Loading an organophosphorous flame retardant into halloysite nanotubes for modifying UV-curable epoxy resin†
Tiancheng Zheng and
Xiuyuan Ni *
*
State Key Laboratory of Molecular Engineering of Polymer, Department of Macromolecular Science, Fudan University, Shanghai 200433, PR China. E-mail: xyni@fudan.edu.cn; Fax: +86-21-65640293; Tel: +86-21-65640982
First published on 27th May 2016
Abstract
Novel flame-resistant UV-curable epoxy (EP) composites were prepared using the organophosphorous flame retardant dimethyl methylphosphonate (DMMP) which was loaded into halloysite nanotubes (HNTs). We used a cone calorimeter to investigate the flame retardancy of the UV-curable epoxy resin containing DMMP-loaded halloysite nanotubes (HNT-D). It was found that compared with the neat epoxy resin the EP/HNT-D system exhibited better flame retardancy with an obvious decrease in the heat release rate (HRR), total heat release (THR), total smoke release (TSR), mass loss rate (MLR) and smoke production rate (SPR). Exploring the flame retardant mechanism revealed two reaction steps in the process of the flame retardant: DMMP gasification followed by a polyphosphoric-HNT carbon layer generating on the condensed phase. It was revealed through measuring the dynamic mechanical properties and tensile properties of the UV-cured EP materials that adding DMMP-loaded halloysite nanotubes (HNT-D) into EP obtained better mechanical properties than adding a simple mixture of halloysite clay and DMMP. With DMMP loaded in HNTs, the HNT-D could reduce the softening influence of DMMP. This method may potentially be applied to improve the flame retardancy of UV-curable epoxy resin in the future.
1 Introduction
As a kind of important halogen-free flame retardant, organophosphorous flame retardants have been extensively used for preparing polymer materials due to their low toxicity, high retarding efficiency and good compatibility with various polar polymers.1–4 During combustion, phosphorus can be foamed with the formation of cellular crusts on the material surface, which can shield the underlying condensed polymeric phase from further heating and also contact with oxygen.5,6 Moreover, organophosphorous flame retardants can release water vapor to dilute flammable gas.7 However, organophosphorous flame retardants usually have a plasticizing effect on polar polymers due to their interaction and therefore, the mechanical properties of the polymers deteriorate unavoidably in terms of the glass transition temperature, storage modulus and tensile strength.8–11 On the basis of this observation, such organophosphorous flame retardants should be encapsulated for overcoming the issue of softening polymers while retaining their superior flame-retardant function. At present, there are few works intended to reduce the softening influences which usually result from using organophosphorous compounds as flame retardants.Halloysite is a type of naturally occurring aluminosilicate clay with a hollow cylindrical structure at the submicron scale.12 With its unique nano-tubular structure, halloysite has been recently used to prepare controlled release systems through loading with various functional reagents such as antimicrobial agents, inhibitors, anticorrosion materials, glycerol, antibiotics and even dyes.13–16 For example, glycerol has been successfully loaded into halloysite nanotubes for cosmetic applications with the loading efficiency exceeding 20 wt%.17 All of these advantages make halloysite nanotubes an ideal nanocontainer for encapsulating reagents. Moreover, halloysite clay can show an effective improvement in the flame retardancy of polymers. Due to their nano-tubular structure, halloysite nanotubes can provide a multi dimensional network to prevent the products of thermal decomposition from diffusing into fire and air during combustion.18 Halloysite nanotubes have been selected as the flame retardants to enhance the flame resistance of polymer matrixes like polypropylene, acrylonitrile butadiene styrene, nitrile-butadiene rubber and polyamide.19–24 Arising from the use of halloysite nanotubes, the resulting flame retardancy was observed with an obvious decrease in the heat release rate, specific extinction area and mass loss rate. It is also important that compared with other nanoclays, halloysite nanotubes can be directly applied to the polymers without an exfoliating treatment,25 and the nanotubes can be well dispersed in the polar polymer matrix. Halloysite nanotubes are available in thousands of tons at a low price, which is attributed to the industrial use.
UV-curable epoxy materials, which convert epoxy oligomers into networks through ultraviolet light, have been extensively used in the field of coatings and the construction industry.26,27 Recently, epoxy resins have been used for preparing three dimensional (3D) printing UV-curable materials because of their high mechanical strength and good curing properties.28,29 It is known that epoxy resins are usually highly combustible in nature. For example, a typical UV-curable epoxide such as 3,4-epoxycyclohexylmethyl has a low limiting oxygen index (LOI), less than 18.30,31 In particular, a small amount of photo-initiators which remain in the cured epoxy materials give radicals when the cured materials are burning, and the radicals can accelerate the process of combustion. As reported, phosphorus flame retardants have been used to enhance the flame retardancy of UV-curable epoxy materials.32,33 The use of organophosphorous compounds reduced the heat release and prevented the epoxide from dripping during burning.34,35 It should be emphasized that as a result of the plasticizing effect, organophosphorous compounds effect the epoxy materials by decreasing the glass transition temperature.36 A decrease in the glass transition temperature means in principle a loss of mechanical strength and also a lower end-use temperature for the polymers. As for the UV-curable epoxy materials, it is crucial to develop efficient strategies for improving the flame retardancy without the loss of the mechanical properties.
