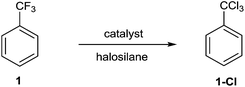
Catalytic halodefluorination of aliphatic carbon–fluorine bonds†
Kelvin K. K. Goh,
Arup Sinha,
Craig Fraser and
Rowan D. Young*
Department of Chemistry, National University of Singapore, 117543, Singapore. E-mail: rowan.young@nus.edu.sg
First published on 25th April 2016
Abstract
A variety of halosilanes, in conjunction with aluminum catalysts, convert fluorocarbons into higher halocarbons. Bromination and iodination of fluorocarbons are more effective than chlorination in terms of yield and activity. The mechanism for the reaction is investigated utilizing experimental and computational evidence and preliminary results suggest an alternate mechanism to that reported for the related hydrodefluorination reaction.
Fluorine–carbon chemistry has established a ‘foot-hold’ as one of the most utilized areas of modern chemistry.1 Fluorine–carbon bonds have found their way into refrigerants, agrochemicals, pharmaceutical drugs and plastics, while the incorporation of fluorine into organic substrates can be very useful given its chemical inertness, electron withdrawing power and ability to act as an NMR spectator.2 As such, a variety of powerful methods for the introduction of fluorine or fluorine-containing groups into organic molecules have recently been developed. However, the late-stage modification of C–F bonds suffers greatly from the inherent chemical stability of the carbon–fluorine bond and catalytic transformation of these bonds to other functional groups remains challenging, especially for sp3 hybridised carbon–fluorine bonds.3
Recent reports have shown the use of silylium, phosphonium and aluminium intermediates as effective hydrodefluorination (HDF) catalysts for aliphatic C–F bonds (Scheme 1).4 Despite the importance of efficient HDF protocols to counter the release of CFCs, HCFCs and HFCs, the substitution of fluorine by hydrogen offers little potential for further functionalization and/or recycling of fluorine compounds. In addition, the conditions for HDF are conducive to Friedel–Crafts alkylations, preventing the clean conversion to product in the presence of aromatic systems. Conversely, the conversion of carbon–fluorine bonds into other halogen–carbon bonds offers novel access to the ability to further functionalise substrates with well-developed synthetic methods that are effective in the presence of chloro-, bromo- and iodo-organic substrates, but largely ineffective with organofluorides. Additionally, the incorporation of higher halides into many materials is of intrinsic value. For example, the ability to access novel brominated flame retardant additives to supersede the current controversially employed additives is of commercial interest.5
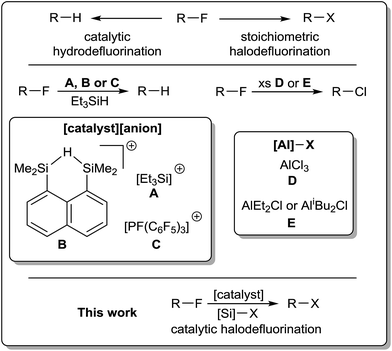 | ||
| Scheme 1 Metal-free hydrodefluorination (HDF) has recently been achieved using (inter alia) catalyst systems A, B and C ([anion] = [B(C6F5)4]− or [HCB11H5Cl6]−).4a,b,c,f Aluminum reagents for stoichiometric halodefluorination (HaloDF), also previously reported, include D and E.7b,c | ||
Indeed, the conversion of fluorine to other halogens has been achieved previously in stoichiometric reactions using group 13 trihalides. Such reactivity was first reported by Henne and Newman as early as 1938 using excess aluminum trichloride,6 and has since been utilized using boron or aluminum chlorides, bromides, iodides and even alkanides (Scheme 1).7 This protocol has also been extended to the formation of C–C bonds from trihydrocarbyl aluminum reagents and the scrambling of C–X/C–F bonds in mixed halofluorocarbons.7b,8 Such reactions suffer from Friedel–Crafts side-reactivity and are thus typically carried out under controlled conditions (e.g. low temperature). In all of these cases, the formation of a very stable group 13 element–fluorine bond has driven reactivity. Silicon is also known to be extremely fluorophilic, with many reported HDF studies using silicon–fluoride bond formation as a thermodynamic sink. As such, we decided to explore the opportunity of driving halodefluorination (HaloDF) using cheap and readily available halosilanes.
