Ti-MIL-125-NH2 membrane grown on a TiO2 disc by combined microwave/ultrasonic heating: facile synthesis for catalytic application†
Yu-Ri Leea,
Sung-Min Choa,
Sung-Hyeon Baecka,
Wha-Seung Ahn*a and
Won-Seung Chob
aDepartment of Chemistry and Chemical Engineering, Inha University, Incheon 402-751, Republic of Korea. E-mail: whasahn@inha.ac.kr; Fax: +82 32 8720959; Tel: +82 32 8607466
bSchool of Materials Science and Engineering, Inha University, Incheon 402-751, Republic of Korea
First published on 28th June 2016
Abstract
A Ti-MIL125-NH2 membrane with a high surface coverage of uniform crystals was prepared on a TiO2 disc using a unique combination of microwave-assisted nucleation followed by ultrasonic irradiation, which produced a highly stable catalytic film for a liquid phase Knoevenagel condensation reaction.
Metal–organic frameworks (MOFs) are a new class of porous crystalline materials that are constructed of metal ions or clusters bridged via rigid organic linker groups.1 Owing to their high surface area in well-ordered porous structures and diverse post-synthetic functionalization,2 these materials have been considered for gas storage,3 adsorption/separation,4 catalysis,5 and other applications.6 Recently, MOF films or membranes supported on a different substrates7 have attracted increasing interest for gas separation8 and catalysis.9
It is difficult to produce a dense and continuous membrane,10 by direct in situ solvothermal preparation,11 in which all nucleation, growth and intergrowth of crystals on a substrate take place during the synthesis step. Therefore, it is usually necessary to introduce a suitable functional group on the support a priori, which can interact either with the metal ions or organic linkers12 to promote nucleation and adhesion on the support.13 On the other hand, it is still a challenge to form strong chemical bonding between the support surface and MOF organic linkers14 to prepare a mechanically robust MOF membrane.7,15 Consequently, improved techniques, such as rubbing,16 dip-coating,17 spin coating,18 wiping,19 microwave-assisted seeding,20 thermal seeding,21 reactive seeding,22 and repeated growth,23 have been proposed to improve the interaction between the seed layer and support. More significantly, a support material made of the same metallic species as the MOF structures was demonstrated to be desirable, because the metal source on the substrate can provide the crystallization nuclei and enhance the adhesion between the MOF layers and substrates, such as in MIL-53(Al) membrane22 on an alumina support and HKUST-1 membrane over a copper net.7
This communication reports for the first time the fabrication of a continuous and defect-free amino-functionalized Ti-MIL125-NH2 membrane on a TiO2 support. TiO2 is a good catalyst support due to the strong metal support interaction, chemical stability, and acid–base property. Heterogeneous TiO2 supported catalysts show a high potential in photocatalyst-related applications.24,25 The Ti-MIL125-NH2 is isostructural to the Ti-MIL125, which is comprised of cyclic octamers constructed from corner- or edge-sharing titanium octahedral units connected to 12 other cyclic octamers through organic linkers,26 except that the benzene dicarboxylate (BDC) ligand is replaced with 2-amino benzene dicarboxylate (BDC-NH2) in the framework. In Ti-MIL125-NH2, the amino groups are free without coordination, and octahedral and tetrahedral cages with free diameters of 10.7 Å and 4.7 Å, respectively, are accessible through triangular 5–7 Å windows (Scheme S1, ESI†). Ti-MIL125-NH2 was recently tested for photocatalytic oxidation of amines.27 Herein, a strong adhesive and uniform seeding layer on the TiO2 support was prepared and subsequently grown into a continuous highly inter-grown Ti-MIL125-NH2 membrane after being subjected to combination of microwave (MW) and ultrasonic (US) irradiation. The physicochemical properties of the prepared membranes were evaluated and compared with those samples prepared by MW or US alone. Finally, the catalytic activity of the Ti-MIL125-NH2 membrane was tested for the Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetate to confirm the membrane stability.