The organophosphorous flame retardants include phosphines, phosphine oxides, phosphonium compounds and phosphonates. Dimethyl methylphosphonate (DMMP) which has a phosphorus content as high as 25% can efficiently enhance the flame resistance of polymer matrixes.37,38 Moreover, DMMP is an appropriate option for loading into halloysite nanotubes with the advantages of a low viscosity (1.75 mPa s) and high boiling point (180 °C).39 In this study, we prepare a nanosized hybrid material through loading DMMP into halloysite nanotubes (HNTs) with the aim of providing a new flame retardant suitable for UV-curable epoxy resins. The new structure of DMMP encapsulated in the hollow cylindrical tubes can protect the epoxy resin matrix from the typical plasticizing effect of phosphorous compounds on polar polymers. In response to combustion, DMMP is released from the HNTs and subsequently retards the flame. The encapsulation of DMMP in the HNTs is carried out under vacuum here. The material structures and properties are investigated using scanning electronic microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), dynamic mechanical analysis (DMA), thermogravimetric analysis (TGA) and mechanical tests. By using cone calorimetry (CC), the flammability of the DMMP–HNTs is measured, and the flame-retarding mechanism of the DMMP–HNTs on UV-curable epoxy resins is analyzed.
2 Experimental
2.1 Materials
Halloysite nanotubes were purchased from Dongming Tianhe Inc. (Shandong, China); dimethyl methylphosphonate (DMMP) was purchased from Darui Chemical Company (Shanghai, China); UV-curable epoxy resin was bought from Royal DSM Inc and used as received. Other reagents and materials were purchased from Sinopharm Chemical Reagent Co., Ltd and used as received.2.2 Preparation of DMMP-loaded HNTs
Halloysite nanotubes were loaded with DMMP through applying vacuum suction. Firstly, 20 wt% halloysite clay was added to distilled water to prepare the water suspension. The mixture was heated to 80 °C for 10 hours to increase the dispersion of the nanotubes. The ultimate powder was separated using centrifugation. Then, 2 g of halloysite nanotubes was added to 50 ml of DMMP and mixed thoroughly using sonication for 1 hour. After that, the mixture was transferred to a vacuum flask for 1 hour. Bubbles out of the mixture showed that air was removed from the cavities of halloysite, which was replaced with DMMP. This vacuum cycle was repeated four times. After this process, the mixture was kept for 2–3 days under ordinary pressure, which can further increase the loading efficiency. Finally, the sample was separated from DMMP through centrifugation, and placed in an oven at 60 °C to dry until recovering the powder.2.3 Preparation of EP/HNT-D and EP/HNTs composites
The preparation of EP/HNT-D and EP/HNTs was as follows: the halloysite nanotubes (HNTs) and DMMP-loaded halloysite nanotubes (HNT-D) were added into UV-curable epoxy resin (EP) in different ratios. Homogenous blends were formed by stirring constantly. After that, the mixture was exposed to UV irradiation for 2 hours at room temperature and then postcured at 60 °C for 5 hours.2.4 Characterization
The DMMP release experiments were carried out using a fluorescence technique. 2 g of DMMP-loaded halloysite nanotubes was dispersed in 100 ml of ethanol under continuous stirring. A 430 nm fluorescence emission peak in the fluorescence spectra of the mixtures at different times was analyzed using an RF-5301PC fluorometer (Shimadzu Japan), with a 380 nm fluorescence excitation wavelength.Thermogravimetric analysis (TGA) was performed on a TGA 1 (Mettler Toledo, Switzerland) thermogravimetric analyzer. The samples were measured at a heating rate of 10 °C min−1 under a nitrogen flow of 40 ml min−1 and a test temperature ranging from 30 °C to 800 °C.
Scanning electron microscopy (SEM) was carried out with an Ultra 55 Field-Emission Scanning Electron Microscope (Zeiss, Germany). The surface of the samples was sputtered with a thin layer of gold prior to the measurements. The SEM measurements were recorded at an accelerating voltage of 10 kV.
Transmission electron microscopy (TEM) was carried out with a Tecnai G2 20 TWIN (FEI, America) at 200 kV. Halloysite nanotube specimens were dropped onto a copper grid with carbon support films and the solvent was completely evaporated before testing.
The FTIR spectra of the samples were recorded on a Nicolet 6700 (Thermofisher, America). The samples were measured using a KBr disk.
The X-ray diffraction (XRD) patterns were recorded with an X’pert PRO (PANalytical, Netherlands) with 2θ ranging from 5° to 80°.
