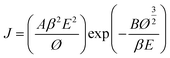Template synthesis of metal tungsten nanowire bundles with high field electron emission performance†
Yong Liu‡
a,
Kun Lan‡a,
Mahir H. Es-Sahebb,
Ahmed A. Elzatahry*c and
Dongyuan Zhao*a
aDepartment of Chemistry, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, State Key Laboratory of Molecular Engineering of Polymers, Laboratory of Advanced Materials, iChEM Collaborative Innovation Center of Chemistry for Energy Materials, Fudan University, Shanghai, 200433, China. E-mail: dyzhao@fudan.edu.cn; Web: http://www.mesogroup.fudan.edu.cn/
bDepartment of Mechanical Engineering, College of Engineering, King Saud University-Mozahmiya Campus, P.O. Box 800, Riyadh 11421, Kingdom of Saudi Arabia
cMaterials Science and Technology Program, College of Arts and Sciences, Qatar University, P.O. Box 2713, Doha, Qatar. E-mail: aelzatahry@qu.edu.qa; Web: http://www.qu.edu.qa/msp/
First published on 23rd June 2016
Abstract
One-dimensional (1D) metal nanowires are of great importance for nanodevices due to their distinctive optical, electronic and mechanical properties. Here, for the first time, we report a simple H2 reduction-assisted hard-templating approach to fabricate metallic tungsten (W) nanowire bundles by using mesoporous silica SBA-15 as a template. The metal W nanowires with a length of 300–500 nm and diameter of 5–8 nm have long-range regularity over large areas because of their inter-wire pillar connections. Each nanowire has body-centred cubic (bcc)-W crystalline structure with a [110] preferential growth direction. As a field electron emitter, the W nanowires bundles show a low turn-on field of 4.1 V μm−1, a high field enhancement factor up to 3563 and good field emission stability (with a fluctuation below 5% over 32 h).
Introduction
In recent years, one-dimensional (1D) nanomaterials have attracted great attention in materials science.1–10 In particular, 1D metal nanowires with an ordered mesostructure and a high surface area have been intensively investigated because of their unique size-and shape-dependent electronic, magnetic and optical properties.11–19 To date, two main synthetic strategies (hard-templating method and soft-templating method) have made wonderful contribution for synthesizing 1D metal nanowires. Especially, the hard-templating method has been considered as the most versatile and straight strategy toward 1D nanowires since the size and shape can be easily directed using a well-defined hard template matrix.20 Typically, Ryoo and co-workers have reported the preparation of Pt nanowires using mesoporous silica MCM-41 and SBA-15 as hard templates.21–23 Stucky and co-workers have reported the synthesis of well-ordered Au, Ag and Pt nanowires using SBA-15 as a hard template.24 And also, we have reported the synthesis of highly ordered In2O3,25 carbon,26 SiC,27 CdS,28 WS2,29 MoS2 (ref. 29) and Co3O4@carbon nanotube arrays30 with hexagonal (p6mm) or cubic (Ia3d) mesostructures replicated from SBA-15 or KIT-6 hard templates. However, up to now, very few work have reported on the synthesis of metallic W nanowire bundles despite their important applications in various electronic devices, switches and metal-dielectric capacitors. In practice, it is difficult to obtain a full density of ordered metallic W nanowire bundles. These may attribute two reasons primarily: (i) the inorganic tungsten precursors are often easily absorbed on the external surface of hard templates rather than a complete impregnation inside their mesopore channels, which causes a disordered frameworks with insufficient internal cross-linkage. (ii) The ultra-high heat treatment (>1000 °C) for tungsten precursor-reduction can easily lead to structural collapse, rather than a continuous metallic frameworks with ordered mesostructures.Up to date, many efforts have been devoted to the exploration of 1D nanostructured field emitters. Typically, carbon nanotubes (CNTs) and Ag nanowires exhibited relatively low turn-on fields. However, the lack of long-term and high-temperature FE stability has greatly hindered their development in practical applications. The metallic W (the most important refractory metal materials) has been used for FE applications for decades, not only because of its high electronic emissivity but also due to its exceptional thermal and chemical stabilities31 (such as the highest melting point, ∼3420 °C, the lowest vapor pressure among all metals, and its excellent corrosion resistance to metal and oxide vapors). Previous studies32 have proved that individual tungsten nanowires possess an ultrahigh field enhancement factor (two orders of magnitude higher than that of CNTs33,34) and a high stability of the FE current density (a standard deviation of less than 1%, in contrast to 24% shown by individual multiwalled CNTs33).