Fig. 1 presents the synthesis steps employed to prepare a continuous and defect-free Ti-MIL125-NH2 membrane supported on a porous TiO2 disc using the two-step combined MW and US irradiation. Although MOFs have usually been prepared via hydrothermal/solvothermal methods,28 there are reports of alternative syntheses, such as MW29 or US30 methods, in which high/intense MW and US waves led to homogeneous nucleation, rapid crystallization, and accelerated synthetic chemical reactions31 during MOFs synthesis. These desirable features can be of equal importance for the preparation of MOFs in film form.14 The experimental details for the preparation/synthesis of the Ti-MIL125-NH2 membrane and in powder form are given in ESI.†
 | ||
| Fig. 1 Schematic diagram of the fabrication steps for the Ti-MIL125-NH2 membrane on a TiO2 support via combined ultrasonic/microwave heating methods. | ||
As illustrated in the figure, the TiO2 support was chemically modified by reacting with the NH2-BDC ligand, which can improve heterogeneous nucleation by inducing covalent bonding between the linker and the support. Subsequently, the pre-treated support was soaked with a precursor solution of Ti-MIL125-NH2 and then to-step secondary crystal growth process was conducted by MW flowed by a US treatment under different synthesis condition. Hereafter, the membrane samples obtained were designated depending on the synthesis method and its treatment duration (M; microwave, U; ultrasonic, and number; duration in min). For example, Ti-MIL125-NH2 (M60/U60) represents a sample prepared by MW followed by a US treatment for 60 min each.
Initially, an attempt was made to prepare directly a Ti-MIL125-NH2 membrane supported on a bare TiO2 support using either MW or US alone. Unfortunately, no Ti-MIL125-NH2 crystals formed on the support despite the extended synthesis for 4 h at the maximum power level of 500 W (Fig. S1†). Only after the seed layer was formed on the support with the aid of the NH2-BDC ligand, were crystals of Ti-MIL125-NH2 detected after MW heating for 60 min (as the MW irradiation time was increased from 30 to 120 min, the characteristic Ti-MIL125-NH2 peaks emerged steadily). A similar US treatment, however, was ineffective in crystallization. Therefore, a seeding step was necessary to fabricate the MOF membrane, which provided the heterogeneous nucleation sites for the initial MOF layer formation. MW irradiation was, in addition, more effective in initiating nucleation than US under the same power level. Haque et al.32 reported that the activation energy for MOF crystal nucleation decreased more sharply with MW than with a US treatment.
Fig. S1† presents SEM images of Ti-MIL125-NH2 membrane growth at different stages. As shown in Fig. S1(b),† the seed layer was formed evenly over the surface of the porous TiO2 substrate while no Ti-MIL125-NH2 peaks were detected. After the MW treatment at 250 W for 60 min, disk-shaped Ti-MIL125-NH2 crystals, approximately 4 μm size, formed but the crystal density at the surface of the Ti-MIL125-NH2 (M60) membrane was rather poor (Fig. S1(e)†). A further increase in synthesis time from 60 to 120 min resulted in higher number density of the MOF crystals in the Ti-MIL125-NH2 (M120) membrane (Fig. S1(f)†), but this was not sufficient to provide complete surface coverage. These results suggest that the construction of a continuous membrane densely populated with Ti-MIL125-NH2 crystals was not possible with MW only in this case. As shown in Fig. S2,† the intensity of the XRD peaks of Ti-MIL125-NH2 (M120) was considerably lower than that of the simulated or powder form of Ti-MIL125-NH2.
