Ionic liquid-based fluorescence sensing paper: rapid, ultrasensitive, and in-site detection of methamphetamine in human urine†
Ruifeng Wangabc,
Xiujuan Qia,
Shimin Liua,
Lei Zhaoc,
Liujin Lua and
Youquan Deng*a
aCentre for Green Chemistry and Catalysis, State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, No. 18, Tianshui Middle Road, Lanzhou, P. R. China. E-mail: ydeng@licp.cas.cn; Tel: +86-931-4968116
bUniversity of Chinese Academy of Sciences, Beijing, 100049, China
cPublic Security Bureau of Ordos, The Inner Mongolia Autonomous Region, 017000, China
First published on 25th May 2016
Abstract
A low cost sensing paper chip supporting an ionic liquid and a fluorescent indicator agent was developed to perform a rapid and ultrasensitive in-site methamphetamine test in human urine. The extraction ability of ionic liquids for methamphetamine, fluorescence behaviour of the system, and performance of the sensing paper were emphasized.
Over the past decades, drugs of abuse have continuously spread around the world, posing danger to both human health and social stability. Owing to public hazard, monitoring of drugs of abuse has become an important topic in the fields of drug control and rehabilitation, field testing, forensic science, etc. Currently, methamphetamine (MA) is the most well-known drug of abuse among young people.1 In general, MA and its metabolites could be assessed through urine analyses, and the results can serve as evidence for identifying drug abusers.2 Until date, several methods have been developed for qualitative or quantitative assessment of MA and its related compounds in human urine samples, such as gas chromatography (GC), high-performance liquid chromatography (HPLC), GC-mass spectrometry (MS), HPLC-MS, capillary electrophoresis (CE), CE-MS, and immunoassays.3 However, most of them are time-consuming, and involve expensive apparatuses or tedious sample pre-treatment. Therefore, development of an efficient, rapid, and simple detection method for such drugs is still necessary in the public safety field.
Fluorescent sensors, which exhibit high sensitivity, selectivity, and fast response, have attracted much attention.4 During the past decades, these chemo-sensors have achieved great success in trace detection of explosives, metal ions, small biomolecules, proteins, DNA, and MA.5 However, these sensors generally have some disadvantages, such as complex preparation process, high costs, and difficulties for practical applications at testing fieldwork.
In recent years, ionic liquids (ILs) have been focussed on in a variety of fields, owing to their unique physicochemical properties including chemical synthesis, catalytic chemistry, electrochemical devices, separation, and biomass dissolution, which are considered green alternatives for traditional volatile organic compounds (VOCs).6 Recently, there have been many reports on the applications of ILs as ideal substitutes for VOCs in separation science, including liquid–liquid extraction, liquid-phase micro-extraction, dispersive liquid–liquid extraction, solid-phase micro-extraction, and electro-migration procedure.7 Moreover, ILs have been used as additives in some systems to increase fluorescence intensity.8
Considering the excellent extraction ability and fluorescence enhancement of ILs, in this work, we aimed to develop a rapid, ultrasensitive, and in-site detection method for MA in human urine. Our strategy includes three steps. First, in order to select a suitable extractant, the extraction efficiency of several hydrophobic ILs for MA were studied in detail. Second, an indicator agent was selected to display the MA enriched by ILs by generating a fluorescent signal. Finally, to demonstrate its possibility for practical applications, a test paper was designed to detect MA in human urine. A schematic illustration of the basic strategy is described in Scheme 1.
