Membrane active cationic cholic acid-based molecular umbrellas†
Julie Kempf and
Andreea Schmitzer*
Département de Chimie, Université de Montréal, Montréal, Québec, Canada. E-mail: ar.schmitzermontreal.ca
First published on 22nd April 2016
Abstract
Herein, we report the synthesis of an umbrella thread and its covalent dimer and their transmembrane transport properties under physiological conditions. The anion selectivity and the transport mechanism of the two compounds are discussed, as well as their interaction with microbial membranes. All the transport results indicate the formation of pores inside the phospholipid membranes. Both umbrella compounds principally favor the anion exchange across the membrane but the umbrella monomer (UM) is more sensitive to the hydrophilicity of the transported anion and favors the H+/Cl− co-transport in the presence of bicarbonate. The umbrella dimer (UD) shows a significant Cl−/HCO3− antiport process. This anion selectivity between the two umbrella compounds is an important finding and can be exploited for biological applications.
Introduction
The cell membrane, composed mainly of phospholipids, is an effective, but yet complex barrier that controls the passage of molecules into or out of a cell. Some neutral compounds such as water or carbon dioxide can easily pass through the phospholipid bilayer, whereas ions and polar solutes are repelled.1 In order to ensure the correct regulation of ion concentration inside and outside the cell, transmembrane proteins present in the cellular membranes transport ions such as chloride or potassium, in very specific and regulated processes. Missing or defective ion channels generate serious diseases called channelopathies and the cystic fibrosis is the most eloquent example of too few open chloride channels in the cellular membrane.2 In systematic efforts to develop strategies to fight these channelopathies, many research groups had focused their efforts on developing synthetic molecules and systems able to compensate the dysfunction of transmembrane proteins.3–9 For example, Gale et al. developed (thio)ureas-based anion transporters exhibiting strong binding affinity for chloride.10–13 They reported that the lipophilicity of the substituents on the (thio)urea scaffold was essential for the transporter to penetrate into the phospholipid bilayer and induce chloride transport via a carrier mechanism.13 On the other hand, Regen's group has extensively studied the cholic acid-based![[left double angle bracket]](https://www.rsc.org/images/entities/char_27ea.gif) molecular umbrellas
molecular umbrellas![[right double angle bracket]](https://www.rsc.org/images/entities/char_27eb.gif) for the transport of hydrophilic ions and molecules.14–16 Depending on the polarity of the media, molecular umbrellas can adopt two different conformations where different parts of the system are exposed to the solvent. Furthermore, Savage et al. had investigated the antimicrobials activity of different ceragenins, which are cationic steroids composed of a sterol backbone functionalized with different substituents, such as amino acids or guanidine groups.17–19 More recently, Chen et al. reported a new family of anion transporters which activity was based on the amphomorphism of the cholic acid. Their systems composed of two steroid moieties functionalized with amino, guanidino or squaramide groups, were able to promote chloride transmembrane transport via a cation/anion symport process.20–22