The flame retardancy of the samples was recorded using a cone calorimeter device (Fire Testing Technology, UK). The dimensions of the samples were 100 mm × 100 mm × 3 mm. All samples were tested at a heat flux of 35 kW m−2. During this test, the cone can record the heat release rate (HRR), the total heat release (THR), the smoke production rate (SPR), the total smoke release (TSR), etc.
The tension test was performed on a CMT4104 (Sans, China). The tensile specimen has a nominal dimension of 30 × 5 mm. The loading rate of this test was 2 mm min−1.
The dynamic mechanical analysis (DMA) was performed on a DMA/SDTA 861e (Mettler-Toledo, Switzerland). The test was performed at a constant frequency of 1 Hz. The heating rate of this test was 2 °C min−1. Measurements were recorded at a temperature ranging from −15 °C to 90 °C.
Shore D hardness was measured using an XHS shore D hardness tester (XHS, China) at room temperature. Measurements were recorded five times and the average shore D hardness values were obtained.
3 Results and discussion
3.1 Flame retardant DMMP loading into halloysite nanotubes
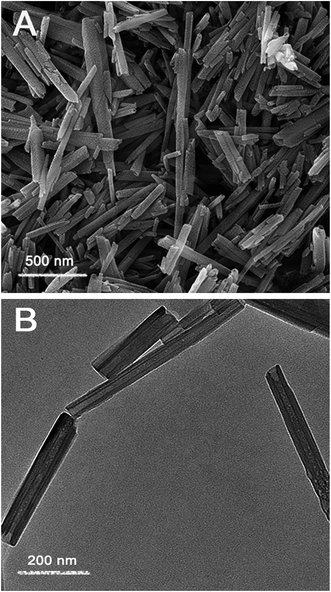
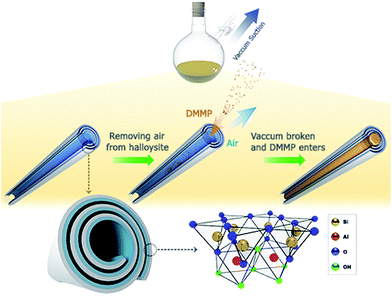
The SEM and TEM images of the empty halloysite nanotubes are shown in Fig. 1. It is observed that the length of halloysite ranges from 500 to 1500 nm (Fig. 1A), and the nanotubes have an outside diameter of 50 ± 20 nm and an inner diameter of 20 ± 5 nm (Fig. 1B). Moreover, the halloysite nanotubes have a uniform nanoparticle size and regular hollow cavities. Such halloysite might have a high loading capacity.As Scheme 1 indicates, DMMP was loaded into the halloysite nanotubes through applying vacuum suction. The air bubbles in the halloysite cavities could be completely removed during this process. Because of the low viscosity of DMMP, the halloysite nanotubes could be dispersed directly in DMMP without other solvents. In order to explore the process of DMMP release from halloysite, we measured the fluorescence spectra at different times for the mixtures of DMMP-loaded halloysite dispersed in ethyl alcohol. Before the release experiments, we measured that DMMP exhibits a fluorescence emission with a peak wavelength of 430 nm. Fig. 2 shows that the peak intensity of the supernate emission increased rapidly until the release time of 50 minutes, followed by a slow increase leading to a constant at 120 minutes. This result indicates that DMMP was released from the halloysite nanotubes in two stages, probably corresponding to two different states of DMMP in the mixture, namely, the adsorption of DMMP onto the surface of the nanotubes and its entrapment inside the cavities.
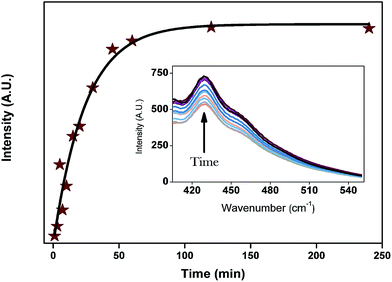 | ||
| Fig. 2 The fluorescence spectra of HNT-D in ethanol at different release times. The excitation wavelength is 380 nm. | ||
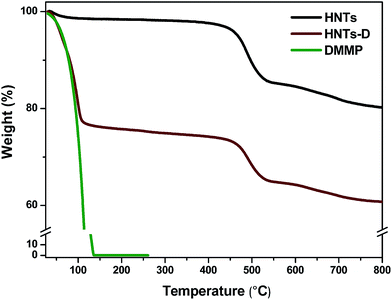
We have compared the X-ray diffraction spectra measured for pure halloysite (HNTs) and halloysite loaded with DMMP (HNT-D), respectively (see the ESI, Fig. S1†). On comparison, no changes are found in the characteristic peaks of the halloysite nanotubes over the range from 6° to 15° for 2θ, which is related to the wall spacing of the halloysite nanotubes. Therefore, the structures of the nanotubes were not influenced by loading with DMMP. The weight loss of the HNTs and HNT-D was measured using thermogravimetric analysis, with the analysis result of TGA shown in Fig. 3 and Table 1. The loading efficiency of DMMP was calculated according to eqn (1).
