Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unexpected LCST-type phase behaviour of a poly(vinyl thiazolium) polymer in acetone†
Konrad
Grygiel
a,
Weiyi
Zhang
a,
Christophe
Detrembleur
b and
Jiayin
Yuan
*a
aDepartment of Colloid Chemistry, Max Planck Institute of Colloids and Interfaces, Am Mühlenberg 1 OT Golm, D-14476 Potsdam, Germany. E-mail: Jiayin.Yuan@mpikg.mpg.de
bCenter for Education and Research on Macromolecules (CERM), Chemistry Department, University of Liege (ULg), Sart-Tilman, B6a, 4000 Liege, Belgium
First published on 1st June 2016
Abstract
We report on an unexpected lower critical solution temperature (LCST)-type phase transition of a poly(vinyl thiazolium) polymer in acetone solution, which was synthesized via radical polymerization of its corresponding ionic monomer bearing no thermoresponsiveness in acetone. The phase transition temperature can be conveniently varied by polymer concentration, ionic strength and addition of a cosolvent.
Stimuli-responsive polymers are a group of “smart” macromolecules that are able to switch between different states in response to certain alterations in the environment. Such transitions are often highly selective and can reversibly respond to subtle perturbations in a specific and precise manner. Research of such materials is not only driven by scientific curiosity, but is also important for various potential applications, including drug delivery, separation, (bio)sensing, catalysis, and “smart” coatings, to just name a few.1
Polymers exhibiting lower critical solution temperature (LCST) are a subclass of these systems and their stimuli-responsive character in solution can be often macroscopically observed as a thermally triggered aggregation of polymer chains above a specific temperature (defined as a cloud point temperature – Tc). Such a reversible behavior can be easily manipulated since the control of temperature is less complicated in lab than tuning other environmental parameters, such as pH, humidity, or pressure. Poly(N-isopropylacrylamide) (PNIPAM) is to date the most commonly studied LCST-type polymer in aqueous phase. Its popularity stems from phase transition near body temperature, which is adjustable by the incorporation of comonomers of different hydrophilicity/hydrophobicity. Nevertheless, other LCST-type polymers have been investigated due to some restricted features of PNIPAM, such as its questionable biocompatibility, phase transition hysteresis, and a significant influence from the end group.2 These polymers include poly(N-vinylalkylamide), lactam/pyrrolidone/pyrrolidine based polymers, poly(dimethylaminoethyl methacrylate), polyoxazolines, and poly(alkyloxide) copolymers, which are dominantly neutral or weakly charged in solution.2
Ionic polymers are often intrinsically responsive towards stimuli such as pH or ionic strength.3–5 To be thermo-responsive, they often require extra structure designs to finely balance the polymer–polymer and polymer–solvent interaction.6–12 Several research groups succeeded in different ways in synthesizing ionic polymers exhibiting LCST-type phase transition in aqueous solutions. A simple, effective approach was to copolymerize ionic monomers with comonomers, such as NIPAM or propylene oxide (PO), whose polymers are known to be thermoresponsive.13–17 The ionic part of these copolymers renders, exemplarily, a strong interaction with surfaces of different materials, while PNIPAM or PPO units make them vulnerable to thermal triggers. An alternative approach encompasses the design of intrinsically thermo-responsive ionic homopolymers. The reported polyanions were typically based on the structure of poly(styrene sulfonate) or poly(3-sulfopropylmethacrylate).18–21 In the case of LCST-type water-soluble polycations, the common ones are polytetraalkylphosphonium or polytetraalkylammonium.22–25 It should be mentioned that supramolecular interactions have also been applied to endow ionic polymers with thermoresponsive function.26
Ionic polymers with LCST-type phase transition in organic solvents are comparably less studied and only a few polycations have been reported.11,27–29 The reason might be related to the difference in intermolecular interaction in organic media and in aqueous phase. In organic media, hydrogen bonding and hydrophobic interactions can be seriously weakened and even cancelled. Aoshima et al. demonstrated that certain poly(vinyl ether)s with imidazolium salts as pendant groups exhibit LCST-type phase transition in chloroform, tetrahydrofuran and acetone.27,29 In the other study, Luis et al. presented chiral ionic homopolymers bearing imidazolium moieties which show LCST-type behavior in chloroform–ethanol mixtures.28
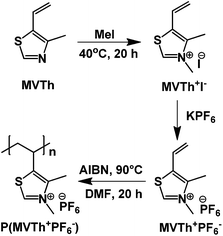
Recently, our group expanded the structural toolbox of thiazolium polymers, a class of ionic polymers that has been so far rarely studied. From a chemical structure point of view, compared to common imidazolium polymers, in the case of the thiazolium polymers a nitrogen atom is simply replaced by sulfur in the heterocyclic cation ring.3 This tiny structural variation alters to some extent their chemical and physical profile and expands property window. We previously reported that the thiazolium polymers possess tunable physical properties and serve as excellent stabilizers for carbon nanotubes and as binders in electrodes of lithium ion batteries.30,31 In spite of these primary achievements, novel properties and functions are expected to emerge from this group of polymers. Herein, we report the synthesis of a vinylthiazolium-type polymer bearing a hexafluorophosphate anion (PF6−), poly(4-methyl-5-vinylthiazolium hexafluorophosphate) [P(MVTh+PF6−)], which exhibits an usual LCST-type phase behavior in acetone.