for the transport of hydrophilic ions and molecules.14–16 Depending on the polarity of the media, molecular umbrellas can adopt two different conformations where different parts of the system are exposed to the solvent. Furthermore, Savage et al. had investigated the antimicrobials activity of different ceragenins, which are cationic steroids composed of a sterol backbone functionalized with different substituents, such as amino acids or guanidine groups.17–19 More recently, Chen et al. reported a new family of anion transporters which activity was based on the amphomorphism of the cholic acid. Their systems composed of two steroid moieties functionalized with amino, guanidino or squaramide groups, were able to promote chloride transmembrane transport via a cation/anion symport process.20–22
Our group has extensively studied imidazolium23,24 and benz-imidazolium25,26 cations in the transport of chloride across phospholipid membranes and demonstrated the antibacterial applications of these anion transporters. However, other anions possess a key role in mammalian physiology. Bicarbonate (HCO3−) is one of the most important inorganic anions present in living systems4 that is essential for many biological processes, such as the regulation of the intracellular pH,27 the maintenance of the cell volume and respiration.28,29 The dysfunction of bicarbonate transporters is involved in many diseases, such as renal tubular acidosis, kidney diseases or cardiac ischemia.30 Because bicarbonate is less polarizable, larger and more hydrophilic than chloride, its transport across phospholipid membranes is more difficult than the chloride and usually occurs via a Cl−/HCO3− antiport process in biological systems. The development of a synthetic system that can mimic a natural transmembrane protein allowing Cl−/HCO3− antiport is still a challenge. In the last years, Davis et al. demonstrated that ceramides were good candidates to mediate Cl−/HCO3− antiport transport across phospholipid membranes.31
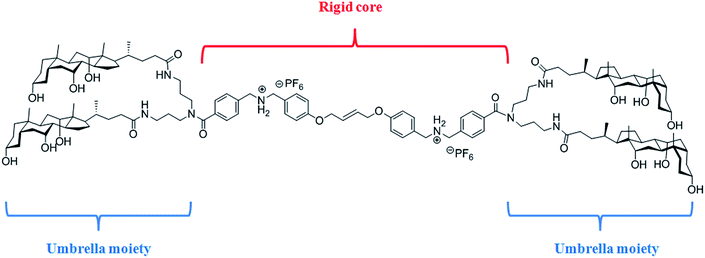
We previously reported an umbrella thread with transmembrane chloride transport properties, its incorporation into a [2]-rotaxane and its capacity to transport small cyclic hydrophilic molecules across phospholipid bilayers.32 We had demonstrated the amphomorphism of this umbrella thread and validated their ability to penetrate and span phospholipid bilayers. We pursued the study of this system by designing a longer umbrella thread that can span the entire phospholipid bilayer, possessing two umbrellas, one at each extremity. Moreover, this thread can be used to assemble [3]-rotaxanes by complexation of cyclic molecules around the two quaternary ammonium sites. We present here the synthesis of a new umbrella thread and its covalent dimer. The transport properties of the umbrella monomer and dimer under physiological conditions were investigated. The anion selectivity and the transport mechanism of the two compounds are discussed here. Finally, the antimicrobial properties towards Gram-positive and Gram-negative bacteria are reported.
Results and discussion
Design
We previously reported the self-association of an umbrella thread inside phospholipid membranes and the formation of a dimeric channel spanning the membrane from one side to the other one. We proposed that inside this channel, anions can pass through by a succession of anion/cation or anion/π interactions with the umbrella thread.33 In order to obtain an umbrella thread that spans the entire phospholipid bilayer, we designed an umbrella monomer that can be used in the synthesis of a homodimer thread by metathesis. The benzyl group used previously to protect the phenol group was replaced by a terminal olefin. As shown in the Fig. 1, the umbrella thread is composed of a rigid core possessing two quaternary ammonium sites and two umbrella extremities formed by four cholic acids, to favour its insertion into a phospholipid bilayer.Synthesis