In this communication, we demonstrate a simple H2 reduction-assisted hard-templating approach to synthesize a metallic W nanowire bundles using SBA-15 as a hard template. To the best our knowledge, this is the first demonstration of synthesizing metallic W nanowire bundles under a low reduction temperature (800 °C) with H2. The typical preparation procedure is shown in Fig. 1. Firstly, a phosphotungstic acid (H3PW12O40·6H2O, PTA) precursor is completely filled into the mesochannels of SBA-15 via a solvent evaporation-induced impregnation process. Subsequently, the impregnated PTA precursor is converted to metallic body-centered cubic (bcc)-W phase using H2 as a reducing agent at a temperature of 800 °C. After etching the SBA-15 template with HF aqueous solution, the metallic W nanowire bundles are successfully obtained with nearly 95% yield.
 | ||
| Fig. 1 Schematic representation of the H2 reduction-assisted hard-templating approach for the preparation of metallic W nanowire bundles from mesoporous silica SBA-15. | ||
Experimental section
Synthesis of mesoporous silica SBA-15 hard template
The mesoporous silica SBA-15 hard template was prepared by hydrothermal synthesis according to the established procedures.36,37 Typically, 25.0 g of triblock copolymer Pluronic P123 was dissolved in 700 mL of water and stirred overnight. The solution was heated to 38 °C and 110 mL of 37 wt% HCl was added. After 1 h, 54.0 g of TEOS was poured in while stirring rigorously, and the mixture was kept at the same temperature for 24 h. The solution was then transferred into a stainless-steel autoclave and heated to 130 °C for 3 days. The white solids were collected by filtration, washed with deionized water, and dried. Finally, the SBA-15 hard template was obtained after the calcination at 550 °C for 5 h at a heating rate of 1.5 °C min−1 in air. The obtained SBA-15 template has a rod-like morphology, with particle diameters of 400–600 nm and lengths of 1–3 μm.Synthesis of metallic tungsten nanowire bundles
Metallic W nanowire bundles were prepared via a H2 reduction-assisted hard-templating method by using SBA-15 as a template. The first step was a nanocasting process. H3PW12O40·6H2O (PTA) precursor was incorporated into the mesochannels of mesoporous silica SBA-15 via a solvent evaporation induced impregnation process. Typically, 0.8 g of SBA-15 template obtained above and 3.5 g of PTA powders were added into an open beaker with 20.0 g of ethanol. The mixture was stirred at room temperature (25 °C) until ethanol was completely evaporated out. The residual powders were dried at 45 °C for 12 h to obtain PTA@SBA-15 composite. Then, the composite PTA@SBA-15 was transferred into a tube furnace for H2 reduction. The tube furnace was swept by H2 to completely remove air, and then heated to 800 °C at a rate of 1 °C min−1 and kept at this temperature for 3 h. When the furnace was cooled down to a room temperature, a dark brown W@SBA-15 powder was collected. The obtained W@SBA-15 powder was stirred with 100 mL of 5 wt% HF aqueous solution for 2 h to etch the mesoporous silica SBA-15 templates. Finally, 2.43 g of the metallic W products was collected after washing with water and acetone, filtering, and drying at 80 °C for 24 h. The final yield was calculated to be 95 wt% on the basis of PTA.Materials characterization
Transmission electron microscopy (TEM) experiments were conducted on a JEOL JEM-2100F (UHR) microscope (Japan) operated at 200 kV. Energy-dispersive X-ray (EDX) and high-angle annular dark-field scanning TEM (HAADF-STEM) analyses were performed with a FEI Talos F200X electron microscope at 200 kV with an EDX detector system. The samples for TEM and EDX measurements were suspended ultrasonically in ethanol and supported onto a carbon-coated copper grid. Field-emission scanning electron microscopy (FESEM) images were collected on the Hitachi Model S-4800 field-emission scanning electron microscope. X-ray diffraction (XRD) patterns were recorded with a Bruker D8 powder X-ray diffractometer (Germany) using Cu Kα radiation (40 kV, 40 mA). Small angle X-ray scattering (SAXS) measurements were taken on a Nanostar U small angle X-ray scattering system (Bruker, Germany) using Cu Kα radiation (40 kV, 35 mA). The d-spacing values were calculated by the formula d = 2π/q. Nitrogen sorption isotherms were measured at 77 K with a Micromeritics Tristar 3020 analyzer. All of the samples were degassed under vacuum at 180 °C for at least 8 h prior to the measurement. The Brunauer–Emmett–Teller (BET) method was utilized to calculate the