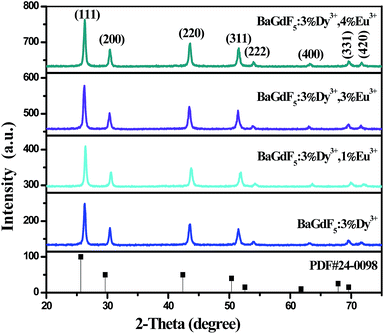 | ||
| Fig. 1 XRD patterns of the as-prepared BaGdF5:Dy3+, BaGdF5:Eu3+ and BaGdF5:Dy3+,Eu3+ samples, the corresponding standard data of BaGdF5 (JCPDS no. 24-0098) is given as reference. | ||
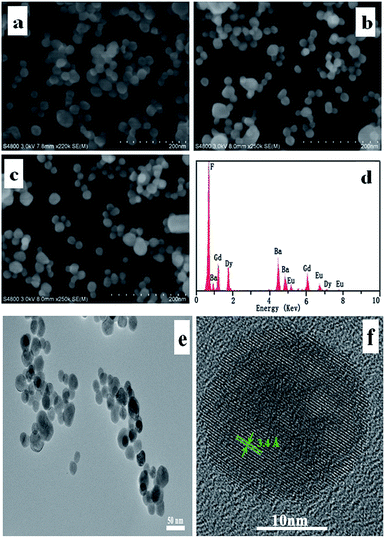
Fig. 2 shows the morphology and composition of the materials prepared by a facile hydrothermal method. From Fig. 2(a)–(c), one can see that the BaGdF5 (a) and BaGdF5:3%Dy3+ (b) and BaGdF5:3%Dy3+,4%Eu3+ (c) samples are composed of a large number of nanoparticles with the size of about 20 nm, which demonstrates that the doping species and the doping quality of rare earth ions do not alter the morphology. Fig. 2(d) gives the EDS spectrum of BaGdF5 nanoparticles doped with 3%Dy3+ and 4%Eu3+. The EDS result reveals that the nanoparticles are mainly composed of Ba, Gd, Dy, Eu, F, which further proved the samples to be BaGdF5:Dy3+,Eu3+. From the TEM image, it can be clearly seen that these nanospheres have an average diameter about 20 nm (Fig. 2(e)). The corresponding HRTEM image shows clearly lattice fringes with interplanar spacing of 3.4 Å ascribed to the (111) plane of BaGdF5 (Fig. 2(f)).29
3.2 Fluorescent performance
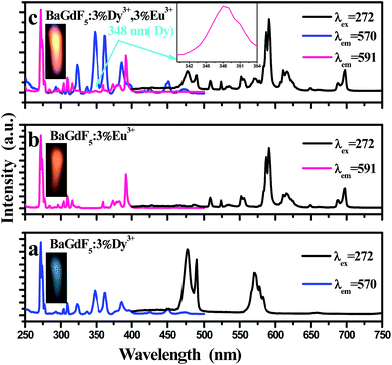
Fig. 3 presents the room temperature photoluminescence excitation and emission spectra for BaGdF5:3%Dy3+ (a), BaGdF5:3%Eu3+ (b), and BaGdF5:3%Dy3+,3%Eu3+ (c). As shown in Fig. 3(a), it can be seen that the photoluminescence excitation spectrum monitored with 570 nm emission (4F9/2 → 6H13/2) of Dy3+ consists of a sharp excitation band at 272 nm and several bands centered at 323, 348, 362, 386, 451 nm, and 474 nm, corresponding to the characteristic f–f transitions of Gd3+ (8S7/2 → 6IJ) and Dy3+ (6H15/2 → 6P3/2, 6H15/2 → 6P7/2, 6H15/2 → 6P5/2, 6H15/2 → 4F7/2, 6H15/2 → 4I15/2, and 6H15/2 → 4F9/2). These excitation peaks indicate that the phosphor can strongly absorb ultraviolet and blue light to obtain Dy3+ emission. Upon excitation with 272 nm, the as-prepared BaGdF5:3%Dy3+ samples exhibit two main emission bands, whose positions are at 477 and 570 nm, corresponding to the magnetic dipole (4F9/2 → 6H15/2) and electric dipole transitions (4F9/2 → 6H13/2) of Dy3+ ions.35 In addition, a bright blue light can be observed in the photo of BaGdF5:3%Dy3+ under excitation at 272 nm.Fig. 3(b) gives excitation and emission spectra of the as-prepared BaGdF5:3%Eu3+ samples. From the Fig. 