US irradiation was then tested to make the Ti-MIL125-NH2 (M60) membrane denser in the crystal population, since the crystallization rates are also known to be accelerated strongly under US conditions both in the nucleation and crystal growth steps compared with the standard solvothermal processes.33 In addition, when crystals already exist on the surface, they can act as templates for the formation of new crystal nuclei or fragment to produce more nucleation sites.34 Therefore, the pre-formed crystals on the surface of the Ti-MIL125-NH2 (M60) can provide “secondary nucleation” sites for subsequent membrane growth, which can increase the crystal density on the surface when subjected to additional US irradiation. The growth of pre-existing crystals on the membrane eliminate the gaps between the particles, and the recrystallization and growth of the crystals with the incorporation of multiple MOF layers occurred by secondary seeded growth.35
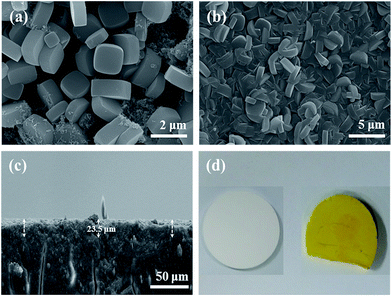
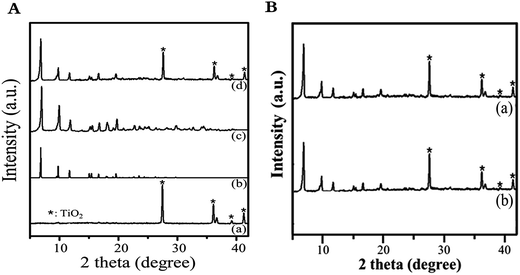
After introducing US irradiation to the Ti-MIL125-NH2 (M60) at 400 W for 30 min, approximately 90% of the surface of the obtained Ti-MIL125-NH2 (M60/U30) membrane was covered with crystals, ca. 4 μm and 1.23 μm in length and width, respectively (Fig. S1(g) and (g-1)†). However, impurities, unreacted precursors, are also present on the surface. Upon further increases in the US irradiation time to 60 min, a homogeneously yellow-coloured Ti-MIL125-NH2 (M60/U60), of which the surface was covered densely and uniformly with highly inter-grown disk-shaped Ti-MIL125-NH2 crystals, ca. 2.5 μm and 1.24 μm in length and width, respectively, were obtained without pinholes or cracks on the support (Fig. 2(b) and S1(h-1)†). The surface crystal morphology of the membrane was similar to that of Ti-MIL125-NH2 powder (Fig. 2(a)), but the Ti-MIL125-NH-2 crystals were grown mostly orientated toward c-axis. Fig. 2(c) shows cross sections of the membrane layer, which was bonded uniformly and tightly to the support with a thickness of approximately 23.5 μm. Unlike those of the Ti-MIL125-NH2 (M60/U30), Ti-MIL125-NH2 (M60/U60) consisted of more dense and uniform crystals without impurities. XRD of the Ti-MIL125-NH2 (M60/U60) membrane (Fig. 3(A)-(d)) revealed the characteristic peaks of both the TiO2 support and Ti-MIL125-NH2 particles (Fig. 3(A)-(a) and (c)), which confirmed that phase-pure crystals formed on the TiO2 support in the absence of other impure phases. Fig. S3† shows the N2 adsorption–desorption isotherms of the Ti-MIL-125-NH2 particles. The sample exhibited type I isotherms at 77 K with no hysteresis and a BET surface area of 1458 m2 g−1 with a pore volume of 0.64 cm3 g−1. As shown in Fig. S4,† EDS showed that there was a soft transition between the Ti-MIL125-NH2 layer (N signal) and TiO2 support (Ti signal), suggesting that the support was covered fully and evenly with the Ti-MIL125-NH2 crystals.