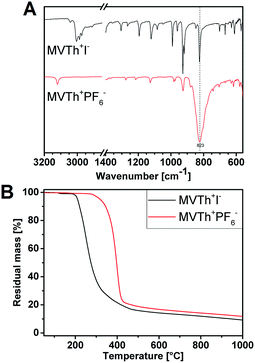
The synthetic method for the thermoresponsive vinylthiazolium polymer is depicted in Fig. 1. Initially, a vinylthiazolium-type monomer bearing iodide as counterion (MVTh+I−) was synthesized, according to our previously reported procedure, in a quaternization reaction of 4-methyl-5-vinyl thiazole (MVTh) with methyl iodide.31 The successful synthesis of MVTh+I− was confirmed via proton nuclear magnetic resonance (1H-NMR) and carbon nuclear magnetic resonance (13C-NMR) spectroscopy (Fig. S1A, B and S2A, B†), respectively. Consequently, the iodide monomer was subjected to an anion exchange reaction with potassium hexafluorophosphate in water, yielding a hydrophobic monomer (MVTh+PF6−) that precipitated out of water. The incorporation of PF6− anions was confirmed by ATR/FTIR spectroscopy (Fig. 2A). Iodide counterion of MVTh+I− is silent in the IR range. Hence, in the IR spectrum of MVTh+I− only the thiazolium cation is responsible for the recorded absorption pattern. When exchanging iodide to PF6− a strong band at 823 cm−1 appears and can be attributed to antisymmetric stretching of PF6−. A further titration test using silver nitrate confirms the quantitative replacement of iodide by PF6−.
 | ||
| Fig. 2 (A) ATR/FTIR spectra. (B) TGA curves (recorded under N2, at a heating rate of 10 K min−1) of MVTh+I− and MVTh+PF6−, respectively. | ||
In the final step, MVTh+PF6− was radically polymerized in dimethylformamide (DMF) to produce the targeted ionic polymer P(MVTh+PF6−). The successful polymerization is accompanied by the disappearance of the 1H-NMR signals of the vinyl protons (at 7.16, 6.03, and 5.69 ppm), which after polymerization are bound to sp3 carbons in the polymer backbone and appear at the high field (Fig. S1C and D†). The disappearance of vinyl group was also confirmed by 13C-NMR spectroscopy (Fig. S2C and D†). The apparent number-average molecular weight is determined by gel permeation chromatography to be 19 kDa with a dispersity index of 2.6.
Anion exchange typically has a significant effect on the thermal properties of ionic monomers and polymers. In the case of thiazolium monomers, substituting PF6− for I− improves thermal stability. The ionic monomer MVTh+PF6− decomposes at 345 °C (determined as temperature of 10% mass loss, Fig. 2B), which is 125 °C higher than MVTh−I+. This is in agreement with our previous reports that thiazolium monomers having large-sized fluorinated counterions as weaker nucleophiles exhibit remarkably better thermal stability than their halide-containing precursors.31 Surprisingly, in terms of thermal stability, MVTh+PF6− slightly outperforms its polymer (310 °C, Fig. S3†). We also subjected MVTh+I− and MVTh+PF6− to differential scanning calorimetry (DSC) measurement, detecting their melting points at 123 °C and 137 °C, respectively (Fig. S4†).