3(b), it can be found that the excitation spectrum of BaGdF5:3%Eu3+ shows some peaks at 316, 364, 384, and 392 nm corresponding to the transitions of Eu3+ ion from the ground level 7F0 to 5H3, 5D4, 5G4 and 5L6 excited levels, respectively. Simultaneously, the sharp excitation band at 272 nm is assigned to the f–f transition of Gd3+. The emission spectrum of BaGdF5:3%Eu3+ phosphor is obtained by exciting at 272 nm. One can see that there are a number of emission peaks in the range of 400–750 nm at 508, 534, 552, 591, 616, and 697 nm, which correspond to 5D2 → 7F3, 5D1 → 7F1, 5D1 → 7F2, 5D0 → 7F1, 5D0 → 7F2, and 5D0 → 7F4 transitions of Eu3+ ions, respectively.36 Generally, the intensity ratio of the electric dipole to magnetic dipole transitions is used to determine the symmetry of the local environment. The strong emission ascribed to the 5D0 → 7F1 magnetic dipole transition (591 nm), is about 3 times stronger than that of the electric dipole transition 5D0 → 7F2 (616 nm), indication that the Eu3+ ions occupy the symmetry sites. Thereby, the orange red emission is often dominant in the emission spectrum. Moreover, it can be seen that the as-prepared BaGdF5:3%Eu3+ show a bright orange red light under UV irradiation, as shown in the inset of Fig. 3(b). As depicted in Fig. 3(a) and (b), the comparison between the excitation spectra of BaGdF5:3%Dy3+ and BaGdF5:3%Eu3+ reveals that there is a sharp excitation band at 272 nm is assigned to the f–f transitions of Gd3+, which indicates that efficient energy transfer appears from Gd3+ to Dy3+ and Eu3+.
From the Fig. 3(c), we can clearly see that a manifest 6H15/2 → 6P7/2 (348 nm) transition of Dy3+ is observed when monitoring by the 5D0 → 7F1 (591 nm) emission of Eu3+ ions for BaGdF5:3%Dy3+,3%Eu3+, which imply that the energy migration from Dy3+ to Eu3+. The emission spectra of BaGdF5:3%Dy3+,3%Eu3 at 272 nm exciting showed the emission of both Dy3+ and Eu3+ ions, which consists of blue and yellow bands corresponding to the 4F9/2 → 6H15/2 (477 nm) and 4F9/2 → 6H13/2 (570 nm) transitions of Dy3+ ions and orange red and red bands attributed to the 5D0 → 7F1 (591 nm), 5D0 → 7F2 (616 nm), and 5D0 → 7F4 (697 nm) transition of Eu3+ ions, respectively. Hence, it is highly valuable to obtain color-tunable emissions through doping Dy3+ along with Eu3+ into BaGdF5 host. As shown in the inset of Fig. 3(c), the photo of BaGdF5:3%Dy3+,3%Eu3+ exhibits orange red light.