Herein, we describe a catalytic process that converts carbon–fluorine bonds into carbon–chloride, bromide and iodide bonds in moderate to high yields. Our process utilizes a variety of halosilanes and catalytic quantities of aluminum halide.
Attempted chlorodefluorination (ChloroDF) of our test substrate, benzotrifluoride (1), using a catalytic amount of aluminum chloride (20 mol%) in combination with excess trimethylsilyl chloride, TMSCl (5 equiv.), resulted in complete consumption of 1, but only trace amounts of the desired product, benzotrichloride (1-Cl) (Table 1-1). Running the reaction with deficient TMSCl resulted in quantitative conversion of 1 to 1-Cl based on TMSCl,9 likely due to a fluoro-aluminum halide catalyst resting state being much less efficient at Friedel–Crafts reactivity.10 As such, the use of stoichiometric, or slightly deficient, halosilane resulted in complete consumption of 1 and high yields of 1-Cl (Table 1-3 and 1-4).
| Entry | Catalyst | Halogen reagent | Solvent | Temp./time (°C/h) | C–F conv. (%) | Yield (%) |
|---|---|---|---|---|---|---|
| a General conditions: 1 (ca. 1 M); reaction volume 1 mL; C6D6 (0.1 mL) added for locking purposes; CF conversion based on C6F6 internal standard; yield determined by GC-MS except where isolated.b Isolated yield in absence of C6D6.c 0.2 equiv. of Et2O added. | ||||||
| 1 | AlCl3 (0.2) | Et3SiCl (5) | PhCl | 80/20 | 100 | Trace |
| 2 | AlCl3 (0.2) | TMSCl (0.66) | PhCl | 80/20 | 24 | 24 |
| 3 | AlCl3 (0.2) | TMSCl (3) | PhCl | 80/20 | 97 | 80 |
| 4 | AlCl3 (0.2) | TMSCl (2.8) | PhCl | 80/20 | 98 | 78 |
| 5 | AlCl3 (0.2) | Et3SiCl (3) | PhCl | 80/42 | 94 | 65 |
| 6 | AlCl3 (0.2) | SiCl4 (0.75) | Neat | 80/20 | 100 | 94b |
| 7 | AlCl3 (0.05) | SiCl4 (0.75) | Neat | 80/20 | 100 | 84 |
| 8 | AlCl3 (0.05) | TMSCl (2.8) | Neat | 80/60 | 87 | 80 |
| 9 | — | TMSCl (3) | PhCl | 80/20 | 0 | 0 |
| 10 | — | SiCl4 (0.75) | PhCl | 80/42 | 0 | 0 |
| 11 | AlCl3 (0.2) | TMSCl (2.8) | C6H4Cl2 | 80/42 | 99 | 71 |
| 12 | AlCl3 (0.2) | TMSCl (2.8) | DCE | 80/42 | 96 | 83 |
| 13 | AlCl3 (0.2) | TMSCl (2.8) | PhH | 80/42 | 96 | 60 |
| 14 | AlCl3 (0.2) | TMSCl (2.8) | PhMe | 80/42 | 88 | 62 |
| 15 | AlCl3 (0.2) | TMSCl (2.8) | n-C8H18 | 80/65 | 32 | 30 |
| 16 | AlCl3 (0.2) | TMSCl (2.8) | PhCl | 80/48 | 34 | 29c |
| 17 | AlF3 (0.2) | TMSCl (2.8) | PhCl | 80/63 | 19 | 10 |
Employing silicon chloride as a halogen source further improved the reaction efficiency. In this case, delivery of multiple chloride atoms resulted in mono, di, tri and tetrafluorides of silicon as by-products – thus complete conversion of 1 to 1-Cl could be accomplished using only 0.75 equiv. of SiCl4. Notably, the loss of silicon fluoride gas by-product from the reaction together with precipitation of hydrolysed aluminum catalyst (i.e. exposure to atmosphere) facilitated the isolation of pure product in high yield (Table 1-6).
Control reactions in the absence of aluminum halide between 1 and TMSCl or SiCl4 showed no evidence of silicon fluorides or 1-Cl formation (Table 1-9 and 1-10).