specific surface areas using adsorption data in a relative pressure range from 0.05 to 0.25. The pore size distributions (PSD) were derived from the adsorption branch of the isotherms using the Barrett–Joyner–Halenda (BJH) model. The total pore volume (Vt) was estimated from the adsorbed amount at a relative pressure P/P0 of 0.995. X-ray photoelectron spectroscopy (XPS) was recorded on an AXIS ULTRA DLD XPS system with MONO Al source (Shimadzu Corp.). Photoelectron spectrometer was recorded by using monochromatic Al KR radiation under vacuum at 5 × 10−9 Pa. All of the binding energies were referenced to the C1s peak at 284.6 eV of the surface adventitious carbon.Field-emission measurements
The field emission measurements of the metallic W nanowire bundles were carried out in a vacuum chamber at a base pressure of 1.0 × 10−6 Pa at room temperature. The W nanowire powder was integrated on silicon substrate by spin-coating method. Typically, the W nanowire powder was dispersed in ethanol solution (4.5 wt%, sonicated for 15 min) and then spin-coated on pre-cleaned n-type silicon (9.0 × 10−3 Ω cm). Finally, the spin-coated silicon was heated to 110 °C on a hotplate for 30 min. The area of the FE cathode is 0.04 cm2. A copper probe was used as an anode and the metallic W nanowire bundles on the Si substrate as a cathode. The area of the FE cathode was 0.04 cm2. The distance between the anode and the metallic W nanowire arrays was maintained at 150 μm. The current–voltage (I–V) characteristics were measured using a Keithley 6485 picoammeter and a Keithley 248 high voltage supply. The Fowler–Nordheim (FN) theory is expressed by the following equation:where J is the field emission current density, E is the mean field between the cathode and anode, Ø is the work function, β is the field enhancement factor, and A and B are two constants (A = 1.54 × 10−6 A eV V−2, B = 6.83 × 103 eV−3/2 V μm−1).
Results and discussions
The high-quality SBA-15 hard template35,36 is synthesized with a hydrothermal treatment at 130 °C for 3 days. This treatment can increase the inter-mesoporosity on the walls of SBA-15 and thus enable more connections that stabilize metal W nanowire bundles. The small-angle X-ray scattering (SAXS) pattern of the mesoporous silica SBA-15 template shows five well-resolved diffraction peaks, which are indexed to the 100, 110, 200, 210 and 220 brag reflections of the hexagonal mesostructure, space group p6mm symmetry (Fig. 2A). After a complete impregnation of PTA, the PTA@SBA-15 composite displays two diffraction peaks. The cell parameter (a0) of PTA@SBA-15 is calculated to be 10.8 nm, which is very close to that of mother SBA-15 template (a0 = 11.0 nm). It implies that the mesostructure is well retained after the impregnation of PTA into the mesochannels of the SBA-15 template. The decrease of the diffraction intensity for the PTA@SBA-15 composite is probably due to the strong X-ray absorption from heavy tungsten atoms.29 The SAXS pattern of the composite W@SBA-15 obtained after H2 reduction at 800 °C shows only one broad diffraction peak. A shift of this diffraction peak to higher 2θ value is observed, indicating a shrinkage of the mesostructure after H2 reduction.37 After etching the SBA-15 template with HF aqueous solution, the W products still exhibit one broad diffraction peak, indicating that W crystal nanowire arrays well replicate the ordered mesostructure of the SBA-15 template. The cell parameter (a0) of W product is calculated to be 10.5 nm. Comparing this value with that of the SBA-15 template (11 nm), the structural shrinkage is estimated to be only 4.5%, suggesting that template-removal process does not cause any further shrinkage in mesostructure.Nitrogen adsorption–desorption isotherms reveal that original SBA-15 template has typical type-IV curves with a high surface area of 604 m2 g−1, a pore volume of 1.16 m3 g−1 and a narrow pore size distribution centred at 6 nm (Fig. 2B). After the impregnation of PTA, the surface area and pore volume decrease to 89.7 m2 g−1 and 0.07 m3 g−1, respectively, suggesting that the mesochannels of the SBA-15 template have been completely filled up. After the H2-reduction at 800 °C for 2 h, the surface area and pore volume further decrease to 47.8 m2 g−1 and 0.04 m3 g−1, respectively, confirming a transformation of PTA to metallic W. After complete removal of the SBA-15 template, the obtained metallic W nanowire bundles exhibit a surface area of 21.3 m2 g−1 and pore volume of 0.02 m3 g−1, respectively, which are very high for metallic W with heavy atomic weight.