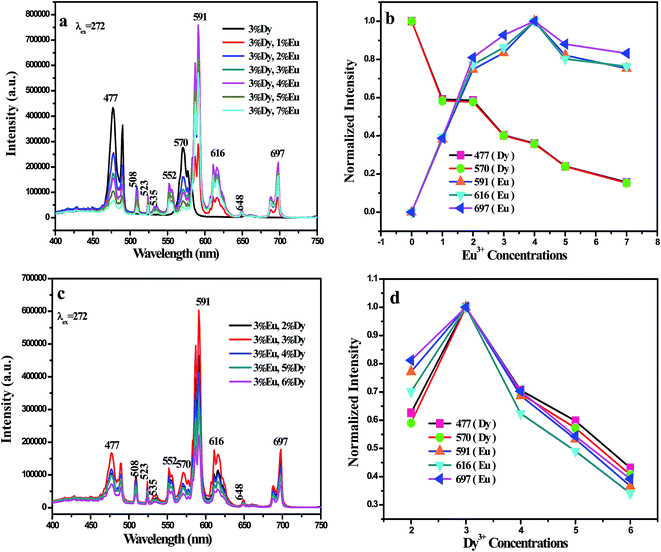
In order to further study the luminescent characteristics, including tunable color and energy transfer in Dy3+, Eu3+ co-doped BaGdF5 host, a series of BaGdF5:3%Dy3+,x%Eu3+ (x = 0, 1, 2, 3, 5, 6, 7, 8, 10) samples have been prepared. From Fig. 4(a), one can see that the characteristic emissions for both Dy3+ and Eu3+ ions can be clearly observed. Simultaneously, as shown in Fig. 4(a) and (b), with increasing Eu3+ concentration, the 4F9/2 → 6H13/2 (477 nm) and 4F9/2 → 6H13/2 (570 nm) transition emission intensities of Dy3+ decrease and the 5D0 → 7F1 (591 nm), 5D0 → 7F2 (616 nm), and (5D0 → 7F4 697 nm) emission intensities of Eu3+ increase, implying that the Eu3+ ions emission is sensitized by Dy3+ ions through energy transfer. However, it is interesting that upon increasing the Eu3+ concentration above 7%, the intensities of the Eu3+ emission begin to decrease as a result of a self-quenching effect due to the interactions between Eu3+ ions. The above results testify that tunable color can be realized by adjusting the concentration of Eu3+ and there is an energy transfer from Dy3+ to Eu3+ in BaGdF5 host.
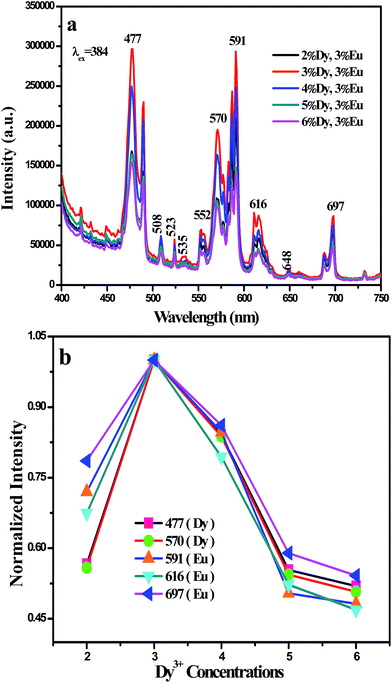
To further confirm the conclusion derived from Fig. 4(a) and (b) that luminescent characteristics and energy transfer both of Dy3+ and Eu3+ in BaGdF5 host, a series of BaGdF5:y%Dy3+,3%Eu3+ (y = 2, 3, 4, 5, 6) samples have been synthesized. The emission spectra for the BaGdF5:y%Dy3+,3%Eu3+ (y = 2, 3, 4, 5, 6) samples have also been showed in Fig. 5. It is clearly that the characteristics emissions of Dy3+ and Eu3+ ions were observed under the 384 nm. In addition, from Fig. 5(a) and (b), one can see that the Dy3+ ions emission increases firstly and then decreases when the concentration of Dy3+ is above 3% as a result of a self-quenching effect due to the interactions between Dy3+ ions. More importantly, although the Eu3+ concentration is fixed, the emission intensity of Eu3+ increases with the Dy3+ of concentration increasing. The above conclusions further support the results that the energy transfer is from the Dy3+ to Eu3+ in BaGdF5 host as well as color-tunable emissions through doping Dy3+ along with Eu3+ into BaGdF5 host can be obtain.