A solvent screen revealed chlorinated alkanes and arenes to be effective solvents, while the reaction tolerated electron rich arenes to a large extent (Table 1-11 to 1-15). Although GC-MS identified Friedel–Crafts products as by-products when benzene, toluene and benzene-d6 were employed as solvents, moderate yields of 1-Cl could still be achieved. The low solubility of aluminum chloride in n-octane solvent resulted in slow conversion, with only 32% HFC conversion after 65 h, however, little by-product was observed with almost quantitative generation of 1-Cl based on HFC conversion.
When aluminum fluoride was employed as a catalyst, negligible reactivity was observed, with a conversion of only 19% after 63 hours (Table 1-17). Aluminum fluoride is less able to act as a molecular Lewis acid as compared to its higher homologues due to its high lattice energy and very low solubility,11 but may become activated by halogen exchange with halosilane. Bromodefluorination (BromoDF) and iododefluorination (IodoDF) were achieved using the homohalogen systems TMSBr/AlBr3 and TMSI/AlI3. BromoDF reactions proceeded to provide comparable, or higher, conversions/yields to ChloroDF reactions. IodoDF provided high C–F conversion of 1, but benzotriiodide was found to be light- and temperature-sensitive, compromising product yield. Running the reaction at room temperature alleviated the decomposition of 1-I, but large quantities of decomposition were still observed (Table 2).
| a General conditions: substrate (ca. 1 M); reaction volume 1 mL, PhCl solvent; C6D6 (0.2 mL) added for locking purposes 80 °C, 21 hours.b General conditions: substrate (ca. 1 M); reaction volume 1 mL, PhCl solvent; C6D6 (0.2 mL) added for locking purposes room temperature, 1 hour.c General conditions: substrate (ca. 1 M); reaction volume 1 mL, PhCl solvent; C6D6 (0.2 mL) added for locking purposes 80 °C, 42 hours.d General conditions: substrate (ca. 1 M); reaction volume 1 mL, PhCl solvent; C6D6 (0.2 mL) added for locking purposes 80 °C, 90 hours.e In the absence of C6D6.f Product was found to be thermally and photolytically unstable and product decomposed during reaction at room temperature.g Yield determined by 1H NMR.h Other bromo-octane isomers account for 52%.i Reaction proceeded prior to addition of catalyst.j Product yield could not be accurately determined using 1H NMR or GC-MS.k Single product detected by GC-MS.l Major product is 1-(CBr3)-4-(CF3)-C6H4 (26% yield).m sp2 C–F bond did not react.n CF conversion based on C6F6 internal standard, yield determined by GC-MS except where isolated. |
|---|
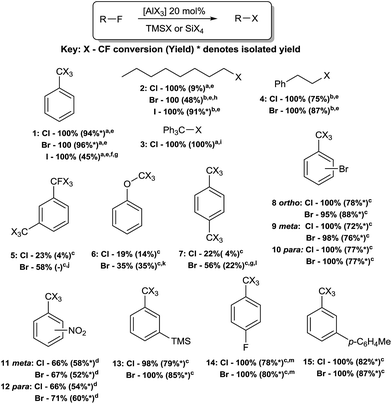 |
Reaction scope was tested using substrates 1–15. High HaloDF conversion was observed in primary fluoroalkanes, triphenylmethyl fluoride and benzylic C–F bonds, even with electron-deficient arenes. Bis(trifluoro)arenes proved exceptions, likely due to the larger number of sp3 C–F bonds to convert. HaloDF of phenyl ether 6 proceeded to low C–F conversions and yields. This result correlates to the reduced HaloDF activity observed in the presence of ether (Table 1-16), and may reflect competing oxygen coordination to the catalyst as opposed to an increased C–F bond strength in 6.
Reaction conversion correlated well with product yield with the exception of alkane 2-Br (100% conversion, 48% yield) and alkane 2-Cl (100 conversion, 9% yield). In these reactions, it was found that the remainder of the 1-fluorooctane was converted into 2-, 3- and 4-halooctanes. In the case of alkyl fluoride 4, rearrangement products were significantly reduced.
Plausible mechanisms of the above described HaloDF reaction were explored through a series of control reactions supported with calculated free energy changes.
Two pathways for the substitution of fluoride with halide present themselves as plausible mechanisms. Aluminum halide acts as a HaloDF agent that is regenerated by halosilane (Scheme 2 mechanism A), or that the halosilane and aluminum halide form an ‘encounter complex’ that activates the silane towards attack of the fluoride (Scheme 2 mechanism B).