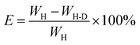 | (1) |
| HNTs | DMMP | HNT-D | |
|---|---|---|---|
| 800 °C residue (wt%) | 80.2 | 0.2 | 60.8 |
3.2 Flame retardancy of EP and its composites
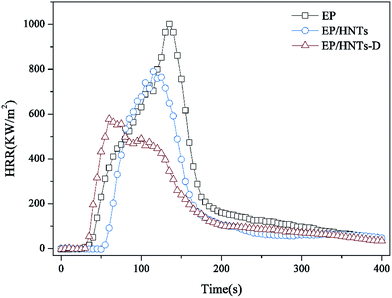
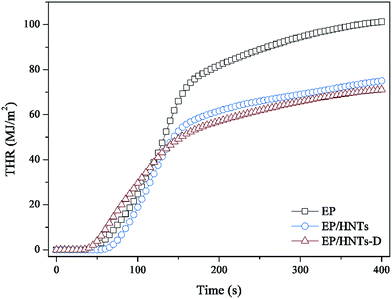
Cone calorimetry (CC) is used for obtaining the important parameters of polymer combustion, which include the time to ignition (TTI), peak of heat release (pHRR), heat release rate (HRR), total heat release (THR), smoke production rate (SPR) and total smoke release (TSR), as well as the CO production rate (COPR). To evaluate the flame retardant effect of HNT-D, we have carried out CC analysis of the composites consisting of EP and HNT-D. Table 2 shows the composition of EP and its composites. The CC results are presented in Fig. 4–8 and Table 3. All samples were tested at a heat flux of 35 kW m−2. It is observed that both the pHRR and THR of the composites are significantly decreased compared to the neat EP resin, as indicated by the results in Fig. 4 and 5, respectively. Compared to EP, with a pHRR of about 1002.4 kW m−2, the composite containing 20 wt% of HNT-D exhibits a lower pHRR (578.1 kW m−2), decreased by 42%. The THR is 104.1 MJ m−2 for neat EP and 73.8 MJ m−2 for the composite containing 20 wt% of HNT-D. Clearly, the use of HNT-D decreased the THR by 29%.| Sample codes | EP (wt%) | HNTs (wt%) | DMMP (wt%) | HNT-D (wt%) |
|---|---|---|---|---|
| EP | 100 | 0 | 0 | 0 |
| EP/HNTs | 80 | 20 | 0 | 0 |
| EP/HNT-D | 80 | 0 | 0 | 20 |
| EP/DMMP | 95.2 | 0 | 4.8 | 0 |
| EP/HNTs/DMMP | 80 | 15.2 | 4.8 | 0 |
| Sample codes | TTI (s) | pHRR (kW m−2) | THR (MJ m−2) | TSR (m2 m−2) | AMLR (g s−1 m−2) | Residue (wt%) |
|---|---|---|---|---|---|---|
| EP | 24 | 1002.4 | 104.1 | 2506.5 | 14.6 | 2.2 |
| EP/HNTs | 43 | 790.2 | 75.2 | 1777.4 | 12.5 | 20.9 |
| EP/HNT-D | 24 | 578.1 | 73.8 | 1391.5 | 10.0 | 20.4 |
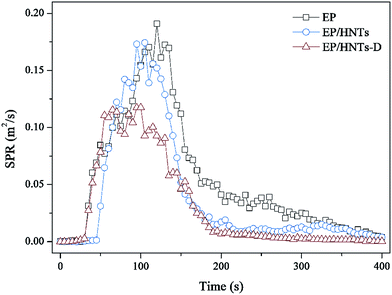
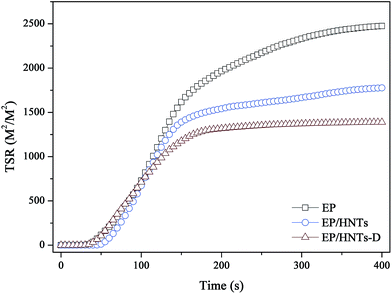
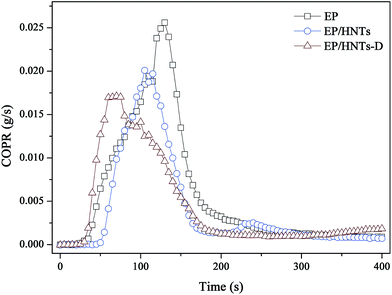
The smoke release data is of great significance for flame-retardant materials. Fig. 6 and 7 show the curves of the smoke production rate (SPR) and total smoke release (TSR) during combustion, respectively. Both the TSR and SPR are decreased significantly, similar to the tendency of the HRR and THR curves. Furthermore, compared with the TSR of neat EP, the TSR of the EP/HNT-D composite is decreased from 2506.5 m2 m−2 to 1391.5 m2 m−2, experiencing a decrease of 44.5%. The COPR curves of these samples are provided in Fig. 8. Compared with neat EP, the composites containing HNT-D exhibit efficiency in inhibiting the CO production rate. The above results indicate that the use of HNT-D dramatically suppressed the production and release of smoke during combustion. It is known that the molecular formula for HNTs is similar to kaolinite with water molecules. The water from the HNTs is released at a temperatures ranging from 110–120 °C. The water vapor inhibits smoke when heated.40 They also produced no smoke during combustion. Moreover, the CC results for the EP/HNTs are presented in Fig. 4–8 and Table 3, respectively. The results show that the EP/HNTs have a lower flame retardancy than EP/HNT-D.