Following the successful fabrication of the densely-packed Ti-MIL125-NH2 (M60/U60) membrane, its heterogeneous catalytic activity for the Knoevenagel condensation reaction between benzaldehyde and ethyl cyanoacetate was investigated (the detailed experimental procedure is given in ESI†). Although the bulk of the MOF membranes have been applied for separation,36 MOF-based film/membrane can also function as a recyclable heterogeneous catalyst with easy catalyst separation compared to the micron-sized low density MOF particles that require rigorous centrifuging for catalyst separation after the chemical reactions.9 The MOF membrane also enables reactions in a continuous mode with a low pressure drop.37 As shown in Fig. 4, the benzaldehyde conversion increased steadily from 41 to 60% as the reaction time was increased from 0.5 to 4 h. After removing the membrane catalyst in a hot filtration experiment, no further reaction took place in the mother liquor, which confirmed the heterogeneous nature of the catalytic reaction. For a further evaluation of the stability of the catalytic membrane in the liquid phase reaction, the reactions were repeated for up to 5 cycles under the same reaction conditions (see Fig. 4(b)). At the end of each reaction cycle, the catalyst membrane was removed from the solution mixture and the catalyst was reused after washing with the solvent.
 | ||
| Fig. 4 (a) Progress of the Knoevenagel condensation reaction over the Ti-MIL-125-NH2 (M60/U60) membrane (■: reaction with catalyst, □: reaction after catalyst removal) and (b) comparison of the recycling tests of the IRMOF-3 (red) and Ti-MIL125-NH2 (M60/U60) (black) membranes in a Knoevenagel condensation reaction. The IRMOF-3 membrane was prepared on an alumina disc at 400 W for 1 h.38 (Amount of the catalysts: ca. 9 mg of Ti-MIL-125-NH2 and ca. 6.2 mg of IRMOF-3 crystals on the TiO2 and Al2O3 support, relatively.) | ||
As shown in Fig. 4(b), the catalytic activity of Ti-MIL125-NH2 (M60/U60) was maintained at 60% conversion after the 5th recycle run in the reaction, suggesting that no structural deterioration or detachment of the membrane layer from the TiO2 disc occurred after the catalytic reaction. XRD showed no obvious decrease in the intensity of the Ti-MIL125-NH2 characteristic peaks (see Fig. 3(B)). On the other hand, the conversion of the IRMOF-3 membrane prepared previously decreased from 87% to 77% after repeated runs due to loss of some weakly bond IRMOF-3 particles to the support.38,39
In conclusion, a densely packed Ti-MIL125-NH2 membrane was prepared on a TiO2 support with a uniform and high surface coverage using MW/US combined heating technique. The key step in this method was to utilize ‘secondary nucleation’ site of the porous support by changing the synthesis method from MW to US, resulting in the rapid heterogeneous nucleation and crystal growth of Ti-MIL125-NH2 crystals. Subsequent multi-secondary growth of the seeded layer produced highly inter-grown Ti-MIL125-NH2 membrane upon US irradiation. The product obtained was applied as a heterogeneous catalyst for the Knoevenagel condensation reaction, and exhibited stable and recyclable catalysis that enables easy catalyst separation.
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant number: NRF-2015R1A4A1042434).Notes and references
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 CrossRef CAS; D. J. Tranchemontagne, J. L. Mendoza-Cortès, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257 RSC.
- L. J. Murray, M. Dinča and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; U. Muller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626 RSC; A. Corma, H. Garcia and F. X. L. Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS PubMed.
- J. L. C. Rowsell, E. C. Spencer, J. Eckert, J. A. K. Howard and O. M. Yaghi, Science, 2005, 309, 1350 CrossRef CAS PubMed.
- S. H. Jhung, J. H. Lee, J. W. Yoon, C. Serre, G. Férey and J. S. Chang, Adv. Mater., 2007, 19, 121 CrossRef CAS.
- S. Bhattacharjee, D. A. Yang and W. S. Ahn, Chem. Commun., 2011, 47, 3637 RSC.
- S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Sensors, 2009, 9, 1574 CrossRef CAS PubMed; S. M. Humphrey and P. T. Wood, J. Am. Chem. Soc., 2004, 126, 13236 CrossRef PubMed; P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef PubMed.