In view of the above studies and take into consideration the characteristic emissions of dysprosium (Dy3+) and terbium (Eu3+) ions, it is expected that the multicolor tunable emissions could be achieved in BaGdF5 system by properly designed activator contents. Therefore, a series of BaGdF5:3%Dy3+,x%Eu3+ (x = 0, 1, 2, 3, 5, 6, 7) and BaGdF5:y%Dy3+,3%Eu3+ (y = 2, 3, 4, 5, 6) samples are prepared. From Fig. 6(a) and (c), we can clearly see that the emission spectrum of the BaGdF5:3%Dy3+,x%Eu3+ (x = 0, 1, 2, 3, 5, 6, 7) and BaGdF5:y%Dy3+,3%Eu3+ (y = 2, 3, 4, 5, 6) products excited at 272 nm includes very similar Dy3+ and Eu3+ emission peaks. As shown in Fig. 6(b), the emission intensities of Eu3+ increase with the increasing of Eu3+ on concentrations. However, the emission intensities of Dy3+ ions decrease monotonously with an increase of Eu3+ ions concentration. From Fig. 6(d), one can see that the intensities of Dy3+ ions gradually increase until the content of Dy3+ ions is above 3%, which is ascribed to the cross relaxation between neighboring Dy3+ ions: Dy3+(4F9/2) + Dy3+(6H15/2) → Dy3+(6F3/2) + Dy3+(6F11/2). More importantly, although the Eu3+ concentration is fixed, the emission intensities of Eu3+ increase with the Dy3+ of concentration increasing at a certain range. All in all, the emission intensity of Dy3+ and Eu3+ varies with changing the doped concentration of Dy3+ and Eu3+ in BaGdF5 phosphors, which is benefit to obtain the multicolor tunable emission lights.
In order to search for a new and economical as well as highly efficient phosphor, many researchers have focused their interests on changing the excitation wavelength. Fig. 7 gives emission spectra of BaGdF5:3%Dy3+,3%Eu3+ sample under different wavelengths excitation. One can see that the sample exhibit the typical emissions of Dy3+ and Eu3+ under 309, 323, 363, 374, 384 and 392 nm excitation. However, the emission intensities of Dy3+ and Eu3+ ions obviously vary. Upon excitation 323 and 363 nm, only the Dy3+ ions characteristic emissions are observed. Nevertheless, when excited at 309 and 392 nm, the emission intensities of Eu3+ ions are distinctly stronger than the emission intensities of Dy3+ ions. The above result easily proved that the multicolor luminescence can be achieved in BaGdF5:Dy3+,Eu3+ system by adopting the appropriate excitation wavelength.
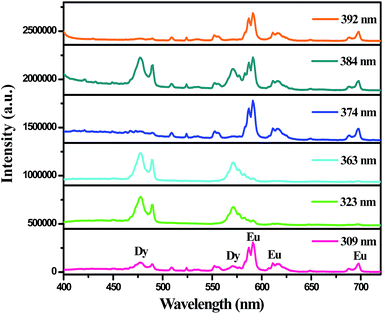 | ||
| Fig. 7 Photoluminescence emission spectra of BaGdF5:3%Dy,3%Eu3+ samples under different wavelengths excitation. | ||
For better understanding the mechanism of energy transfer phenomena, the fluorescent decay curves of Dy3+ ions emission in the BaGdF5:3%Dy3+,x%Eu3+ samples under monitored at 477 nm with irradiation of 384 nm were measured. From Fig. 8, one can see that all the luminescent decay times of Dy3+ in BaGdF5:Dy3+,Eu3+ can be fitted well with a single exponential function as24
| I = I0 + Ae−t/τ | (1) |
To further understand the energy transfer process in depth, the energy transfer efficiencies (ηT) from Dy3+ to Eu3+ are calculated using the following formula:37
| ηT = 1 − τ/τ0 | (2) |
According to Dexter and Schulman, concentration quenching is in many cases due to energy sink in the lattice is reached. As suggested by Blasse, the average separation RDy−Eu can be expressed by38
| RDy−Eu = 2 × [3V/(4πxZ)]1/3 | (3) |
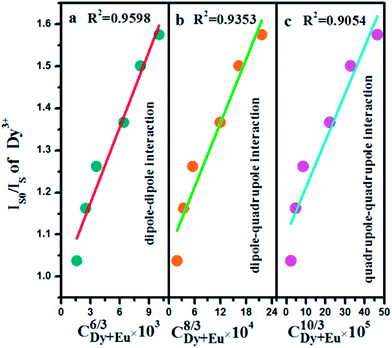
As a more detailed analysis method for the energy transfer mechanism, according to Dexter's energy transfer expressions of multipolar interaction, the following relation can be obtained:39,40
| IS0/IS ∝ Cn/3 | (4) |
Fig. 10exhibits the proposed energy transfer mechanism between Dy3+ and Eu3+, which is speculated according to literature.31,41,42 Although the energy level 4F9/2 of Dy3+ (21.14 × 103 cm−1) is slightly higher than the energy level 5D0 of Eu3+ (17.29 × 103 cm−1), the energy transfer (ET) is possible due to the phonon-aided non-radiative relaxation from 4F9/2 level of Dy3+ to the 5D0 level of Eu3+.43,44 When the samples were excited under UV irradiation, the Dy3+ firstly absorbed the energy, and then emitted blue and yellow lights due to the transition of the 4F9/2 → 6H15/2 (477 nm) and 4F9/2 → 6H13/2 (570 nm). Simultaneously, it delivered some of the energy to the Eu3+ ions, which emitted the red light based on the transition of the 5D0 → 7F1 (590 nm), 5D0 → 7F2 (616 nm) and 5D0 → 7F4 (697 nm).