3.3 The flame retardant mechanism of DMMP-loaded halloysite nanotubes
Fig. 9 shows the digital photographs of the residues from the EP and EP/HNT-D composite combustion. As can be seen, there is no residue left for EP after the CC test. As for the EP/HNT-D composite, the residue after combustion exhibits a continuous char layer at the surface. We have measured the char mass and average MLR of EP and the EP/HNT-D composites and listed the data in Table 3. The results show that the residue left in the materials is 2.0 wt% for neat EP and 20.4 wt% for EP/HNT-D. Apparently, the char residue mass of EP/HNT-D was much higher than that of neat EP. Moreover, when 20 wt% of HNT-D was added, the average MLR dropped from 14.6 g s−1 m−2 to 10.0 g s−1 m−2, experiencing a decrease of 31.5%. Obviously, both the char residue mass and MLR consistently prove that the combustion rate of the composite was decreased by the HNT-D. Fig. 10 presents the SEM images of the combustion residues. It can be seen that lots of large holes are generated on the surfaces of the EP residue. The large holes might easily transfer the flame and heat during combustion. Compared with the residue of neat EP, the residues of EP/HNT-D have more continuous and compact surfaces with the absence of holes. To further analyze the microstructure of the residues, we have compared the surface morphologies of the combusted EP/HNTs and EP/HNT-D. It can be seen from Fig. 10C and D that the surface of the EP/HNT-D residue is more flat and compact than that of the EP/HNT residue. Moreover, the halloysite nanotubes in the EP/HNT-D residue retain their complete shape and structure, while the halloysite nanotubes in the EP/HNT residue are much thinner. The above results indicate that the stability of the combustion residues was significantly improved with HNT-D.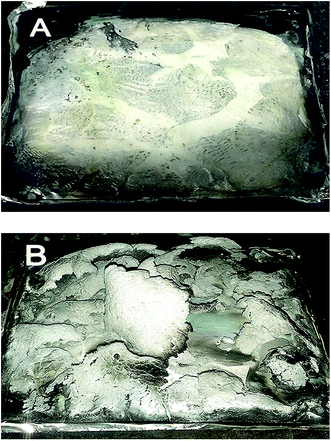 | ||
| Fig. 9 Digital photographs of the residues of (A) EP and (B) EP/HNT-D after the cone calorimeter test. | ||
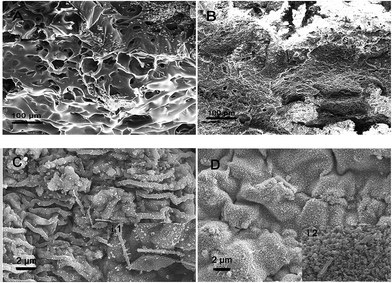 | ||
| Fig. 10 SEM images of the residues of (A) EP, (B and D) EP/HNT-D and (C) EP/HNTs after combustion. The insets to (C) and (D) are the magnified morphologies. | ||
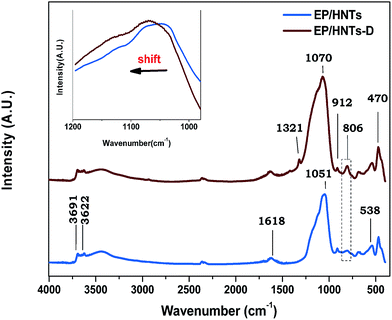
In order to explore the mechanism of the flame retardancy in the composites, we have compared the FTIR spectra that are measured for the combustion residues of the composites containing untreated halloysite nanotubes (HNTs) and HNT-D. The compositions of the EP/HNTs and EP/HNT-D are shown in Table 2. As shown in Fig. 11, the peaks ranging from 3622 cm−1 to 3696 cm−1 are attributed to the stretching vibrations of the inner-surface hydroxyl groups. Inter-layer water is demonstrated by the deformation vibration ranging from 1617 cm−1 to 1635 cm−1. The peak at 470 cm−1 is assigned to the deformation of Si–O–Si and the peak at 538 cm−1 is assigned to the stretching vibration of Al–O–Si.41 It is important to find some different peaks in the two FTIR spectra. Firstly, compared with EP/HNT-D, the symmetric stretching peak of Si–O at 806 cm−1 for the EP/HNTs was unapparent, which might be due to the shape and structure of the HNTs being damaged during combustion. Secondly, a new peak appears at 1321 cm−1 in the FTIR spectrum of the EP/HNT-D residue, which is assigned to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Accordingly, during the process of combustion, there was still a part of DMMP, in addition to the gasification, that formed a polymetaphosphate and adhered to the condensed phase. Thirdly, there is an obvious red shift in the peak of in-plane Si–O stretching at 1051 cm−1. This phenomenon is caused by halloysite clay reacting with DMMP to generate silicoaluminophosphate (SAPO).42 As a kind of high-efficiency acidic catalyst, SAPO could promote the formation of phosphoric acid and enhance the process of oxidative dehydration crosslinking charring, which might then make the surface of the residue more compact.43–45
O groups. Accordingly, during the process of combustion, there was still a part of DMMP, in addition to the gasification, that formed a polymetaphosphate and adhered to the condensed phase. Thirdly, there is an obvious red shift in the peak of in-plane Si–O stretching at 1051 cm−1. This phenomenon is caused by halloysite clay reacting with DMMP to generate silicoaluminophosphate (SAPO).42 As a kind of high-efficiency acidic catalyst, SAPO could promote the formation of phosphoric acid and enhance the process of oxidative dehydration crosslinking charring, which might then make the surface of the residue more compact.43–45
Scheme 2 shows the possible flame retardant mechanism of HNT-D. During the combustion process, the HNTs act as physical isolators which slow down the heat and mass transfer. Due to the decrease in the HRR and THR, more HNTs with complete shape and structure survived and entrapped the polymer decomposition products into the cavities. This helps to reduce the mass loss rate of the composites. Meanwhile, the hydrate water from the HNTs is also released and this water vapor could inhibit the release of flammable gas and toxic smoke. That is the reason why the SPR and TSP decreased after adding HNT-D. Moreover, DMMP is gasified rapidly from the HNTs and generates a polyphosphoric-HNT carbon layer. It is a synergistic effect between the HNTs and DMMP during catalysis that forms a compact and dense char layer covering the surface of the residues. This carbon layer could slow the flame and the transfer of combustible volatiles between the gas and condensed phases, and protect the substrate from further combustion. Eventually, the flame retardancy of EP is improved using HNT-D.
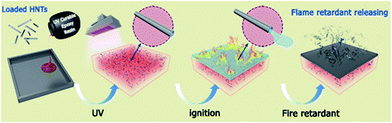 | ||
| Scheme 2 Schematic illustration of the mechanism for the enhanced flame resistance of the EP/HNT-D composite. | ||
3.4 Mechanical properties of flame retardant EP composites
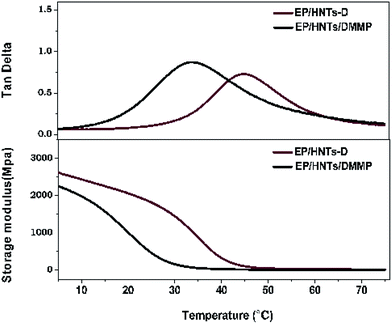
We have compared the mechanical properties measured for the composites with HNT-D and the composites in which halloysite and DMMP were added. The compositions of EP/HNTs/DMMP and EP/HNT-D are shown in Table 2. The dynamic mechanical analysis results are shown in Fig. 12 and Table 4. Compared with EP/HNTs/DMMP, the storage modulus of EP/HNT-D is significantly higher, especially at room temperature (25 °C). The storage modulus at 25 °C is 285.8 MPa for EP/HNTs/DMMP and 1685.7 MPa for the composite containing 20 wt% of HNT-D. Moreover, the peak temperatures of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves determine the glass transition temperature (Tg) of the composites. Compared with EP/HNTs/DMMP, the Tg value of the EP/HNT-D composite is increased from 32.2 °C to 43.3 °C. From the results of the dynamic mechanical analysis, it is revealed that the composite containing 20 wt% of HNT-D exhibits higher storage modulus and Tg values.
δ curves determine the glass transition temperature (Tg) of the composites. Compared with EP/HNTs/DMMP, the Tg value of the EP/HNT-D composite is increased from 32.2 °C to 43.3 °C. From the results of the dynamic mechanical analysis, it is revealed that the composite containing 20 wt% of HNT-D exhibits higher storage modulus and Tg values.
| EP/DMMP | EP/HNTs/DMMP | EP/HNT-D | |
|---|---|---|---|
| Tg (°C) | 29.3 | 32.2 | 43.3 |
| Storage modulus at 25 °C (MPa) | 84.3 | 285.8 | 1685.7 |
| Shore D hardness | 42.4 | 43.8 | 58.1 |
| Tensile strength (MPa) | 3.9 | 4.8 | 13.6 |
Hardness and tensile strength are important parameters for analyzing the softening influences imparted by plasticizers on EP. We have measured the stress–strain test and shore D hardness of the EP/HNTs/DMMP and EP/HNT-D composites and listed the data in Table 4. It is evident that the composite containing 20 wt% of HNT-D exhibits a higher tensile strength and shore D hardness. In particular, compared with the shore D hardness of EP/HNTs/DMMP, the shore D hardness of the EP/HNT-D composite is increased from 43.8 to 58.1, experiencing an increase of 32.6%. As we all know, DMMP is a kind of organophosphorous flame retardant. Organophosphorous compounds effect epoxy materials by decreasing the glass transition temperature when directly added into the UV-curable epoxy resin. Moreover, a decrease in the glass transition temperature means in principle a loss of mechanical strength and also a lower end-use temperature for the composite. However, for EP/HNT-D, DMMP is loaded into the halloysite nanotubes and this softening influence is reduced. This phenomenon indicates that HNT-D could restrain the softening influences of DMMP. Moreover, the mechanical properties of EP/DMMP are listed in Table 4. The results indicate that EP/HNT-D has better mechanical properties than EP/DMMP.
4 Conclusions
We prepared a nanosized hybrid material through loading DMMP into halloysite nanotubes with the aim of providing a new flame retardant suitable for UV-curable epoxy resins. The HNT-D containing UV-cured epoxy resin was analyzed with a cone calorimeter to evaluate the flame retardancy. It was found that compared with neat EP, the EP/HNT-D composites performed better flame retardancy with an obvious decrease in the HRR, THR, TSR, MLR, SPR and COPR. As for the epoxy resin containing 20 wt% of HNT-D, in particular, the pHRR and THR were reduced by 42% and 29%, respectively.The SEM micrographs and digital photographs showed that compared with the residues of both neat EP and EP/HNTs, the carbon layer of the EP/HNT-D residue was more compact and stable. The flame retardant mechanism of HNT-D contains two reaction steps: DMMP gasification followed by a polyphosphoric-HNT carbon layer generating on the condensed phase. Moreover, the dynamic mechanical analysis results and stress–strain test showed that the UV-cured EP composites with HNT-D had better mechanical properties than the composites in which halloysite clay and DMMP were added directly. From the results of this study, it is revealed that HNT-D improves the flame resistance of UV-curable epoxy resin and also reduces the softening influences of DMMP. The preparation method here may potentially be used to develop other smart epoxy resin systems in the future.
Acknowledgements
This work was supported by the Science and Technology Commission of Shanghai Municipality (STCSM) under Grant No. 13DZ1108904.References
- S. Y. Lu and I. Hamerton, Prog. Polym. Sci., 2002, 27, 1661–1712 CrossRef CAS.
- L. J. Chen, L. Song, P. Lv, G. X. Jie, Q. L. Tai, W. Y. Xing and Y. Hu, Prog. Org. Coat., 2011, 70, 59–66 CrossRef CAS.
- P. M. Hergenrother, C. M. Thompson, J. G. Smith, J. W. Connell, J. A. Hinkley, R. E. Lyon and M. Moulton, Polymer, 2005, 46, 5012 CrossRef CAS.
- X. D. Qian, L. Song, Y. Hu, R. K. Yuen, L. J. Chen, Y. Q. Guo, N. N. Hong and S. H. Jiang, Ind. Eng. Chem. Res., 2011, 50, 1881 CrossRef CAS.
- S. W. Zhu and W. F. Shi, Polym. Degrad. Stab., 2003, 80, 217–222 CrossRef CAS.
- A. Dasari, Z. Z. Yu, G. P. Cai and Y. W. Mai, Prog. Polym. Sci., 2013, 38, 1357–1387 CrossRef CAS.
- W. Xu, G. J. Wang and X. R. Zheng, Polym. Degrad. Stab., 2015, 111, 142–150 CrossRef CAS.
- G. D. Feng, P. Y. Jia, L. Q. Zhang, L. H. Hu, M. Zhang and Y. H. Zhou, Korean J. Chem. Eng., 2015, 32, 1201–1206 CrossRef CAS.
- P. Y. Jia, M. Zhang, L. H. Hu, C. G. Liu, G. D. Feng, X. H. Yang, C. Y. Bo and Y. H. Zhou, RSC Adv., 2015, 5, 76392 RSC.
- P. Y. Jia, M. Zhang, L. H. Hu, C. G. Liu, G. D. Feng, C. Y. Bo, C. G. Liu and Y. H. Zhou, Ind. Crops. Prod., 2015, 76, 590–630 CrossRef CAS.
- P. Y. Jia, M. Zhang, L. H. Hu, J. Zhou, G. D. Feng and Y. H. Zhou, Polym. Degrad. Stab., 2015, 121, 292–302 CrossRef CAS.
- S. Hendricks, Nature, 1938, 142, 38 CrossRef CAS.
- N. G. Veerabadran, R. R. Price and Y. M. Lvov, Nano, 2007, 2, 115–120 CrossRef CAS.
- V. Vergaro, E. Abdullayev, Y. M. Lvov, A. Zeitoun, R. Cingolani, R. Rinaldi and S. Leporatti, J. Mater. Chem. B, 2013, 1, 2894–2903 RSC.
- E. Abdullayev, R. Price, D. Shchukin and Y. Lvov, ACS Appl. Mater. Interfaces, 2009, 1, 1437–1443 CAS.
- P. Yuan, P. D. Southon, Z. W. Liu and C. J. Kepert, Nanotechnology, 2012, 23, 1–5 Search PubMed.
- Y. J. Suh, D. S. Kil, K. S. Chung, E. Abdullayev, Y. M. Lvov and D. Mongayt, J. Nanosci. Nanotechnol., 2011, 11, 661–665 CrossRef CAS PubMed.
- M. X. Liu, Z. X. Jia, D. M. Jia and C. R. Zhou, Prog. Polym. Sci., 2014, 39, 1498–1525 CrossRef CAS.
- Z. X. Jia, Y. F. Luo, B. C. Guo, B. T. Yang, M. L. Du and D. M. Jia, Polym.–Plast. Technol. Eng., 2009, 48, 607–613 CrossRef CAS.
- M. L. Du, B. C. Guo and D. M. Jia, Eur. Polym. J., 2006, 42, 1362–1369 CrossRef CAS.
- N. F. Attia, M. A. Hassan, M. A. Nourb and K. E. Geckeler, Polym. Int., 2014, 63, 1168–1173 CrossRef CAS.
- B. Lecouvet, M. Sclavons, S. Bourbigot and C. Bailly, Polym. Degrad. Stab., 2013, 98, 1993–2004 CrossRef CAS.
- P. Rybinski and G. Janowska, Thermochim. Acta, 2013, 557, 24–30 CrossRef CAS.
- R. Nakamura, A. N. Netravali, A. B. Morgan, M. R. Nyden and J. W. Gilman, Fire Mater., 2013, 37, 75–90 CrossRef CAS.
- Y. Fu, D. Zhao, P. J. Yao, W. C. Wang, L. Q. Zhang and Y. Lvov, ACS Appl. Mater. Interfaces, 2015, 7, 8156–8165 CAS.
- F. L. Jin, X. Li and S. J. Park, J. Ind. Eng. Chem., 2015, 29, 1–11 CrossRef CAS.
- J. Sun, X. D. Wang and D. Z. Wu, ACS. Appl. Mater. Interfaces, 2012, 4, 4047–4061 CAS.
- B. G. Compton and J. A. Lewis, Adv. Mater., 2014, 26, 5930–5935 CrossRef CAS PubMed.
- Y. Chen, X. Q. Jia, M. Q. Wang and T. Wang, RSC Adv., 2015, 5, 33171–33176 RSC.
- S. V. Levchik and E. D. Weil, Polym. Int., 2004, 57, 1901 CrossRef.
- W. S. Liu, Z. G. Wang, L. Xiong and L. N. Zhao, Polymer, 2010, 51, 4776 CrossRef CAS.
- Y. J. Li, X. Y. Gu, J. Zhao, P. Jiang, J. Sun and T. Wang, J. Appl. Polym. Sci., 2014, 40011 Search PubMed.
- P. J. Chao, Y. J. Li, X. Y. Gu, D. D. Han, X. G. Jia, M. Q. Wang, T. F. Zhou and T. Wang, Polym. Chem., 2015, 6, 2977 RSC.
- J. Deng and W. F. Shi, Eur. Polym. J., 2004, 40, 1137 CrossRef CAS.
- G. H. Chen, B. Yang and Y. Z. Wang, J. Appl. Polym. Sci., 2006, 102, 4978 CrossRef CAS.
- A. S. Zerda and A. J. Lesser, J. Appl. Polym. Sci., 2002, 84, 302–309 CrossRef CAS.
- F. F. Feng and L. J. Qian, Polym. Compos., 2014, 35, 301–309 CrossRef CAS.
- C. Zhang, S. M. Liu, J. Y. Huang and J. Q. Zhou, Chem. Lett., 2010, 39, 1270–1272 CrossRef CAS.
- C. Q. Wang, F. Y. Ge, J. Sun and Z. S. Cai, J. Appl. Polym. Sci., 2013, 130, 916–926 CrossRef CAS.
- M. Ying, Additives for Plastics Handbook, Jiangsu Science and Technology Press, Jiangsu, China, 2002 Search PubMed.
- P. Yuan, P. D. Southon, Z. W. Liu, M. E. R. Green, J. M. Hook, S. J. Antill and C. J. Kepert, J. Phys. Chem. C, 2008, 112, 15742–15751 CAS.
- N. F. Attia, M. A. Hassan, M. A. Nourb and K. E. Geckeler, Polym. Int., 2014, 63, 1168–1173 CrossRef CAS.
- H. Y. Ma, L. F. Tong, Z. B. Xu and Z. P. Fang, Appl. Clay Sci., 2008, 42, 238–245 CrossRef CAS.
- X. Zhang, J. W. Wang, J. Zhong, A. Liu and J. Gao, Microporous Mesoporous Mater., 2008, 108, 13–21 CrossRef CAS.
- Y. F. Ma, N. Li, X. T. Ren, S. H. Xiang and N. J. Guan, J. Mol. Catal. A: Chem., 2006, 250, 9–14 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08178a |
| This journal is © The Royal Society of Chemistry 2016 |