A series of hydrophobic ILs with low viscosity, including EMImNTf2, BMImNTf2, BMMImNTf2, BMImPF6, BPyNTf2, PP1,4NTf2, and P1,4NTf2 (structures are shown in Scheme 2; the abbreviations are defined as Table 1 shown) were employed to extract MA from the aqueous solution. A stock solution of MA–HCl (0.1 mg mL−1) was prepared in ultrapure water. The extraction was performed at room temperature, with pH 11 maintained by NaOH (see ESI1† for extraction procedure details). The parameter E was determined to compare the extraction efficiency of ILs, and was calculated by the equation: E = 1 − Ai/Ao, where Ao and Ai are the peak area of MA in water phase before and after extraction, respectively.
| ILs | Aia | Ea (%) |
|---|---|---|
| a Repeated 3 times, taking the average. EMIm: 1-ethyl-3-methylimidazolium; BMIm: 1-butyl-3-methylimidazolium; BMMIm: 1,2,3-trimethylimidazolium; BPy: N-butylpyridinium; PP1,4: N-butyl-N-methyl-piperidinium: P1,4: N-butyl-N-methylpyrrolidinium; NTf2: bis((trifluoromethyl)sulfonyl)amide; PF6: hexafluorophosphate. | ||
| EMImNTf2 | 216.696 | 90.4 |
| BMImNTf2 | 291.808 | 87.1 |
| BMMImNTf2 | 461.400 | 79.6 |
| BMImPF6 | 522.228 | 76.9 |
| BPyNTf2 | 194.734 | 91.4 |
| PP1,4NTf2 | 403.810 | 82.1 |
| P1,4NTf2 | 239.230 | 89.4 |
| MA | Ao = 2260.54 | |
The results of the extraction are listed in Table 1. It was shown that BPyNTf2 displayed significant extraction ability for MA (E = 91.4%), however, BMImPF6 gave the lowest value (E = 76.9%). Other NTf2 type ILs gave an E value from 79.6 to 90.4%. This suggested that the type of anions of ILs significantly affected the extraction ability. Another important factor that influenced the extraction performance was the cation type of ILs. For example, among BMImNTf2 (chosen as a typical representative of alkylimidazolium NTf2 salts), BPyNTf2, PP1,4NTf2, and P1,4NTf2, BPyNTf2 yielded the highest E value. Then, the order of E value from high to low was P1,4NTf2 > BMImNTf2 > PP1,4NTf2. To investigate the mechanism of extraction, control experiments were carried out by comparing the extraction efficiency of EMImNTf2, BMImNTf2, and BMMImNTf2. The results showed that BMImNTf2 exhibited higher extraction efficiency than BMMImNTf2 and EMImNTf2 was more efficient than BMImNTf2. By comparing the chemical structure of these ILs, it is apparent that the activated hydrogen (H) atom of the imidazolium cation had a positive impact on the extraction procedure. For example, the E value of BMImNTf2 decreased from 87.1% to 79.6% after the H of 2C was substituted by methyl. Furthermore, the E value of BMImNTf2 increased by 3.3% after butyl was replaced by ethyl. This was because ethyl had a weaker steric hindrance effect that made the activated H atom easier to capture MA. Although pyridinium was different with imidazolium, it was inferred that the interaction between the activated H atom on the pyridinium cation of BPyNTf2 and the N atom of MA might play an important role in this process. In addition, it is worth mentioning that the pre-concentration procedure only took approximately 15 s, indicating that an efficient and rapid process for the detection of MA could be achieved.
Early reports9 have shown that dansyl chloride (DNC) could be used as the derivatization agent in the detection of MA, owing to advantages such as: (1) the fluorescence of MA-derivatized DNC is readily apparent under UV light, (2) good specificity could be obtained (many other abused drugs, such as opiates, cocaine, and methylephedrine, yielded negative results using the DNC-derivatizing system), and (3) there were few urinary components that interfered with the detection of MA. In this manner, DNC was introduced in the system of BPyNTf2-proconcentrated MA as indicator agent to apperceive it more simply and rapidly. The indicator agent could react with the MA in BPyNTf2 very quickly under ambient conditions and then form a fluorescent product (5-(dimethylamino)-N-methyl-N-(1-phenylpropan-2-yl)naphthalene-1-sulfonamide, referred to as FSP, shown in Scheme 3).