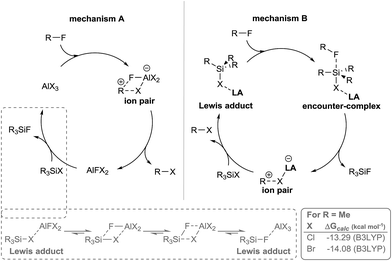 | ||
| Scheme 2 HaloDF is postulated to occur in one of two distinct cycles. Mechanism A: aluminum halide acts as a HaloDF agent that is regenerated by halosilane (see ESI for computational details†), mechanism B: aluminum halide, acting as a Lewis acid, activates halosilane towards HaloDF. | ||
The formation of hydrosilane-borane adducts has been postulated to occur in hydrosilylation reactions of carbonyls and olefins, and may play a role in specific HDF reactions.12,13 Recently crystallographic evidence of both a borane–silane adduct and an alane–silane adduct were reported supporting the ability of Lewis acids to activate hydrosilanes.14 Strong Lewis acids and halosilanes have also been reported to form such adducts.15 Recently, there have been reports of aluminum Lewis acids acting as catalysts for HDF via the formation of aluminum–hydrosilane adducts.14a,16
Conversely, aluminum halides are known to facilitate HaloDF in a stoichiometric manner in the absence of halosilane. Regeneration of aluminum halides from silicon halides warranted investigation, given that the generation of aluminum halides from the reaction of alumina and silicon halides is a well-known industrial process.17 However, reports also exist for the chlorination of fluorosilanes facilitated by aluminum chloride.18
The formation of halosilane–aluminum halide Lewis adducts is pivotal to both postulated mechanisms (A and B). Such adducts were spectroscopically observed by us to form prior to addition of HFC substrate and have been studied previously by NMR in solution, and typically result in deshielding of the silicon nucleus.15b,15c,19 However, it is unclear as to whether such adducts act as pre-catalysts, catalyst resting states or as active intermediates in the catalytic cycle.
Few data are available on calculated or experimentally observed geometries of aluminum–silicon halide bridged adducts, and none on simple homoleptic aluminum systems.20 As such geometries and bond energies of TMS–X–AlX3 adducts were calculated (X = F, Cl, Br, I) (Table S1, ESI†). Calculated free energy determined the overall HaloDF reaction to be exergonic (Table S2, ESI†).
Halide exchange between aluminum fluoride and TMSX (X = Cl, Br) was observed to generate TMSF. Heating samples of aluminum fluoride with 1.05 equivalents of either TMSCl or TMSBr in benzene-d6 for 48 hours produced small amounts of TMSF (<10% conversion). Although these reactions were observed to occur slowly in respect to HaloDF reaction times, the strong lattice network of solid AlF3, restricting its ability to act as a molecular species, and the relative insolubility of AlF3 as compared to AlFX2 may account for the slow observed exchange.12 Indeed, when aluminum fluoride was employed as a catalyst, slow reactivity was observed, with a conversion of only 19% after 63 hours (Table 1-17).
We were not able to obtain TMSF to establish the exchange equilibrium constant between TMSF and higher aluminum halides, however, reported syntheses relying upon this exchange would seem to indicate the presence of an equilibrium with relatively little free energy.18
To support this proposal, free energies for silicon halide/aluminum halide exchanges were calculated. In agreement with the exchanges observed by NMR, the change in free energies supports the favorable regeneration of TMSX from AlX3 and TMSF (Table S3, ESI†). HaloDF is likely to be kinetically assisted using TMSX and SiCl4 reagents, given that both TMSF and SiF4 are gases at SATP. This may also explain the reactivity differences between TMSCl and Et3SiCl as HaloDF reagents, given that Et3SiF has a boiling point of 107 °C.
The nature of the reaction invokes comparison with the related SN2 Finkelstein reaction, however, the generation of an intermediate carbocation species, pertinent to both mechanisms A and B, is supported by a number of observations. (1) The use of Et3SiCl or Et3SiBr as HaloDF agents in the HaloDF of benzotrifluoride also generates small amounts of benzyl halide and dihalide, presumably via hydride abstraction from the alkyl silane; (2) benzylic fluorides prove to be better substrates than aliphatic fluorides; (3) the HaloDF of primary alkylfluorides results in rearrangement products. To account for the low amounts of Friedel–Crafts by-products, we propose that aluminum tetrahalide-carbocation ‘ion pairs’ are formed that are able to combine at a faster rate than arene attack of carbocation intermediates.