Anion exchange can strongly affect hydrophilicity/hydrophobicity of ionic monomers and polymers.3 In this work, the solubility of MVTh+PF6− and P(MVTh+PF6−) in a wide range of solvents was tested at ambient condition and compared with their I− counterparts (Table 1).31 Polar organic solvents of high boiling point, such as DMF and DMSO, are in general good solvents and ethyl acetate (EtOAc) and chloroform (CHCl3) are bad solvents for all the samples, independent on the anion type. MVTh+I− is overall hydrophilic and soluble in water and methanol (MeOH), while MVT+PF6− turns insoluble in water and only slightly soluble in MeOH. MVT+PF6− is fairly soluble in other common organic solvents of low boiling point, such as acetone and acetonitrile (ACN), but a limited solubility in tetrahydrofuran (THF) was observed. Its polymer is soluble in acetone and ACN.
| Compound | H2O | MeOH | Acetone | THF | ACN | DMF/DMSO | EtOAc/CHCl3 |
|---|---|---|---|---|---|---|---|
| MVTh+I− | + | + | − | − | − | + | − |
| MVTh+PF6− | − | +/− | + | +/− | + | + | − |
| P(MVTh+PF6−) | − | − | + | − | + | + | − |
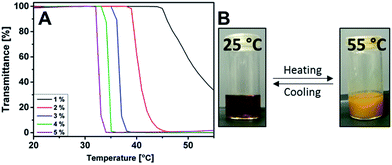
The uniqueness of the herein presented thiazolium polymer over its previously reported counterparts stems from the unexpected stimuli-responsive behaviour in acetone solution. At an elevated temperature, P(MVTh+PF6−) undergoes thermally triggered aggregation and precipitation in acetone, i.e. LCST-type phase transition, at Tc (defined as the temperature at 80% transmittance at λ = 700 nm). It is worth mentioning that LCST-type phase transition is so far observed in polymers with imidazolium, pyridinium, tetraalkylphosphonium and tetraalkyammonium cations in their repeating units. The current example is the first reported in the poly(vinyl thiazolium) family, which bears a LCST-type phase behavior in acetone.6 Additionally, some recently observed LCST-type polycations were discovered actually from their corresponding thermoresponsive monomers in the same solvent. It becomes a popular trend to screen LCST-type ionic monomers to find ionic polymers bearing the same solution property. Our example here indicates that LCST-type ionic polymers do not necessarily require their monomeric species to be thermoresponsive.
In the turbidity test, Tc of P(MVTh+PF6−) exhibits concentration-dependence (Fig. 3A and B). At 5 wt% Tc is 33 °C with a sharp phase transition. At lower polymer concentrations, Tc gradually increases and is accompanied by a noticeable broadening in phase transition. At the lowest concentration measured here at 1 wt%, Tc is 47 °C with a transmittance of 30% at 55 °C, which is the upper liquid temperature of acetone. The dependence of Tcs on polymer concentration can be associated with the turbidity method, in which higher polymer concentrations trigger faster nucleation and produce more macroscopically detectable aggregates at the same given transition temperature.32 This behavior is commonly observed for the polymer–solvent systems showing LCST-type phase transition at the polymer concentrations lower than the critical concentration.33
The Tcs of P(MVTh+PF6−) solutions can be tuned by addition of external salts as well. Here, three salts, lithium bis(trifluoromethane sulfony)imide (LiTFSI), potassium hexafluorophosphate (KPF6), and lithium tetrafluoroborate (LiBF4) were tested at a polymer concentration of 3 wt%. The concentration of the given salts in the final solutions varied from 0.001 to 0.01 M, except for LiBF4 from 0.001 to 0.008 M due to its limited solubility in acetone (Fig. 4A and S5–S7†). It is observed that addition of LiTFSI increases Tcs from 37 °C (pure polymer solution) to 45 °C (at 0.01 M LiTFSI). By contrast, the presence of KPF6 and LiBF4 decreases Tcs. In the case of KPF6, a gradual decrease in Tc down to 30 °C at 0.01 M KPF6 occurs. Tc seems more sensitive towards the addition of LiBF4 (down to 23 °C at 0.008 M LiBF4). This behavior is understandable since typically phase transition strongly depends on the solvophilicity/solvophobicity balance of the polymer as well as the ionic strength.28 The noticed change in Tcs results from the common salting-out effect (governed by the solubility product law) and the anion exchange effect which alters solvophobicity of the polymer. In the case of KPF6, variation in Tc is purely driven by salting-out effect, as the anions are identical. As for LiTFSI and LiBF4, the salting-out effect is accompanied by the competing anion exchange event. From our previous experiments, it is known that P(MVTh+BF4−) is insoluble in acetone (thus “acetone-phobic”) while P(MVTh+TFSI−) is fairly soluble in acetone (thus “acetone-philic”). Thereby, it is understandable that P(MVTh+PF6−) solution which undergoes partial anion exchange with LiBF4 or LiTFSI, contains partial P(MVTh+BF4−) and P(MVTh+TFSI−) units, respectively. Therefore, the former polymer turns less soluble and precipitates out at lower Tcs, while the latter exhibits the opposite effect, showing increased solubility in acetone (consequently precipitation caused by salting-out effect is only visible at higher Tcs).