For comparison, a reference experiment was performed. A human urine sample (5.0 mL) was spiked with MA–HCl (400 ng mL−1), and then extracted by 0.5 mL BPyNTf2. The IL phase was separated from the sample and dissolved into 5 mL CH2Cl2 containing DNC (10−2 mmol mL−1). After a 30 s reaction, the solution was treated with UV light and characterised by fluorescence spectroscopy. Blank urine (5.0 mL) from the same volunteer was also treated in the same way. As shown in Fig. 1, the MA-added sample emitted a strong green fluorescence excited by UV light (Fig. 1B), while no obvious phenomenon was observed in the blank urine (Fig. 1C). The fluorescence spectra also gave consistent results, as shown in Fig. 1A. This suggested that the ILs-indicator system was feasible and effective to detect MA in human urine.
 | ||
| Fig. 1 Comparison of fluorescence intensity for blank (A red line/C) and MA-contained (A blue line/B) solution. A: fluorescence spectra; B/C: real picture. Excited at 315 nm. | ||
The fluorescence behaviour of the FSP solution was studied in the 305–395 nm wavelength range with steps of 10 nm. As shown in Fig. 2, FSP can emit observable fluorescence in a wide range excitation wavelength (from 305 to 365 nm), which is the main wavelength range of UV light. The strongest emission peak was observed at ∼540 nm when the excitation wavelength was 315 nm. Changes in the excitation wavelength did not results in significant shifts in the emission wavelength (∼540 nm) and no other emission peaks were observed, indicating that the detection of MA was not interfered by urinary components. In addition, the range of emission wavelength was from ∼450 nm to ∼700 nm, which covers the main wavelength of visible light, indicating that the fluorescence could be identified by naked eyes.
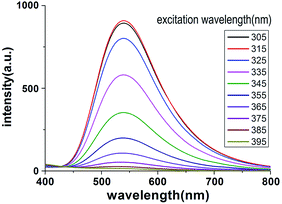 | ||
| Fig. 2 Fluorescence intensity changes with different excitation wavelength (from 305 to 395 nm). The initial concentration of MA in human urine was 100 ng mL−1. | ||
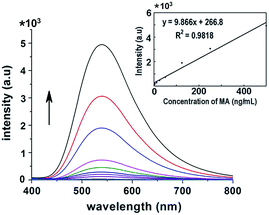
Subsequently, the sensitivity of this system was investigated. The initial concentration of MA in blank human urine was varied from 0.5–500 ng mL−1. Then, after extraction with 500 μL BPyNTf2, the IL phase was diluted by 5 mL CH2Cl2/DNC solution. The fluorescence spectra (excited at 315 nm) of these solutions are shown in Fig. 3. Results show that increasing the MA concentration leads to an increase in fluorescence intensity, accordingly. The fluorescence intensity changes of these different concentration solutions of MA were monitored. Among these changes, it is worth noting that the fluorescence intensity was 726.5 a.u. (green line in Fig. 3) when the concentration of MA was 50 ng mL−1. The fluorescence emitted by the sensing paper (fabricated as the way described below) could be seen by naked eyes under UV light when the concentration of MA was over 50 ng mL−1, otherwise, the fluorescent light could not be appreciated clearly (details described below). Moreover, as shown in Fig. 3, the fluorescence increase was significant and constant, which led us to investigate the linear relationship between the MA content in human urine and fluorescence intensity. As the insert in Fig. 3 shows, the intensity enhancing plots were well-fitted by the linear equation (y = 9.866x + 266.8, R2 = 0.9818), and permitted to calculate that with a fluorescence intensity increase of 100 a.u., the detection for MA was 101 ng mL−1. Based on this result, a semi-quantitation of the MA content in human urine could be achieved using the sensing paper and visualized by naked eyes.