As Fig. 11(A) depicted, BaGdF5:Dy3+ samples emitted blue light and the CIE chromaticity coordinates was calculated to be (0.238, 0.275) as result from the blue emission stronger than yellow emission in Dy3+ singly activated BaGdF5 phosphors. The pure Eu3+-activated BaGdF5 emitted orange-red light. From the CIE diagram of BaGdF5:3%Dy3+,x%Eu3+ (x = 0, 1, 2, 3, 4, 5, 7, 8, 10) phosphors, it can be seen that when excited at 272 and 384 nm, the trend of their color tones changes from blue to orange red light by adjusting the doping concentration of Eu3+, the corresponding selected images are presented in Fig. 11(A) (point 1–6). In addition, for the BaGdF5:y%Dy3+,3%Eu3+ (y = 0, 2, 3, 4, 5, 6) phosphors, one can see that upon excited 272 and 384 nm, the trend of their color tones changes from red to white light by adjusting the doping concentration of Dy3+, the corresponding selected images are presented in Fig. 11(B) (point 15–23). More importantly, BaGdF5:3%Dy3+,3.0%Eu3+ excited at 309, 323, 363, 374, 384 and 392 nm, the corresponding CIE coordinates for all the excitation wavelengths are determined to be (24, 0.388, 0.189), (25, 0.262, 0.276), (26, 0.270, 0.287), (27, 0.344, 0.287), (28, 0.325, 0.302) and (29, 0.451, 0.365), which are located in light red, blue green, white and red regions, respectively. The corresponding selected images under corresponding excitation wavelengths are obtained by taking pictures when the Jobin Yvon FluoroMax-4 Fluorescence Spectrophotometer is exciting the products. So the surrounding environment and light source will influence the results of the picture. All of the results indicate that multicolor luminescence can be achieved through adopting different excitation wavelengths or adjusting appropriate concentration of Dy3+ and Eu3+ in BaGdF5. Finally, the specific CIE chromaticity coordinates of BaGdF5:Dy3+,Eu3+ samples are given in the Table 1.
| Labels | Samples | Excitation (nm) | CIE (x, y) |
|---|---|---|---|
| 1 | BaGdF5:0.03Dy3+ | 272 | (0.264, 0.303) |
| 7 | BaGdF5:0.03Dy3+ | 384 | (0.253, 0.267) |
| 2 | BaGdF5:0.03Dy3+,0.01Eu3+ | 272 | (0.358, 0.345) |
| 8 | BaGdF5:0.03Dy3+,0.01Eu3+ | 384 | (0.285, 0.288) |
| 3 | BaGdF5:0.03Dy3+,0.02Eu3+ | 272 | (0.401, 0.362) |
| 9 | BaGdF5:0.03Dy3+,0.02Eu3+ | 384 | (0.308, 0.299) |
| 4 | BaGdF5:0.03Dy3+,0.03Eu3+ | 272 | (0.438, 0.376) |
| 10 | BaGdF5:0.03Dy3+,0.03Eu3+ | 384 | (0.325, 0.302) |
| 5 | BaGdF5:0.03Dy3+,0.04Eu3+ | 272 | (0.451, 0.379) |
| 11 | BaGdF5:0.03Dy3+,0.05Eu3+ | 384 | (0.351, 0.310) |
| 6 | BaGdF5:0.03Dy3+,0.07Eu3+ | 272 | (0.465, 0.365) |
| 12 | BaGdF5:0.03Dy3+,0.07Eu3+ | 384 | (0.377, 0.319) |
| 13 | BaGdF5:0.03Dy3+,0.08Eu3+ | 384 | (0.395, 0.329) |
| 14 | BaGdF5:0.03Dy3+,0.10Eu3+ | 384 | (0.420, 0.338) |
| 15 | BaGdF5:0.03Eu3+ | 272 | (0.492, 0.400) |
| 16 | BaGdF5:0.03Eu3+,0.02Dy3+ | 272 | (0.424, 0.368) |
| 20 | BaGdF5:0.03Eu3+,0.02Dy3+ | 384 | (0.320, 0.297) |
| 17 | BaGdF5:0.03Eu3+,0.04Dy3+ | 272 | (0.417, 0.367) |
| 21 | BaGdF5:0.03Eu3+,0.04Dy3+ | 384 | (0.326, 0.308) |
| 18 | BaGdF5:0.03Eu3+,0.05Dy3+ | 272 | (0.392, 0.348) |
| 22 | BaGdF5:0.03Eu3+,0.05Dy3+ | 384 | (0.297, 0.280) |
| 19 | BaGdF5:0.03Eu3+,0.06Dy3+ | 272 | (0.386, 0.344) |
| 23 | BaGdF5:0.03Eu3+,0.06Dy3+ | 384 | (0.305, 0.289) |
| 24 | BaGdF5:0.03Dy3+,0.03Eu3+ | 309 | (0.388, 0.335) |