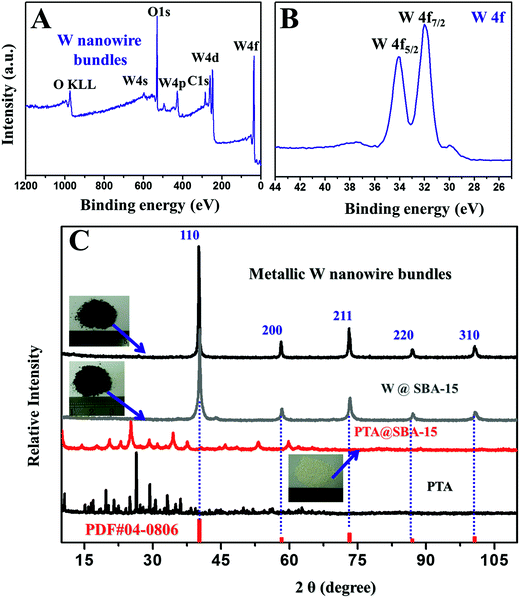
The scanning electron microscopy (SEM) image (Fig. S1A and B†) reveal that the mother SBA-15 template has a continuous rod-like morphology, with a particle diameter of about 300–500 nm and lengths of about 0.5–3 μm. The transmission electron microscopy (TEM) images (Fig. S1C and D†) show that the SBA-15 template possesses well-ordered mesochannels with a hexagonal p6mm symmetry. The pore size of the SBA-15 template is estimated to be 11.2 nm, in agreement with that determined from SAXS data. This remarkable mesostructural feature of SBA-15 makes it ideal as the template for the synthesis of metal W nanowire bundles. After the PTA impregnation followed by H2 reduction at 800 °C for 2 h, the W product appears as bright-white wire-like objects throughout the whole mesochannels of the SBA-15 (Fig. 3A and S2–S4†). No apparent W bulk deposition on the outer surface of the SBA-15 particles is observed, indicating that all PTA precursors are impregnated into the mesopore channels via the solvent evaporation-induced impregnation process. After etching the SBA-15 template, W nanowire bundles in large domains are observed (Fig. 3B and Fig. S5†). High-magnification TEM images show that these W bundles are constructed by interconnected aligned and regularly packed nanowires in whole domains (Fig. 3C). The diameters of the metal W nanowires are all in range of 8–10 nm and the lengths of 300–1500 nm, which is analogous to the mother SBA-15 template. The obtained W nanowire bundles are retained even after an ultrasonic treatment for 1 h, showing a good mechanical stability. The TEM images recorded along [001] direction clearly show hexagonal cylinder mesostructured of metal W nanowire bundles in large domains (Fig. 3C). The high-revolution TEM (HRTEM) images show perfect 2D atomic lattices with a spacing of 0.238 and 0.158 nm (Fig. 3D and S6†), corresponding to the (110) and (200) planes of body-centred cubic (bcc)-W crystalline phase, respectively. The selected area electronic diffraction (SAED) pattern recorded on the W nanowire bundles clearly shows well-resolved diffraction rings and many diffraction spots assigned to bcc-W crystalline phase (Fig. 3E), further revealing that the nanowires are composed of crystalline nanoparticles. The elemental mapping from the HAADF-STEM image shows that only W element is uniformly distributed on the whole nanowire arrays (Fig. 3F–G), further confirming the formation of pure metallic W phase. Only characteristic peaks of tungsten, surface adventitious carbon and oxygen are observed in the X-ray photoelectron spectra (XPS) of W nanowires (Fig. 4A), confirming that the product was pretty pure W. The high resolution XPS spectrum of W 4f shows two peaks at 33.6 and 31.4 eV (Fig. 4B), respectively, which is assigned to pure metallic W. No peaks assigned to tungsten carbonate, oxides or Si are observed. Theoretically, 1 g of PTA can yield 0.73 g of metallic W. In our synthesis, 2.43 g metallic W nanowire arrays is collected from 3.5 g PTA precursor and 0.8 g SBA-15 template. The yield is calculated to be 95% based on W content. Such a high conversion yield can lead to a capability of large production of W nanowire bundles in our synthesis.
