Magnetic hyperthermia-induced drug release from ureasil-PEO-γ-Fe2O3 nanocomposites†
Abstract
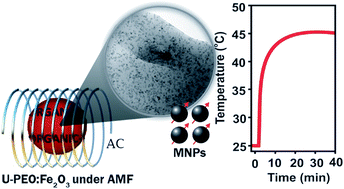
A multifunctional material suitable for cancer therapy, which combines stimuli-responsive properties for drug delivery and magnetic hyperthermia prepared by a one-pot sol–gel synthesis from the conjugation of ureasil cross-linked poly(ethylene oxide) (U-PEO) hybrid materials with superparamagnetic nanoparticles (γ-Fe2O3), is reported in this communication.

 Please wait while we load your content...
Please wait while we load your content...