| 25 | BaGdF5:0.03Dy3+,0.03Eu3+ | 323 | (0.262, 0.276) |
| 26 | BaGdF5:0.03Dy3+,0.03Eu3+ | 363 | (0.270, 0.287) |
| 27 | BaGdF5:0.03Dy3+,0.03Eu3+ | 374 | (0.344, 0.287) |
| 28 | BaGdF5:0.03Dy3+,0.03Eu3+ | 392 | (0.451, 0.365) |
3.3 Magnetic property
The inorganic compounds which include Gd atom exhibit paramagnetic properties. The seven unpaired inner 4f electrons of Gd3+ ions are closely bound to the nucleus and can be effectively shield by the outer closed-shell 5s2 5p6 electrons from the crystal field, which gives rise to the magnetic properties of Gd3+ ions. The magnetic moments related to Gd3+ ions are all localized and non-interacting, which led to paramagnetism of Gd3+ ions.45 Hence, the as-prepared BaGdF5:Dy3+,Eu3+ samples not only exhibit excellent fluorescent properties but also magnetic properties. The magnetic properties of the as-prepared samples were analyzed by a VSM. Fig. 12(A) and (B) show the hysteresis loops of BaGdF5:3%Dy3+,3%Eu3+ nanospheres at room temperature (300 K) and low temperature (2 K), respectively. From Fig. 12(A) and (B), one can see that the BaGdF5:3%Dy3+,3%Eu3+ exhibit a good paramagnetism in the magnetic range of −30 to 30 kOe at 300 K due to no coercivity or remanence and the magnetization was 2.02 emu g−1 (Fig. 12(A)), which approaches the value reported for nanoparticles used for common bio-separation.46,47 Whereas BaGdF5:3%Dy3+,3%Eu3+ at low temperature (2 K) show superparamagnetism with a saturation magnetization value of 83.39 emu g−1 (Fig. 12(B)). The increase in the magnetic susceptibility at low temperature might be due to the reduction in thermal fluctuation, which is typical behaviour in paramagnetic materials described by Curie's law.48 | ||
| Fig. 12 Magnetization–applied magnetic field curves of BaGdF5:3%Dy3+,3%Eu3+ nanospheres at 300 K (A) and 2 K (B), respectively. | ||
4. Conclusions
In summary, Dy3+ and Eu3+ co-doped nanophosphors have been synthesized through a hydrothermal method and the as-synthesized samples are in particle shape with the diameter of about 20 nm. Upon UV light excitation, the Dy3+ or Eu3+ ions singly activated BaGdF5 phosphors exhibit excellent emission properties in their respective regions. In addition, the energy transfer process of Dy3+ → Eu3+ has been systematically investigated in the BaGdF5 host. The energy migration from Dy3+ to Eu3+ has been verified to be an electric dipole–dipole mechanism, of which the critical distance (RDy−Eu) is estimated to be 12.59 Å. By adopting different excitation wavelengths or adjusting appropriate concentration of Dy3+ and Eu3+ ions, tunable photoluminescence are realized in Dy3+ and Eu3+ co-doped BaGdF5 system. These single-component phosphors exhibit color-tunable emissions, which may have application in full-color display. In addition, the obtained samples also exhibit paramagnetic properties at room temperature and low temperature.Acknowledgements
This present work was financially supported by the National Natural Science Foundation of China (Grant No. 21171066), and the Opening Research Funds Projects of the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry and College of Chemistry, Jilin University (2010–05).Notes and references
- W. Yin, L. Zhou, Z. Gu, G. Tian, S. Jin, L. Yan, X. Liu, G. Xing, W. Ren and F. Liu, J. Mater. Chem. C, 2012, 22, 6974–6981 RSC.