- H. Bux, A. Feldhoff, J. Cravillon, M. Wiebcke, Y. S. Li and J. Caro, Chem. Mater., 2011, 23, 2262 CrossRef CAS; L. Fan, M. Xue, Z. Kang, H. Li and S. Qiu, J. Mater. Chem., 2012, 22, 25272 RSC; H. Bux, C. Chmelik, R. Krishna and J. Caro, J. Membr. Sci., 2011, 369, 284 CrossRef; K. Huang, S. Liu, Q. Li and W. Jin, Sep. Purif. Technol., 2013, 119, 94 CrossRef; H. Guo, G. Zhu, I. J. Hewitt and S. Qiu, J. Am. Chem. Soc., 2009, 131, 1646 CrossRef PubMed.
- Y. Liu, Z. Ng, E. A. Khan, H. K. Jeong, C. Ching and Z. Lai, Microporous Mesoporous Mater., 2009, 118, 296 CrossRef CAS; L. Diestel, H. Bux, D. Wachsmuth and J. Caro, Microporous Mesoporous Mater., 2012, 164, 288 CrossRef; S. Zhou, X. Zou, F. Sun, H. Ren, J. Liu, F. Zhang, N. Zhao and G. Zhu, Int. J. Hydrogen Energy, 2013, 38, 5338 CrossRef; J. A. Bohrman and M. A. Carreon, Chem. Commun., 2012, 48, 5130 RSC.
- S. Aguado, J. Canivet and D. Farrusseng, J. Mater. Chem., 2011, 21, 7582 RSC; R. Jin, Z. Bian, J. Li, M. Ding and L. Gao, Dalton Trans., 2013, 42, 3936 RSC; S. Aguado, J. Canivet and D. Farrusseng, Chem. Commun., 2010, 46, 7999 RSC; E. V. Ramos-Fernandez, M. Garcia-Domingos, J. Juan-Alcañiz, J. Gascon and F. Kapteijn, Appl. Catal., A, 2011, 391, 261 CrossRef CAS; Y. Mao, J. Li, W. Cao, Y. Ying, P. Hu, Y. Liu, L. Sun, H. Wang, C. Jin and X. Peng, Nat. Commun., 2014, 5, 5532 CrossRef PubMed.
- Y. P. Yampolskii, I. Pinnau and B. D. Freeman, Materials Science of Membranes for Gas and Vapor Separation, John Wiley & Sons, Ltd., New York, 2006 Search PubMed.
- M. Shah, M. C. McCarthy, S. Sachdeva, A. K. Lee and H. K. Jeong, Ind. Eng. Chem. Res., 2012, 51, 2179 CrossRef CAS.
- R. Semino, N. A. Ramsahye, A. Ghoufi and G. Maurin, ACS Appl. Mater. Interfaces, 2016, 8, 809 CAS.
- F. Schreiber, Prog. Surf. Sci., 2000, 65, 151 CrossRef CAS; J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef PubMed; A. Huang, W. Dou and J. Caro, J. Am. Chem. Soc., 2010, 132, 15562 CrossRef PubMed; E. Biemmi, C. Scherb and T. Bein, J. Am. Chem. Soc., 2007, 129, 8054 CrossRef PubMed.
- Y. Yoo and H. K. Jeong, Chem. Commun., 2008, 2441 RSC.
- J. Gascon and F. Kaptejin, Angew. Chem., Int. Ed., 2010, 49, 1530 CrossRef CAS PubMed.
- R. Ranjan and M. Tsapatsis, Chem. Mater., 2009, 21, 4920 CrossRef CAS; K. Tao, C. Kong and L. Chen, Chem. Eng. J., 2013, 220, 1 CrossRef.
- Z. Zhao, X. Ma, Z. Li and Y. S. Lin, J. Membr. Sci., 2011, 382, 82 CrossRef CAS.
- J. Hu, H. Cai, H. Ren, Y. Wei, Z. Xu, H. Liu and Y. Hu, Ind. Eng. Chem. Res., 2010, 49, 12605 CrossRef CAS.
- Y. C. Pan, B. Wang and Z. P. Lai, J. Membr. Sci., 2012, 292, 421 Search PubMed.