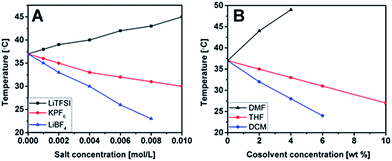 | ||
| Fig. 4 T cs measured in a 3 wt% P(MVTh+PF6−) solutions in acetone: (A) at different concentrations of external salts; (B) at different concentration of cosolvents. | ||
Solvophobic interactions (apart from coulombic interactions) play an important role in LCST behavior of ionic polymers.34–36 Therefore, we explored the possibility of tuning solute/solvent interactions by the addition of a cosolvent to acetone to impact the Tcs of P(MVTh+PF6−) solutions. A 3 wt% P(MVTh+PF6−) solution in acetone was used as a reference and three common organic solvents, THF, DMF, and dichloromethane (DCM) were tested as the additives (Fig. 4B and S8–S10†). DMF is a good solvent for P(MVTh+PF6−). It is expected that the addition of DMF will promote solute/solvent interactions due to the increased solvophilicity of P(MVTh+PF6−) to the solvent mixture and possible hydrogen bonding between the C2 proton of the thiazolium ring and solvent molecules. It is indeed the case, since addition of 2 and 4 wt% of DMF resulted in the Tc elevated from 37 °C (the reference sample) up to 43 and 49 °C, respectively. At 6 wt% Tc goes beyond the investigated temperature range. Oppositely, Tcs decrease by mixing the polymer solution with a poor solvent, such as THF and DCM. With the addition of THF, a gradual downturn of Tc is detected (Tc ∼ 31 °C at 6 wt% and 27 °C at 10 wt% of THF). The analogous but more pronounced behaviour is observed when DCM, a non-solvent for P(MVTh+PF6−), is used as a cosolvent additive (Tc ∼ 24 °C at 6 wt% of DCM).
Conclusions
In summary, for the first time a poly(vinyl thiazolium) polymer was reported to present a LCST-type phase transition in acetone. The phase transition varies from room temperature to the boiling point of acetone in response to alterations in the polymer concentration and the addition of salts or cosolvents. The findings in this work introduce above all a new thermoresponsive ionic polymer structure and additionally offer a model system to analyze the polymer–solvent interaction that is of future research interest. The work also announces that LCST-type ionic polymers may be prepared from a monomer non-thermoresponsive in the same solvent.Acknowledgements
The authors acknowledge financial support from the Max Planck Society and the Marie Curie Actions of EU's 7th Framework Program (REA grant agreement no. 289347). C. D. is Research Director by F. R. S.-FNRS and thanks FNRS for financial supports.Notes and references
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- D. Mecerreyes, Prog. Polym. Sci., 2011, 36, 1629–1648 CrossRef CAS.
- M. Geoghegan, L. Ruiz-Perez, C. C. Dang, A. J. Parnell, S. J. Martin, J. R. Howse, R. A. L. Jones, R. Golestanian, P. D. Topham, C. J. Crook, A. J. Ryan, D. S. Sivia, J. R. P. Webster and A. Menelle, Soft Matter, 2006, 2, 1076–1080 RSC.
- C. Chassenieux and C. Tsitsilianis, Soft Matter, 2016, 12, 1344–1359 RSC.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163–2178 RSC.
- X. Han, Z. Xiong, X. Zhang and H. Liu, Soft Matter, 2015, 11, 2139–2146 RSC.
- S. T. Hemp, A. E. Smith, W. C. Bunyard, M. H. Rubinstein and T. E. Long, Polymer, 2014, 55, 2325–2331 CrossRef CAS PubMed.