- L. Yang, G. Li, M. Zhao, J. Zheng, D. Luo, Y. Zheng and L. Li, Eur. J. Inorg. Chem., 2013, 5999–6008 CrossRef CAS.
- Y. Liu, G. Liu, J. Wang, X. Dong and W. Yu, Inorg. Chem., 2014, 53, 11457–11466 CrossRef CAS PubMed.
- Y. Yan, J. Wang, M. Hojamberdiev, Z. Lu, B. Ren and Y. Xu, J. Alloys Compd., 2014, 597, 282–290 CrossRef CAS.
- F. Meiser, C. Cortez and F. Caruso, Angew. Chem., Int. Ed., 2004, 43, 5954–5957 CrossRef CAS PubMed.
- S. Sivakumar, P. R. Diamente and F. C. van Veggel, Chem.–Eur. J., 2006, 12, 5878–5884 CrossRef CAS PubMed.
- G. Yi, H. Lu, S. Zhao, Y. Ge, W. Yang, D. Chen and L.-H. Guo, Nano Lett., 2004, 4, 2191–2196 CrossRef CAS.
- S. Sivakumar, F. C. M. van Veggel and M. Raudsepp, J. Am. Chem. Soc., 2005, 127, 12464–12465 CrossRef CAS PubMed.
- J. W. Stouwdam and F. C. van Veggel, Nano Lett., 2002, 2, 733–737 CrossRef CAS.
- J. W. Stouwdam, G. A. Hebbink, J. Huskens and F. C. van Veggel, Chem. Mater., 2003, 15, 4604–4616 CrossRef CAS.
- X. Zhang, J. Zhang and M. Gong, Opt. Mater., 2014, 36, 850–853 CrossRef CAS.
- T. Grzyb, A. Gruszeczka, R. J. Wiglusz and S. Lis, J. Mater. Chem. C, 2013, 1, 5410–5418 RSC.
- M. Jiao, N. Guo, W. Lü, Y. Jia, W. Lv, Q. Zhao, B. Shao and H. You, Dalton Trans., 2013, 42, 12395–12402 RSC.
- Y. Jia, W. Lü, N. Guo, W. Lü, Q. Zhao and H. You, Phys. Chem. Chem. Phys., 2013, 15, 6057–6062 RSC.
- S. Gai, P. Yang, D. Wang, C. Li, N. Niu, F. He and X. Li, CrystEngComm, 2011, 13, 5480–5487 RSC.
- S. Huang, J. Xu, Z. Zhang, X. Zhang, L. Wang, S. Gai, F. He, N. Niu, M. Zhang and P. Yang, J. Mater. Chem. C, 2012, 22, 16136–16144 RSC.
- C. Gong, Q. Li, R. Liu, Y. Hou, J. Wang, X. Dong, B. Liu, X. Yang, Z. Yao and X. Tan, Phys. Chem. Chem. Phys., 2013, 15, 19925–19931 RSC.
- M. Pang, X. Zhai, J. Feng, S. Song, R. Deng, Z. Wang, S. Yao, X. Ge and H. Zhang, Dalton Trans., 2014, 43, 10202–10207 RSC.
- H. Guan, G. Liu, J. Wang, X. Dong and W. Yu, Dalton Trans., 2014, 43, 10801–10808 RSC.
- P. Ghosh and A. Patra, J. Phys. Chem. C, 2008, 112, 19283–19292 CAS.
- H. Guan, G. Liu, J. Wang, X. Dong and W. Yu, RSC Adv., 2015, 5, 50611–50616 RSC.
- F. Wang, X. Xue and X. Liu, Angew. Chem., Int. Ed., 2008, 47, 906–909 CrossRef CAS PubMed.