- H. T. Kwon and H. K. Jeong, Chem. Commun., 2013, 49, 3854 RSC.
- V. V. Guerrero, Y. Yoo, M. C. McCarthy and H. K. Jeong, J. Mater. Chem., 2010, 20, 3938 RSC.
- Y. Hu, X. Dong, J. Nan, W. Jin, X. Ren, N. Xu and Y. M. Lee, Chem. Commun., 2011, 47, 737 RSC.
- M. C. McCarthy, V. Varela-Guerrero, G. V. Barnett and H. K. Jeong, Langmuir, 2010, 26, 14636 CrossRef CAS PubMed.
- Y. Liu, J. Li, B. Zhou, H. Chen, Z. Wang and W. Cai, Chem. Commun., 2011, 47, 10314 RSC.
- Y. Liu, J. Li, B. Zhou, S. Lv, X. Li, H. Chen, Q. Chen and W. Cai, Appl. Catal., B, 2012, 485, 111 Search PubMed.
- M. A. Moreira, J. C. Santos, A. F. P. Ferreira, J. M. Loureiro, F. Ragon, P. Horcajada, P. G. Yot, C. Serre and A. E. Rodrigues, Langmuir, 2012, 28, 3494 CrossRef CAS PubMed.
- D. Sun, L. Ye and Z. Li, Appl. Catal., B, 2015, 164, 428 CrossRef CAS.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- S. H. Jhung, J. H. Lee, J. W. Yoon, C. Serre, G. Férey and J. S. Chang, Adv. Mater., 2007, 19, 121 CrossRef CAS; H. Y. Cho, D. A. Yang, J. Kim, S. Y. Jeong and W. S. Ahn, Catal. Today, 2012, 185, 35 CrossRef.
- J. Kim, S. T. Yang, S. B. Choi, J. Sim, J. Kim and W. S. Ahn, J. Mater. Chem., 2011, 21, 3070 RSC; H. Y. Cho, J. Kim, S. N. Kim and W. S. Ahn, Microporous Mesoporous Mater., 2013, 169, 180 CrossRef CAS.
- K. Suslick, Science, 1990, 247, 1439 Search PubMed; K. H. Kim and K. B. Kim, Ultrason. Sonochem., 2008, 15, 1019 CrossRef CAS PubMed.
- E. Haque, N. A. Khan, J. H. Park and S. H. Jhung, Chem.–Eur. J., 2010, 16, 1046 CrossRef CAS PubMed.
- N. A. Khan and S. H. Jhung, Coord. Chem. Rev., 2015, 285, 11 CrossRef CAS.
- M. D. Luque de Castro and F. P. Capote, Ultrason. Sonochem., 2007, 14, 717 CrossRef CAS PubMed.
- J. A. Bohrman and M. A. Carreon, Chem. Commun., 2012, 48, 5130 RSC.
- S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116 RSC; S. R. Venna and M. A. Carreon, Chem. Eng. Sci., 2015, 124, 3 CrossRef CAS.
- A. E. W. Beers, T. A. Nijhuis, N. Aalders, F. Kapteijn and J. A. Moulijn, Appl. Catal., A, 2003, 243, 237 CrossRef CAS.
- Y. R. Lee, S. M. Cho, W. S. Ahn, C. H. Lee, K. H. Lee and W. S. Cho, Microporous Mesoporous Mater., 2015, 213, 161 CrossRef CAS.
- The amount of the catalytic NH2 species attached to IRMOF-3 particles is ca. 1.4 times higher than that of Ti-MIL-125-NH2 particles for equal weight of the sample used due to different chemical structures.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, experimental details, characterizations, catalytic reaction, schematic structure of Ti-MIL-125-NH2, SEM, XRD, N2 adsorption–desorption isotherm, SEM-EDX, and recycling test. See DOI: 10.1039/c6ra09438d |
| This journal is © The Royal Society of Chemistry 2016 |