To give further evidence for the formation of metal W nanowires bundles in the confined mesochannels of SBA-15 templates, the crystal phase transformation during the entire synthesis process is monitored by wide-angle XRD patterns (WXRD). The WXRD pattern of the PTA precursors shows all diffraction peaks correspond to a pure H3PW12O40·6H2O phase (JCPDS: 50-0403) without any detectable impurity (Fig. 4C). After a complete impregnation of PTA in the mesochannels of SBA-15, the positions of the diffraction peaks remain the same, but the intensities become comparatively weak and the width gets wide. These phenomena imply that the PTA precursors are successfully incorporated into the mesochannels of the SBA-15 template with the maintenance of the crystal phase. After the H2-reduction at 800 °C for 2 h, the obtained W@SBA-15 composites show five diffraction peaks at 2θ = 40.3, 58.3, 73.2, 87.0, and 100.6°, which can be indexed to the 110, 200, 211, 220 and 310 reflections of body-centred cubic (bcc) structured tungsten with the lattice parameter a = 0.316 nm (JCPDS: 04-0806). No other diffraction peaks from WO3 or WO are found, suggesting a complete transformation of PTA to metallic W. The WXRD pattern almost remains unchanged upon etching the SBA-15 template with HF aqueous solution, indicating a good stability of the W nanowire bundles. From the (110) peak of the XRD pattern, the average crystalline size is calculated to be about 10 nm using the Scherrer equation, in agreement with the domain size of the W nanowires estimated from TEM result.
The field-electron emission property of the W nanowires bundles are measured in a vacuum chamber at a base pressure of 1.0 × 10−6 Pa at room temperature. A typical plot of field-emission current density vs. electric field (J–E) shows that the metal W nanowires bundles have a low turn-on field of 4.1 V μm−1 (defined as the emission current density reaching 10 μA cm−2) (Fig. 5A). The FE performance of the W nanowires bundles is compared with other tungsten nanostructures (Table S1†). Notably, the turn-on field of the present W nanowires bundles (4.1 V μm−1) is lower than the reported values for other tungsten nanostructures, such as nanowires (5.0 V μm−1, diameters: 10–50 nm; lengths: ∼1 μm),32 nanorods (8.0 V μm−1, diameters: 100 nm; lengths: 1–2 μm),38 and nanothorns (6.2 V μm−1, diameters: 20–60 nm; lengths: <500 nm).39 The field electron emission property of the W nanowires bundles are further studied using the Fowler–Nordheim (FN) theory.38–40 The linear behaviour of F–N plot (Fig. 5B) confirms that the electron emission of the W nanowire array tips is proceeded by a field emission process. Using the work function of bulk tungsten (4.5 eV), the field enhancement factor (β) of the W nanowire arrays is calculated to be 3563. Moreover, no obvious emission degradation is observed over a period of 32 h, and the fluctuation in the emission current density is less than 5%. This suggests that the field electron emission from the W nanowires bundles is very stable. The excellent field-electron emission property of the ordered W nanowire bundles can be well understood as the results of the high surface area (21.3 m2 g−1) high aspect ratio (110 ± 20) and favorable crystal structure (bcc structured β-W).