- H. Guan, G. Liu, J. Wang, X. Dong and W. Yu, New J. Chem., 2014, 38, 4901–4907 RSC.
- Y. Liu, G. Liu, X. Dong, J. Wang and W. Yu, Phys. Chem. Chem. Phys., 2015, 17, 26638–26644 RSC.
- E. Pavitra, G. S. R. Raju, W. Park and J. S. Yu, New J. Chem., 2014, 38, 163–169 RSC.
- S. Som, P. Mitra, V. Kumar, V. Kumar, J. Terblans, H. Swart and S. Sharma, Dalton Trans., 2014, 43, 9860–9871 RSC.
- S. Zeng, M.-K. Tsang, C.-F. Chan, K.-L. Wong, B. Fei and J. Hao, Nanoscale, 2012, 4, 5118–5124 RSC.
- L. Guo, Y. Wang, Y. Wang, J. Zhang and P. Dong, CrystEngComm, 2012, 14, 3131–3141 RSC.
- D. Yang, C. Li, G. Li, M. Shang, X. Kang and J. Lin, J. Mater. Chem. C, 2011, 21, 5923–5927 RSC.
- G. Ajithkumar, B. Yoo, D. E. Goral, P. J. Hornsby, A.-L. Lin, U. Ladiwala, V. P. Dravid and D. K. Sardar, J. Mater. Chem. B, 2013, 1, 1561–1572 RSC.
- Q. Ju, D. Tu, Y. Liu, R. Li, H. Zhu, J. Chen, Z. Chen, M. Huang and X. Chen, J. Am. Chem. Soc., 2011, 134, 1323–1330 CrossRef PubMed.
- S. Rodriguez-Liviano, A. I. Becerro, D. Alcántara, V. Grazú, J. M. De la Fuente and M. Ocaña, Inorg. Chem., 2013, 52, 647–654 CrossRef CAS PubMed.
- D.-K. Ma, S.-M. Huang, Y.-Y. Yu, Y.-F. Xu and Y.-Q. Dong, J. Phys. Chem. C, 2009, 113, 8136–8142 CAS.
- C. Cao, H. K. Yang, J. W. Chung, B. K. Moon, B. C. Choi, J. H. Jeong and K. H. Kim, Mater. Res. Bull., 2012, 47, 1704–1708 CrossRef CAS.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- G. Yang, S. Gai, F. Qu and P. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 5788–5796 CAS.
- G. Li, D. Geng, M. Shang, C. Peng, Z. Cheng and J. Lin, J. Mater. Chem. C, 2011, 21, 13334–13344 RSC.
- G. Blasse and B. C. Grabmaier, Uminescent Materials, 1994, pp. 1–9 Search PubMed.
- D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063–1070 CrossRef CAS.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836–850 CrossRef CAS.
- D. Yang, X. Kang, M. Shang, G. Li, C. Peng, C. Li and J. Lin, Nanoscale, 2011, 3, 2589–2595 RSC.
- C. Lorbeer, J. Cybinska and A.-V. Mudring, Chem. Commun., 2010, 46, 571–573 RSC.
- S. Das, C.-Y. Yang and C.-H. Lu, J. Am. Ceram. Soc., 2013, 96, 1602–1609 CrossRef CAS.
- W. Xu, Q. Peng, J. Dong, Y. Gao, X. Liang, S. Wang and G. Chen, J. Am. Ceram. Soc., 2010, 93, 3064–3067 CrossRef CAS.
- H.-T. Wong, H. L. Chan and J. Hao, Appl. Phys. Lett., 2009, 95, 1–3 CrossRef.
- D. Dosev, M. Nichkova, R. K. Dumas, S. J. Gee, B. D. Hammock, K. Liu and I. M. Kennedy, Nanotechnology, 2007, 18, 055102 CrossRef PubMed.
- Z. Liu, G. Yi, H. Zhang, J. Ding, Y. Zhang and J. Xue, Chem. Commun., 2008, 694–696 RSC.
- H.-T. Wong, F. Vetrone, R. Naccache, H. L. W. Chan, J. Hao and J. A. Capobianco, J. Mater. Chem. C, 2011, 21, 16589–16596 RSC.
| This journal is © The Royal Society of Chemistry 2016 |