In HDF, the hydride is presumed to be delivered to the carbocation intermediate directly by the hydrosilane. To determine whether a similar mechanism may operate in HaloDF, we added an equivalent of TMSCl to [CPh3][B(C6F5)4], after which no evidence of trityl chloride formation was observed by NMR spectroscopy or GC-MS spectrometry. Indeed, reaction of [Et3Si][B(C6F5)4] (generated in situ from Et3SiH and [CPh3][B(C6F5)4]) with 5 equivalents of benzotrifluoride and 15 equivalents of either Et3SiCl or TMSCl produced one equivalent of Et3SiF or TMSF,21 but no benzotrichloride. These reactions imply that in catalysis, halosilane does not quench the carbocation intermediate directly.
It was also found that the strong Lewis acid, B(C6F5)3 devoid of higher halogen substituents, did not promote HaloDF, even though fluoride abstraction by B(C6F5)3 has been reported.12
In conclusion, we have developed a catalytic method to effectively convert aliphatic fluorocarbons into higher halocarbons in high yield using cheap and accessible aluminum catalysts and silicon halide reagents under mild, practical conditions. Such conversions may have potential applications in the processing of HFCs, the production of brominated flame retardants and the late-stage-functionalization of carbon–fluorine bonds. Preliminary mechanistic studies suggest an alternate mechanism as compared to those reported for HDF reactions.
Acknowledgements
We thank the National University of Singapore and the Singapore Ministry of Education for financial support (WBS R-143-000-586-112).References
- R. N. Perutz, Science, 2008, 321, 1168 CrossRef CAS PubMed.
- G. Sandford, Philos. Trans. R. Soc., A, 2000, 358, 455 CrossRef CAS.
- T. G. Richmond, In Activation of Unreactive Bonds and Organic Synthesis, Topics in Organometallic Chemistry, ed. P. S. Murai, H. Alper, R. A. Gossage, V. V. Grushin, M. Hidai, Y. Ito, W. D. Jones, F. Kakiuchi, G. van Koten, Y.-S. Lin, Y. Mizobe, S. Murai, M. Murakami, T. G. Richmond, A. Sen, M. Suginome and A. Yamamoto, Springer Berlin Heidelberg, 1999, pp. 243–269 Search PubMed.
- (a) V. J. Scott, R. Çelenligil-Çetin and O. V. Ozerov, J. Am. Chem. Soc., 2005, 127, 2852 CrossRef CAS PubMed; (b) C. Douvris and O. V. Ozerov, Science, 2008, 321, 1188 CrossRef CAS PubMed; (c) R. Panisch, M. Bolte and T. Müller, J. Am. Chem. Soc., 2006, 128, 9676 CrossRef CAS PubMed; (d) C. Douvris, C. M. Nagaraja, C.-H. Chen, B. M. Foxman and O. V. Ozerov, J. Am. Chem. Soc., 2010, 132, 4946 CrossRef CAS PubMed; (e) M. Klahn, C. Fischer, A. Spannenberg, U. Rosenthal and I. Krossing, Tetrahedron Lett., 2007, 48, 8900 CrossRef CAS; (f) C. B. Caputo, L. J. Hounjet, R. Dobrovetsky and D. W. Stephan, Science, 2013, 341, 1374 CrossRef CAS PubMed.
- Y. R. Kim, F. A. Harden, L. M. L. Toms and R. E. Norman, Chemosphere, 2014, 106, 1 CrossRef CAS PubMed.
- A. L. Henne and M. S. Newman, J. Am. Chem. Soc., 1938, 60, 1697 CrossRef CAS.
- (a) J. Riera, J. Castañer, J. Carilla and A. Robert, Tetrahedron Lett., 1989, 30, 3825 CrossRef CAS; (b) J. Terao, M. Nakamura and N. Kambe, Chem. Commun., 2009, 45, 6011 RSC; (c) J. Terao, S. A. Begum, Y. Shinohara, M. Tomita, Y. Naitoh and N. Kambe, Chem. Commun., 2007, 43, 855 RSC; (d) Y. Mizukami, Z. Song and T. Takahashi, Org. Lett., 2015, 17, 5942 CrossRef CAS PubMed.