Conclusions
In summary, metallic W nanowire bundles have successfully been synthesized with yields higher than 90% using a simple H2 reduction-assisted SBA-15 templating method. The replicated W nanowire bundles have long-range hexagonal regularity over large area, high surface area and highly crystalline metallic bcc-W phase structure. Owing to their unique mesostructured features, the resultant metallic W nanowire bundles show a low turn-on field (4.1 V μm−1), high field-enhancement factor (3563) and good field emission stability (with a fluctuation below 5% over 20 h). This H2 reduction-assisted SBA-15 templating method overcomes previous limitations for synthesizing metallic W nanowire bundles with ordered mesostructures. In addition, this H2 reduction-assisted SBA-15 templating method can make metal precursors completely filled into the mesochannels of SBA-15 via the solvent evaporation-induced impregnation process, which can easily be extended to the synthesis of other ordered pure metal nanowire arrays and their mixture alloys for advanced applications on quantum-electronic devices, switches and metal-dielectric capacitors, etc.Acknowledgements
This work is supported by the State Key Research Program of China (2013CB934104 and 2012CB224805), the National Science Foundation (21210004), Science & Technology Commission of Shanghai Municipality (14JC1400700), China Postdoctoral Science Foundation (2015M580295) and the International Postdoctoral Exchange Fellowship Program (No. 20160051), Shanghai Leading Academic Discipline Project (B108) and the National Plan for Science and Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Research Project Number ADV1718-02.References
- L. Zang, Y. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596–1608 CrossRef CAS PubMed.
- Z. L. Wang, ACS Nano, 2008, 2, 1987–1992 CrossRef CAS PubMed.
- Z. Tang and N. A. Kotov, Adv. Mater., 2005, 17, 951–962 CrossRef CAS.
- Y. Liu, R. Che, G. Chen, J. Fan, Z. Sun, Z. Wu, M. Wang, B. Li, J. Wei, Y. Wei, G. Wang, G. Z. Guan, A. A. Elzatahry, A. A. Bagabas, A. M. Al-Enizi, Y. H. Deng, H. S. Peng and D. Y. Zhao, Sci. Adv., 2015, 1, e1500166 CrossRef PubMed.
- W. Zhao, P. Yuan, X. She, Y. Xia, S. Komarneni, K. Xi, Y. Che, X. Yao and D. Yang, J. Mater. Chem. A, 2015, 3, 14188–14194 RSC.
- Y. Zhou, L. Wang, S. Chen, S. Qin, X. Liu, J. Chen, D.-J. Xue, M. Luo, Y. Cao and Y. Cheng, Nat. Photonics, 2015, 9, 409–415 CrossRef CAS.
- Y. Yang, K. Wang, H.-W. Liang, G.-Q. Liu, M. Feng, L. Xu, J.-W. Liu, J.-L. Wang and S.-H. Yu, Sci. Adv., 2015, 1, e1500714 CrossRef PubMed.
- J. Levinsen, P. Massignan, G. M. Bruun and M. M. Parish, Sci. Adv., 2015, 1, e1500197 CrossRef PubMed.
- J. Resasco, H. Zhang, N. Kornienko, N. Becknell, H. Lee, J. Guo, A. L. Briseno and P. Yang, ACS Cent. Sci., 2016, 2, 80–88 CrossRef CAS PubMed.
- Y. Liu, J. Hu, T. Zhou, R. Che and J. Li, J. Mater. Chem., 2011, 21, 16621–16627 RSC.
- A. I. Boukai, Y. Bunimovich, J. Tahir-Kheli, J.-K. Yu, W. A. Goddard III and J. R. Heath, Nature, 2008, 451, 168–171 CrossRef CAS PubMed.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- A. I. Hochbaum, R. Chen, R. D. Delgado, W. Liang, E. C. Garnett, M. Najarian, A. Majumdar and P. Yang, Nature, 2008, 451, 163–165 CrossRef CAS PubMed.
- B. Tian, X. Zheng, T. J. Kempa, Y. Fang, N. Yu, G. Yu, J. Huang and C. M. Lieber, Nature, 2007, 449, 885–888 CrossRef CAS PubMed.
- B. Deng, P.-C. Hsu, G. Chen, B. Chandrashekar, L. Liao, Z. Ayitimuda, J. Wu, Y. Guo, L. Lin and Y. Zhou, Nano Lett., 2015, 15, 4206–4213 CrossRef CAS PubMed.
- X. Wang, R. Wang, L. Shi and J. Sun, Small, 2015, 11, 4737–4744 CrossRef CAS PubMed.
- H. Lee, I. Kim, M. Kim and H. Lee, Nanoscale, 2016, 8, 1789–1822 RSC.
- X. Gao, F. Lu, B. Dong, Y. Liu, Y. Gao and L. Zheng, Chem. Commun., 2015, 51, 843–846 RSC.
- M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. D. Yang, Nat. Mater., 2005, 4, 455–459 CrossRef CAS PubMed.