- H. Yoshimitsu, E. Korchagina, A. Kanazawa, S. Kanaoka, F. M. Winnik and S. Aoshima, Polym. Chem., 2016, 7, 2062–2068 RSC.
- J. Zhang, J. Liu, Y. Zuo, R. Wang and Y. Xiong, Molecules, 2015, 20, 17378 CrossRef CAS PubMed.
- Y. Xiong, J. Liu, Y. Wang, H. Wang and R. Wang, Angew. Chem., Int. Ed., 2012, 51, 9114–9118 CrossRef CAS PubMed.
- E. Karjalainen, V. Aseyev and H. Tenhu, Macromolecules, 2014, 47, 7581–7587 CrossRef CAS.
- N. Weber, J. Texter and K. Tauer, Macromol. Symp., 2011, 302, 224–234 CrossRef CAS.
- J. Texter, V. A. Vasantha, R. Crombez, R. Maniglia, L. Slater and T. Mourey, Macromol. Rapid Commun., 2012, 33, 69–74 CrossRef CAS PubMed.
- Y. Zuo, N. Guo, Z. Jiao, P. Song, X. Liu, R. Wang and Y. Xiong, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 169–178 CrossRef CAS.
- E. Karjalainen, N. Chenna, P. Laurinmaki, S. J. Butcher and H. Tenhu, Polym. Chem., 2013, 4, 1014–1024 RSC.
- A. Tudor, L. Florea, S. Gallagher, J. Burns and D. Diamond, Sensors, 2016, 16, 219 CrossRef PubMed.
- Y. Kohno and H. Ohno, Aust. J. Chem., 2012, 65, 91–94 CrossRef CAS.
- Y. Men, X.-H. Li, M. Antonietti and J. Yuan, Polym. Chem., 2012, 3, 871–873 RSC.
- Y. Kohno, Y. Deguchi and H. Ohno, Chem. Commun., 2012, 48, 11883–11885 RSC.
- B. Ziolkowski and D. Diamond, Chem. Commun., 2013, 49, 10308–10310 RSC.
- Y. Men, H. Schlaad and J. Yuan, ACS Macro Lett., 2013, 2, 456–459 CrossRef CAS.
- Y. Kohno, Y. Deguchi, N. Inoue and H. Ohno, Aust. J. Chem., 2013, 66, 1393–1398 CrossRef CAS.
- G. Wang and P. Wu, Soft Matter, 2015, 11, 5253–5264 RSC.
- W. Li and P. Wu, Polym. Chem., 2014, 5, 5578–5590 RSC.
- O. Kretschmann, C. Steffens and H. Ritter, Angew. Chem., Int. Ed., 2007, 46, 2708–2711 CrossRef CAS PubMed.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Macromolecules, 2012, 45, 9427–9434 CrossRef CAS.
- S. Montolio, L. Gonzaez, B. Altava, H. Tenhu, M. I. Burguete, E. Garcia-Verdugo and S. V. Luis, Chem. Commun., 2014, 50, 10683–10686 RSC.
- K.-I. Seno, S. Kanaoka and S. Aoshima, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5724–5733 CrossRef CAS.
- K. Grygiel, L. Chabanne, Y. Men and J. Yuan, Macromol. Symp., 2014, 342, 67–77 CrossRef CAS.
- K. Grygiel, J.-S. Lee, K. Sakaushi, M. Antonietti and J. Yuan, ACS Macro Lett., 2015, 4, 1312–1316 CrossRef CAS.
- Y. Men, H. Schlaad, A. Voelkel and J. Yuan, Polym. Chem., 2014, 5, 3719–3724 RSC.
- D. J. Phillips and M. I. Gibson, Polym. Chem., 2015, 6, 1033–1043 RSC.
- H. R. Dittmar and W. H. Schröer, J. Phys. Chem. B, 2009, 113, 1249–1252 CrossRef CAS PubMed.
- H. Weingärtner, T. Merkel, U. Maurer, J.-P. Conzen, H. Glasbrenner and S. Käshammer, Ber. Bunsen-Ges., 1991, 95, 1579–1586 CrossRef.
- H. Weingärtner, M. Kleemeier, S. Wiegand and W. Schröer, J. Stat. Phys., 1995, 78, 169–196 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and instruments, experimental details, and additional characterization. See DOI: 10.1039/c6ra09023k |
| This journal is © The Royal Society of Chemistry 2016 |