- (a) W. Gu, M. R. Haneline, C. Douvris and O. V. Ozerov, J. Am. Chem. Soc., 2009, 131, 11203 CrossRef CAS PubMed; (b) H. Bozorgzadeh, E. Kemnitz, M. Nickkho-Amiry, T. Skapin and J. M. Winfield, J. Fluorine Chem., 2001, 107, 45 CrossRef CAS; (c) V. A. Petrov, C. G. Krespan and B. E. Smart, J. Fluorine Chem., 1996, 77, 139 CrossRef CAS; (d) E. Kemnitz and D. H. Menz, Prog. Solid State Chem., 1998, 26, 97 CrossRef CAS; (e) L. E. Manzer and V. N. Rao, Adv. Catal., 1993, 39, 329 CAS.
- The yield of 1-Cl based on available TMSCl was 110%, indicating that aluminium chloride is responsible for the balance of excess product.
- N. O. Calloway, J. Am. Chem. Soc., 1937, 59, 1474 CrossRef CAS.
- T. Krahl and E. Kemnitz, J. Fluorine Chem., 2006, 127, 663 CrossRef CAS.
- (a) D. J. Parks and W. E. Piers, J. Am. Chem. Soc., 1996, 118, 9440 CrossRef CAS; (b) S. Rendler and M. Oestreich, Angew. Chem., Int. Ed., 2008, 47, 5997 CrossRef CAS PubMed.
- C. B. Caputo and D. W. Stephan, Organometallics, 2012, 31, 27 CrossRef CAS.
- (a) J. Chen and E. Y.-X. Chen, Angew. Chem., Int. Ed., 2015, 54, 6842 CrossRef CAS PubMed; (b) A. Y. Houghton, J. Hurmalainen, A. Mansikkamäki, W. E. Piers and H. M. Tuononen, Nat. Chem., 2014, 6, 983 CrossRef CAS PubMed.
- (a) Y. S. Song, B. R. Yoo, G. H. Lee and I. N. Jung, Organometallics, 1999, 18, 3109 CrossRef CAS; (b) G. A. Olah and L. D. Field, Organometallics, 1982, 1, 1485 CrossRef CAS; (c) G. A. Olah, L. Heiliger, X. Y. Li and G. K. S. Prakash, J. Am. Chem. Soc., 1990, 112, 5991 CrossRef CAS.
- M. Ahrens, G. Scholz, T. Braun and E. Kemnitz, Angew. Chem., Int. Ed., 2013, 52, 5328 CrossRef CAS PubMed.
- D. Gencev, K. S. Mogyorosi, S. Riederauer and J. Szepvolgyi, Process for the preparation of aluminum trichloride and silicon dioxide by chlorination of alumina with silicon tetrachloride, US4416862 A, November 22, 1983.
- (a) C. Eaborn, J. Chem. Soc., 1953, 494 RSC; (b) R. Müller and C. Dathe, Z. Anorg. Allg. Chem., 1961, 313, 207 CrossRef; (c) U. Wannagat, H. Bürger and E. Ringel, Monatsh. Chem., 1962, 93, 1363 CrossRef CAS; (d) W. C. Schumb and D. W. Breck, J. Am. Chem. Soc., 1952, 74, 1754 CrossRef CAS.
- Z. Xie, D. J. Liston, T. Jelínek, V. Mitro, R. Bau and C. A. Reed, J. Chem. Soc., Chem. Commun., 1993, 384 RSC.
- (a) J. Xu and Y. H. Ding, Aust. J. Chem., 2014, 67, 740 CrossRef CAS; (b) M. Rohde, L. O. Müller, D. Himmel, H. Scherer and I. Krossing, Chem.–Eur. J., 2014, 20, 1218 CrossRef CAS PubMed.
- Formed presumably via reaction between benzotrifluoride and a chlorine bridged disilyl cation {i.e. [(Me3Si)(μ-Cl)(SiR3)]+; R = Me, Et}, see: M. Lehmann, A. Schulz and A. Villinger, Angew. Chem., Int. Ed., 2009, 48, 7444 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures, calculated structures and free energy calculations. See DOI: 10.1039/c6ra09429e |
| This journal is © The Royal Society of Chemistry 2016 |