Panchromatic engineering for efficient zinc oxide flexible dye-sensitized solar cells using porphyrin and indoline dyes†
Abstract
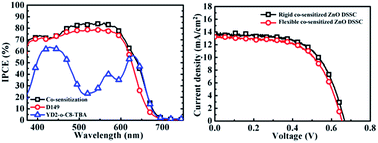
In this work, panchromatic engineering of room temperature (RT) chemically assembled ZnO anodes has been investigated using low acidity porphyrin and indoline dyes, i.e. YD2-o-C8-TBA and D149, for application in flexible dye-sensitized solar cells (DSSCs). The photovoltaic performance of the YD2-o-C8-TBA/D149 co-sensitized ZnO DSSC was optimized using ZnO anodes prepared on indium tin oxide (ITO)-coated glass substrates. The short-circuit current density of the optimized co-sensitized ZnO DSSC is 10% and 75% higher than those of the individual D149-sensitized and YD2-o-C8-TBA-sensitized cells, respectively. Compared to the D149-sensitized cell, the YD2-o-C8-TBA/D149 co-sensitized ZnO DSSC with a wavelength ranging from 475–700 nm exhibited improved photon-to-current conversion efficiencies. The optimized cell efficiency of 5.6% is accounted for by the rigid co-sensitized ZnO DSSC. Due to the RT fabrication of the ZnO anode, a comparable photovoltaic performance is attained with the co-sensitized ZnO DSSC fabricated using the ITO-coated plastic substrates. An efficiency of 5.3% is monitored in the flexible co-sensitized ZnO DSSC.

 Please wait while we load your content...
Please wait while we load your content...