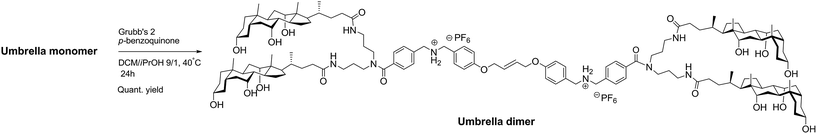
The synthesis of the umbrella thread was based on our already reported procedure.32 The 4-(allyloxy)benzaldehyde 1 and the 4-aminobenzoate 2 were condensed to give the first moiety of the thread 3. The secondary amine group was then protected with a N-Boc group and the ester moiety was hydrolyzed to obtain compound 4. Only the use of HOOBt as coupling agent allowed the coupling between 4 and 6 to give compound 7 (DCC and EDC were also tested), which was deprotected in acidic conditions before the anion metathesis step, yielding to the umbrella monomer (UM). The overall yield for the synthesis of UM after 11 steps was 25%, which represents a significant increase compared to the one we previously reported32 (Scheme 1).The synthesis of the umbrella dimer (UD) was realized between two equivalents of UM in the presence of the Grubbs' 2nd generation catalyst (Scheme 2).34 The use of an alcohol as solvent was necessary for the solubilization of UM, but a polar solvent, associated with a high reaction temperature led often to the decomposition of the catalyst.35 The ruthenium hydride complex formed in these conditions can also be responsible for the isomerization of the double bond. To prevent this olefin iso-merization, as suggested by Grubbs et al., we used an electron deficient p-benzoquinone as additive in catalytic amount.36 The terminal allyl ether group on UM is a type I olefin that undergoes rapid homo-dimerization,37 producing ethylene that can be easily removed under vacuum. Only the trans isomer possesses the proper geometry to allow the dimer to span the entire phospholipid bilayer and even if the Grubbs's 2 catalyst doesn't usually favour the formation of one isomer relative to the other one, we were very pleased to observe that the bulky umbrella moiety disfavored the formation of the cis isomer. 13C NMR spectra of the UD, revealed only a chemical shift at 67.6 ppm for the carbon α, confirming the trans relative configuration38 (see ESI, Fig. S4†).
Interaction with the phospholipid bilayer and chloride transport properties
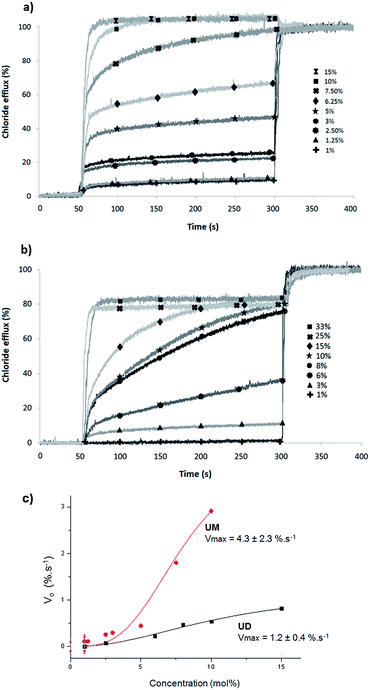
The transport efficiencies of UM and UD were first compared in large unilamellar vesicles (LUVs) egg-yolk phosphatidylcholine (EYPC) liposomes containing lucigenin. Lucigenin is a fluorescent probe which fluorescence is quenched by the presence of chloride (Fig. 2).39 Transport experiments were conducted with liposomes containing 100 mM NaCl, 2 mM lucigenin and 10 mM phosphate buffer solution (pH 6.2), suspended in an isotonic 100 mM NaNO3 and 10 mM phosphate buffer solution (pH 6.2). The concentrations of UM and UD were varied from 1 to 30 mol% relative to EYPC concentration (5 mM) and the increase of lucigenin's fluorescence was followed, this being characteristic of a chloride efflux from the LUVs. At the end of each experiment, Triton X was added to lyse the liposomes and obtain the maximum of lucigenin's fluorescence (Fig. 3a and b).Based on the kinetic experiments realized at different concentrations of UM or UD, a comparison of the initial rates (Vo) at less than 10% of the maximum transport process for UM and UD was realized. The Hill analysis clearly shows that UM penetrates more rapidly in the phospholipid membrane, compared to UD (Fig. 3c). The presence of the two umbrella extremities may be unfavourable for the fast insertion of UD in the hydrophobic layer of the membrane.
An additional Hill analysis was performed to determine the half maximum effective concentration (EC50,250 s) for chloride transport after 250 s. The EC50 values obtained were respectively 0.25 mM (5.7 ± 0.5 mol% relative to EYPC concentration) for UM and 0.28 mM (6.4 ± 0.25 mol%) for UD.