- Y. Liu, K. Lan, A. A. Bagabas, P. Zhang, W. Gao, J. Wang, Z. Sun, J. Fan, A. A. Elzatahry and D. Y. Zhao, Small, 2016, 12, 860–867 CrossRef CAS PubMed.
- Z. Liu, Y. Sakamoto, T. Ohsuna, K. Hiraga, O. Terasaki, C. H. Ko, H. J. Shin and R. Ryoo, Angew. Chem., Int. Ed., 2000, 39, 3107–3110 CrossRef CAS PubMed.
- H. J. Shin, R. Ryoo, Z. Liu and O. Terasaki, J. Am. Chem. Soc., 2001, 123, 1246–1247 CrossRef CAS PubMed.
- H. J. Shin, C. H. Ko and R. Ryoo, J. Mater. Chem., 2001, 11, 260–261 RSC.
- Y.-J. Han, J. M. Kim and G. D. Stucky, Chem. Mater., 2000, 12, 2068–2069 CrossRef CAS.
- H. Yang, Q. Shi, B. Tian, Q. Lu, F. Gao, S. Xie, J. Fan, C. Yu, B. Tu and D. Y. Zhao, J. Am. Chem. Soc., 2003, 125, 4724–4725 CrossRef CAS PubMed.
- B. Tian, S. Che, Z. Liu, X. Liu, W. Fan, T. Tatsumi, O. Terasaki and D. Y. Zhao, Chem. Commun., 2003, 2726–2727 RSC.
- Y. Shi, Y. Meng, D. Chen, S. Cheng, P. Chen, H. Yang, Y. Wan and D. Y. Zhao, Adv. Funct. Mater., 2006, 16, 561–567 CrossRef CAS.
- F. Gao, Q. Lu and D. Y. Zhao, Adv. Mater., 2003, 15, 739–742 CrossRef CAS.
- Y. Shi, Y. Wan, R. Liu, B. Tu and D. Y. Zhao, J. Am. Chem. Soc., 2007, 129, 9522–9531 CrossRef CAS PubMed.
- D. Gu, W. Li, F. Wang, H. Bongard, B. Spliethoff, W. Schmidt, C. Weidenthaler, Y. Xia, D. Y. Zhao and F. Schüth, Angew. Chem., Int. Ed., 2015, 54, 7060–7064 CrossRef CAS PubMed.
- S. Wang, Y. He, X. Fang, J. Zou, Y. Wang, H. Huang, P. M. Costa, M. Song, B. Huang and C. T. Liu, Adv. Mater., 2009, 21, 2387–2392 CrossRef CAS.
- Y.-H. Lee, C.-H. Choi, Y.-T. Jang, E.-K. Kim, B.-K. Ju, N.-K. Min and J.-H. Ahn, Appl. Phys. Lett., 2002, 81, 745–747 CrossRef CAS.
- C. Jin, J. Wang, M. Wang, J. Su and L.-M. Peng, Carbon, 2005, 43, 1026–1031 CrossRef CAS.
- J.-M. Bonard, K. A. Dean, B. F. Coll and C. Klinke, Phys. Rev. Lett., 2002, 89, 197602 CrossRef PubMed.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- Y. Liu, Y. Luo, A. A. Elzatahry, W. Luo, R. Che, J. Fan, K. Lan, A. M. Al-Enizi, Z. Sun, B. Li, Z. W. Liu, D. K. Shen, Y. Ling, C. Wang, J. X. Wang, W. J. Gao, C. Yao, K. P. Yuan, H. S. Peng, Y. Tang, Y. H. Deng, G. F. Zheng, G. Zhou and D. Y. Zhao, ACS Cent. Sci., 2015, 1, 400–408 CrossRef CAS PubMed.
- J. Zhou, S. Z. Peng, J. Chen, J. C. She and N. S. Xu, presented at IVMC & IFES 2002, Lyon, France, 2002, available at http://ifes2002.univ-lyon1.fr/Abstract/EA_147.pdf Search PubMed.
- Y. Baek, Y. Song and K. Yong, Adv. Mater., 2006, 18, 3105–3110 CrossRef CAS.
- H. Huang, Y. Li, Q. Li, B. Li, Z. Song, W. Huang, C. Zhao, H. Zhang, S. Wen and D. Carroll, Nanoscale, 2014, 6, 8306–8310 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09308f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |