Coupling catalytic hydrolysis and oxidation on metal-modified activated carbon for HCN removal
Abstract
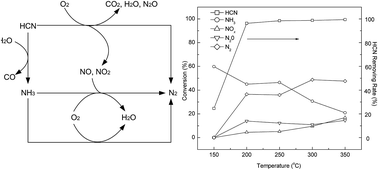
A method of coupling catalytic hydrolysis and oxidation for HCN removal by metal-modified activated carbon (denoted as AC-M) was studied using a dynamic method in a fixed bed reactor. The results showed that impregnation of metal oxides on the activated carbon significantly enhanced the removal capacity for HCN. Among the different types of metal-modified catalysts, AC-Cu exhibited the highest catalytic activity. The AC-Cu catalyst showed >96% conversion of HCN at 200–350 °C. The selectivity of N2 in the conversion of HCN reached 48.8% at 300 °C. Oxygen concentration, relative humidity and calcination temperature can greatly influence the catalytic activity. In particular, the reaction temperature was determined to be a crucial factor. The detailed characterization of the catalyst was performed using X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), X-ray photoelectron spectroscopy (XPS), temperature-programmed reduction (TPR), and temperature programmed desorption (TPD). The Cu 2p XPS spectra and XRD patterns indicated that CuO was formed as an active species for the catalytic removal of HCN. We concluded that AC-Cu could be used as a catalyst for the removal of HCN by coupling catalytic hydrolysis and oxidation.

 Please wait while we load your content...
Please wait while we load your content...