As the chloride transport inside the membrane requires the formation of the active species in this process, the EC50 value of UM is relative to its dimeric assembly inside the membrane. Thus, the similar EC50 values for UM and UD confirm our previous hypothesis on the dimeric assembly of UM inside the phospholipid bilayer.33
The identification of the transport mechanism, the formation of transmembrane pores versus a mobile carrier mechanism, was performed in EYPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol (7
cholesterol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) liposomes. Cholesterol rigidifies and orders the membrane by increasing the energy barrier of the movement of phospholipids inside the bilayer (rotation, lateral diffusion or phospholipid flip-flop).40 The transport efficiency of a transmembrane channel is not influenced by the rigidity of the membrane, whereas a mobile transporter encounters more difficulties to diffuse across a more rigid phospholipid/cholesterol membrane. The same chloride efflux was induced by UM and UD in the presence or absence of cholesterol, suggesting the formation of a transmembrane channel in both cases (see ESI, Fig. S7†).
3) liposomes. Cholesterol rigidifies and orders the membrane by increasing the energy barrier of the movement of phospholipids inside the bilayer (rotation, lateral diffusion or phospholipid flip-flop).40 The transport efficiency of a transmembrane channel is not influenced by the rigidity of the membrane, whereas a mobile transporter encounters more difficulties to diffuse across a more rigid phospholipid/cholesterol membrane. The same chloride efflux was induced by UM and UD in the presence or absence of cholesterol, suggesting the formation of a transmembrane channel in both cases (see ESI, Fig. S7†).
Since UM and UD are large enough to disrupt the integrity of the membrane and induce the lysis of liposomes, carboxyfluorescein (CF) leakage assays were performed to confirm the stability of the liposomes during the transport experiments. CF is a fluorescent probe that self-quenches its fluorescence when encapsulated at high concentrations into liposomes. In the case where the membrane of the liposomes is disrupted, a very fast increase of fluorescence is observed, due to the fast release of the CF and its dilution into the extravesicular media. On the other hand, as CF is a large and membrane impermeable dye, a slow increase of fluorescence is often characteristic of the formation of large pores in the bilayer. CF leakage experiments were performed on liposomes containing 20 mM CF and 100 mM NaCl dispersed in a 100 mM NaCl extravesicular solution, to prevent chloride self-diffusion. No CF leakage was observed even with 13.5 mol% of UM and UD (concentration inducing 100% of chloride efflux in the previous assays), demonstrating the stability and the integrity of the membrane during the transport experiments. Regen's and Chen's groups have already reported that umbrella systems form large pores across the membranes.41 In our case, only a very high concentration of UD (60 mol%) allowed a slow CF release, while for UM almost no CF leakage was observed even at this concentration. These results suggest that UD forms larger pores than UM (Fig. 4).
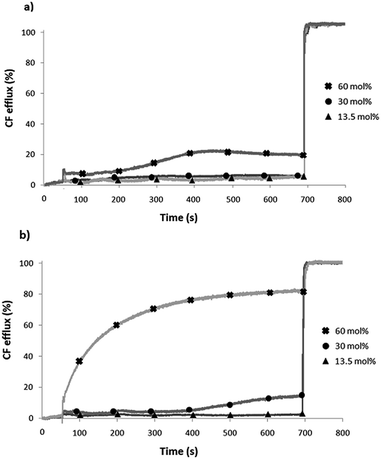 | ||
| Fig. 4 Carboxyfluorescein leakage assays in EYPC liposomes. (a) UM and (b) UD were added at 50 s and Triton X was added at 700 s. Each curve is the average of three independent measurements. | ||
The transport of ions by synthetic transmembrane channels usually occurs via passive transport mechanisms in which the charge balance across the phospholipid membrane is usually maintained.1 This is achieved by either a symport mechanism, where both an anion and a cation are transported, or by an antiport mechanism, where two anions are transported or exchanged across the membrane in opposite directions.42 To identify this mechanism and ion selectivity of UM and UD, chloride efflux experiments were first performed using different chloride salts (MCl with M+: Na+, Cu2+, Ca2+) as intravesicular solutions using the lucigenin-based assay. The chloride efflux was not influenced by the nature of the different cations used, suggesting only a slow contribution of the Cl−/M+ symport transport process (see ESI, Fig. S6†).
Further Cl−/X− antiport mechanism studies were performed by changing the nature of the extravesicular anions. The permeability of the phospholipid membrane to different external anions influences the chloride efflux if the antiport mechanism is dominant. Nitrate, perchlorate and bicarbonate are more hydrophobic than sulfate.43 In the case of UD, no variation was observed when these different anions were present in the extravesicular solution. However, the chloride transport efflux induced by UM was decreased when sulfate was used as external anion, which is characteristic to an antiport process (see ESI, Fig. S5†). Hydrated anions such as bicarbonate or sulfate favour the co-transport of proton/chloride due to their difficulty to diffuse across the membranes.
Further transport experiments were performed with liposomes containing 8-hydroxy-1,3,6-pyrenetrisulfonate (HPTS), a pH-fluorescent probe possessing a protonated (I0) and a deprotonated (I1) form, with excitation wavelengths at 403 nm and 460 nm respectively. The variation of the ratio I1/I0 is a direct evidence of a proton/chloride co-transport across the membrane. Hence, an increase of the ratio I1/I0 is indicative of protons transport outside the liposome. Experiments were performed with liposomes containing an HPTS solution (HPTS 0.1 mM, 100 mM NaCl, 10 mM phosphate buffer solution, pH = 6.4). As observed in the previous experiments, the variation of the extravesicular solution influences the efficiency of the anion transport, but also the transport of protons (Fig. 5). A more important increase of the ratio I1/I0 was observed when sulfate was used as external anion for both UM and UD. This fast increase of the ratio I1/I0 in presence of UD was followed by a slow decrease, corresponding to the return at an equilibrated anions concentration between the intra- and the extravesicular solutions.44 On the other hand, the co-transport of protons was more pronounced when UM was used in the bicarbonate solution. This suggests a lower efficiency of UM to translocate bicarbonate across phospholipid bilayers, compared to UD. The slight increase of the I1/I0 ratio induced by UD corresponds to a very slow alkalinisation of the intravesicular solution, indicating a minor amount of HCl transported across the bilayer.44 The important result that deserves to be pointed out is the selectivity of UD to transport bicarbonate in a Cl−/HCO3− antiport process, similar to the natural systems.
All these transport results indicate the formation of pores inside the phospholipid membranes. Both umbrella compounds principally favor the anion exchange across the membrane. However, UM and its self-assembled dimer formed in the bilayer are more sensitive to the hydrophilicity of the transported anion and favor the H+/Cl− co-transport in the presence of bicarbonate. UD favours the Cl−/HCO3− antiport process. This anion selectivity between the two umbrella compounds is an important finding and can be exploited for biological applications, if able to penetrate more sophisticated cellular membranes.
Antibacterial studies
The capacity of UM and UD to penetrate more complex phospholipid bilayers was evaluated in Gram-negative (E. coli SK037) and Gram-positive bacteria (B. thuringiensis HD73). As we previously reported the capacity of benzimidazolium salts to alter the permeability of the bacterial membranes and induce cellular death,25 the minimal inhibitory concentrations (MIC) for these two strains were determined and are reported in the Table 1.| Gram-positive B. thuringiensis | Gram-negative E. coli | Liposomes | ||
|---|---|---|---|---|
| MIC (μM) | Bacteriostatic concentration (μM) | MIC (μM) | EC50,250 s (mol%) | |
| UM | 8–10 | 5–30 | >100 | 5.7 ± 0.5 |
| UD | >10 | n.d. | >10 | 6.4 ± 0.25 |
Even if in liposomes UM and UD showed the same EC50,250 s, they do not have the same antimicrobial activity in Gram-negative and Gram-positive bacteria. Bacterial membranes are more complex environments, containing other components than phospholipids, such as proteins or peptidoglycans. In the case of UD, due to its poor solubility in the biological conditions, the higher concentration studied was 10 μM. The MIC values for B. thuringiensis and E. coli are higher than this concentration.
For UM, a lower MIC value was obtained for B. thuringiensis, compared to E. coli. This value is similar to those previously reported for other cholic acid derivatives17,18,45 Savage et al. have already discussed the mechanism of interaction of cholic acid derivatives with bacterial membranes.46 They proposed an initial association step between the sterol and the lipopolysaccharides at the microbial surface, before the insertion of the hydrophobic part of the compound into the phospholipid bilayer.
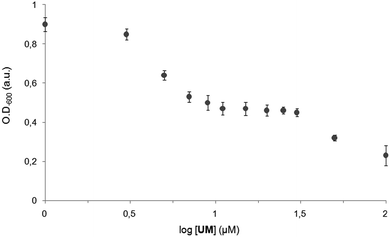
The presence of a second umbrella moiety in the structure of UD, may lead to a stronger interaction with the negatively charged bacterial surface, preventing its insertion. In this regard, the number of cholic acid units seems to dictate the capacity of the compound to penetrate and destabilize the bacterial membrane. Even if in liposomes both UM and UD showed the ability to penetrate the phospholipid bilayer, the mass and volume of the transporter seem to affect this ability in a more complex environment, as a bacterial membrane. Another observation that can be outlined from this study is the considerable bacteriostatic effect of UM for Gram-negative E. coli at concentrations between 5 and 30 μM (Fig. 6). This bacteriostatic activity in E. coli47,48 may be due to an interference of UM in the bacterial respiration which usually reduces bacterial division, without stopping it.49 The more pronounced antibacterial activity of UM that can be observed above 50 μM may correspond to the concentration at which UM starts to self-assemble as pore-forming dimers in the bacterial membrane and trigger bacterial death.
Conclusions
In conclusion, new umbrella monomer UM and dimer UD threads have been synthesized and their capacities to promote chloride transmembrane transport were investigated. Transport experiments in synthetic liposomes showed the penetration and formation of pores into phospholipid membranes. The larger UD transporter forms larger pores in phospholipid bilayers, but shows no significant improvement in the chloride transport process. However, UD is more efficient than UM for the transport of large anions such as bicarbonate, via an antiport mechanism. Nevertheless, the smaller UM shows a more pronounced antibacterial activity for Gram-negative and Gram-positive bacteria, demonstrating its ability to penetrate complex phospholipid environments. This is an important result and work is under progress in our group to assemble a [3]-rotaxane by complexation of biologically active macrocycles on the quaternary ammonium sites of UM and UD. The exploitation of these mechanically interlocked systems as a vehicle to favour the translocation of a macrocyclic drug through phospholipid bilayers remains an interesting challenge that we are currently addressing.Acknowledgements
We gratefully acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC), the Fonds Québécois de la Recherche sur la Nature et les Technologies (FRQ-NT) and the Université de Montréal for the financial support. We also thank Le Centre Régional de RMN and Le Centre Régional de Spectrométrie de masse of the Université de Montréal.Notes and references
- J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843 RSC.
- M. J. Welsh, FASEB J., 1990, 4, 2718 CAS.
- H. Valkenier, C. M. Dias, K. L. Porter Goff, O. Jurcek, R. Puttreddy, K. Rissanen and A. P. Davis, Chem. Commun., 2015, 14235 RSC.
- M. Lisbjerg, H. Valkenier, B. M. Jessen, H. Al-Kerdi, A. P. Davis and M. Pittelkow, J. Am. Chem. Soc., 2015, 137, 4948 CrossRef CAS PubMed.
- L. González-Mendoza, B. Altava, M. I. Burguete, J. Escorihuela, E. Hernando, S. V. Luis, R. Quesada and C. Vicent, RSC Adv., 2015, 5, 34415 RSC.
- J. H. Lee, J. H. Lee, Y. R. Choi, P. Kang, M. G. Choi and K. S. Jeong, J. Org. Chem., 2014, 79, 6403 CrossRef CAS PubMed.
- X. D. Wang, S. Li, Y. F. Ao, Q. Q. Wang, Z. T. Huang and D. X. Wang, Org. Biomol. Chem., 2016, 14, 330 CAS.
- N. L. Mora, A. Bahreman, H. Valkenier, H. Li, T. H. Sharp, D. N. Sheppard, A. P. Davis and A. Kros, Chem. Sci., 2016, 7, 1768 RSC.
- N. Rodriguez-Vazquez, M. Amorin, I. Alfonso and J. R. Granja, Angew. Chem., 2016, 55, 4506 Search PubMed.
- C. J. E. Haynes, N. Busschaert, I. L. Kirby, J. Herniman, M. E. Light, N. J. Wells, I. Marques, V. Félix and P. A. Gale, Org. Biomol. Chem., 2014, 12, 62 CAS.
- R. B. Elmes, N. Busschaert, D. D. Czech, P. A. Gale and K. A. Jolliffe, Chem. Commun., 2015, 10107 RSC.
- M. Olivari, R. Montis, L. E. Karagiannidis, P. N. Horton, L. K. Mapp, S. J. Coles, M. E. Light, P. A. Gale and C. Caltagirone, Dalton Trans., 2015, 44, 2138 RSC.
- M. J. Spooner and P. A. Gale, Chem. Commun., 2015, 4883 RSC.
- L. L. Cline, V. Janout, M. Fisher, R. L. Juliano and S. L. Regen, Bioconjugate Chem., 2011, 22, 2210 CrossRef CAS PubMed.
- V. Janout, L. L. Cline, B. P. Feuston, L. Klein, A. O'Brien, T. Tucker, Y. Yuan, L. A. O'Neill-Davis, R. L. Peiffer, S. S. Nerurkar, V. Jadhav, D. M. Tellers and S. L. Regen, Bioconjugate Chem., 2014, 25, 197 CrossRef CAS PubMed.
- V. Janout, W. A. Schell, D. Thevenin, Y. Yu, J. R. Perfect and S. L. Regen, Bioconjugate Chem., 2015, 26, 2021 CrossRef CAS PubMed.
- C. Li, M. R. Lewis, A. M. Gilbert, M. D. Noel, D. H. Scoville, G. W. Allman and P. B. Savage, Antimicrob. Agents Chemother., 1999, 43, 1347 CAS.
- E. J. Schmidt, J. S. Boswell, J. P. Walsh, M. M. Schellenberg, T. W. Winter, C. Li, G. W. Allman and P. B. Savage, J. Antimicrob. Chemother., 2001, 47, 671 CrossRef CAS PubMed.
- X. Z. Lai, Y. Feng, J. Pollard, J. N. Chin, M. J. Rybak, R. Bucki, R. F. Epand, R. M. Epand and P. B. Savage, Acc. Chem. Res., 2008, 41, 1233 CrossRef CAS PubMed.
- Y.-M. Lu, L.-Q. Deng and W.-H. Chen, RSC Adv., 2014, 4, 43444 RSC.
- L. Q. Deng, Z. Li, Y. M. Lu, J. X. Chen, C. Q. Zhou, B. Wang and W. H. Chen, Bioorg. Med. Chem. Lett., 2015, 25, 745 CrossRef CAS PubMed.
- Z. Li, L. Q. Deng, J. X. Chen, C. Q. Zhou and W. H. Chen, Org. Biomol. Chem., 2015, 13, 11761 CAS.
- J. Gravel, J. Kempf and A. Schmitzer, Chem.–Eur. J., 2015, 21, 18642 CrossRef CAS PubMed.
- M. Vidal, C.-R. Elie, S. Campbell, A. Claing and A. R. Schmitzer, MedChemComm, 2014, 5, 463 RSC.
- C. R. Elie, G. David and A. R. Schmitzer, J. Med. Chem., 2015, 58, 2358 CrossRef CAS PubMed.
- J. Kempf, N. Noujeim and A. R. Schmitzer, RSC Adv., 2014, 4, 42293 RSC.
- D. Sterling and J. R. Casey, Biochem. J., 1999, 344, 221 CrossRef CAS PubMed.
- P. T. Bonar and J. R. Casey, Channels, 2014, 2, 337 CrossRef.
- P. A. Gale, Acc. Chem. Res., 2011, 44, 216 CrossRef CAS PubMed.
- E. Cordat and J. R. Casey, Biochem. J., 2009, 417, 423 CrossRef CAS PubMed.
- W. A. Harrell Jr, M. L. Bergmeyer, P. Y. Zavalij and J. T. Davis, Chem. Commun., 2010, 3950 RSC.
- C. Chhun, J. Richard-Daniel, J. Kempf and A. R. Schmitzer, Org. Biomol. Chem., 2013, 11, 6023 CAS.
- C. Chhun and A. R. Schmitzer, MedChemComm, 2011, 2, 987 RSC.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953 CrossRef CAS PubMed.
- M. Hassam, A. Taher, G. E. Arnott, I. R. Green and W. A. van Otterlo, Chem. Rev., 2015, 115, 5462 CrossRef CAS PubMed.
- S. H. Hong, D. P. Sanders, C. W. Lee and R. H. Grubbs, J. Am. Chem. Soc., 2005, 127, 17160 CrossRef CAS PubMed.
- A. K. Chatterjee, T. L. Choi, D. P. Sanders and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 11360 CrossRef CAS PubMed.
- F. Grellepois, B. Crousse, D. Bonnet-Delpon and J. P. Begue, Org. Lett., 2005, 7, 5219 CrossRef CAS PubMed.
- B. A. McNally, A. V. Koulov, B. D. Smith, J. B. Joos and A. P. Davis, Chem. Commun., 2005, 1087 RSC.
- M. R. Krause and S. L. Regen, Acc. Chem. Res., 2014, 47, 3512 CrossRef CAS PubMed.
- W. H. Chen, X. B. Shao and S. L. Regen, J. Am. Chem. Soc., 2005, 127, 12727 CrossRef CAS PubMed.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038 CrossRef CAS PubMed.
- N. Busschaert, P. A. Gale, C. J. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis and W. A. Harrell Jr., Chem. Commun., 2010, 6252 RSC.
- S. K. Berezin and J. T. Davis, J. Am. Chem. Soc., 2009, 131, 2458 CrossRef CAS PubMed.
- A. J. Rasras, T. H. Al-Tel, A. F. Al-Aboudi and R. A. Al-Qawasmeh, Eur. J. Med. Chem., 2010, 45, 2307 CrossRef CAS PubMed.
- C. Li, L. P. Budge, C. D. Driscoll, B. M. Willardson, G. W. Allman and P. B. Savage, J. Am. Chem. Soc., 1999, 121, 931 CrossRef CAS.
- M. N. Melo, R. Ferre and M. A. Castanho, Nat. Rev. Microbiol., 2009, 7, 245 CrossRef CAS PubMed.
- D. Roversi, V. Luca, S. Aureli, Y. Park, M. L. Mangoni and L. Stella, ACS Chem. Biol., 2014, 9, 2003 CrossRef CAS PubMed.
- M. A. Lobritz, P. Belenky, C. B. Porter, A. Gutierrez, J. H. Yang, E. G. Schwarz, D. J. Dwyer, A. S. Khalil and J. J. Collins, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterizations of the compounds. Experimental details for measurement of chloride transport using lucigenin and HPTS assays, antibacterial protocols. See DOI: 10.1039/c6ra07663g |
| This journal is © The Royal Society of Chemistry 2016 |