Based on the results, a sensing paper chip supporting an ionic liquid and a fluorescent indicator agent was schemed out for in-site testing of MA in human urine. The filter paper pieces were added into the CH2Cl2 solution containing BPyNTf2 and DNC to infuse for 1 minute; the manufacturing process for the ionic liquid-based fluorescence sensing paper (ILBF-sp) in this way is simple and efficient. In addition, it was found that DNC could dissolve completely into BPyNTf2 (ESI2†), indicating that BPyNTf2 not only extracted MA efficiently, but also provided a hydrophobic environment to keep DNC from hydrolysing. To evaluate the performance of the ILBF-sp, a series of human urine (from healthy volunteers) spiked with different solutions of MA (500, 250, 100, 50, 25 ng mL−1, respectively) were employed. After ILBF-sp was dipped in these urine solutions, we observed the colour changes under a portable UV lamp. As Fig. 4 shows, the ILBF-sp generated green fluorescence and the intensity increased with increase in the MA concentration; green changed from pale to bright, while the blue-like colour in the blank ILBF-sp gradually disappeared. Therefore, the concentration of MA in urine could be predicted by visually comparing the fluorescence intensity in the ILBF-sp. Another point investigated was the qualitative performance of the ILBF-sp. Fig. 4 also shows that the fluorescence intensity was weak when the concentration was 25 ng mL−1, making it difficult to distinguish from the blank. By comparison, the fluorescence was sufficiently intense when the concentrations were higher than 50 ng mL−1. Therefore, to assure a determination with accuracy and the objectivity, 50 ng mL−1 of MA was defined as the detection threshold value of the ILBF-sp.
 | ||
| Fig. 4 Colours of ILBF-sp before and after testing different concentrations of MA (25 to 500 ng mL−1) in human urine under a portable UV lamp. | ||
Currently, the immune colloidal gold testing paper (IMCG-tp) method is widely applied for screening of drugs of abuse.10 However, the IMCG-tp usually includes time-consuming production process steps with expensive regents; in addition, it has a higher detection threshold value. For the development of a practical detection paper, here we describe a successful case to detect the presence of MA in a MA abuser's urine. Meanwhile, a comparative test was carried out, using the IMCG-tp to test the same samples. Urine (60 μL) was dropped on the IMCG-tp, and ILBF-sp was dipped in the urine sample, respectively. Consequently, the ILBF-sp gave a satisfactory result with a strong fluorescent signal, while the IMCG-tp showed negative result (ESI3†).
The detection selectivity of the sensing paper is a very important feature that should elicit attention. In fact, the detection selectivity could be guaranteed in part by: (1) the good extraction performance of the ionic liquid, which had been discussed in our manuscript; (2) the outstanding selective reaction between dansyl chloride (employed as fluorescence agent) and methamphetamine, which has been investigated by other researchers in detail.9 However, it was not enough to ensure that the paper was a useful sensor. Therefore, four typical medicines that could be metabolized by kidney and detected in urine (chlorpheniramine, ephedrine, phenobarbital, and lorazepam) were chosen to test the detection selectivity of the paper.11 In the control experiment, a series of ultrapure water solutions of these compounds, and methamphetamine as reference, were prepared at the concentration of 100 ng mL−1, and treated by the sensing paper. The results (ESI4†) showed no obvious fluorescence for chlorpheniramine, phenobarbital, or lorazepam, with the exception of ephedrine. This indicated that if a person dosed the ephedrine-containing medicine, it would influence the detection selectivity of the sensing paper. Nevertheless, considering the structure similarity between ephedrine and methamphetamine, the interference was reasonable. In addition, because of individual differences, the diversity of diet and uncertainty of the drug used, which lead to the complexity of metabolites, made it impossible to ensure that the results were accurate. Consequently, our research aimed to develop a low-cost paper as a useful tool for screening “ice” abusers. Studies to elucidate the chances of error are underway in our lab.
In summary, herein we report a rapid, ultrasensitive, and in-site detection method of MA in human urine, using ILBF-sp. In our approach, the use of BPyNTf2 plays a key role because it provides: (1) a medium that pre-concentrates MA in human urine efficiently and rapidly, (2) a hydrophobic environment in which DNC could react with MA undisturbed and rapidly, (3) a non-volatile liquid medium that enables simple invention of practical sensing paper. Another key element is the use of DNC, which serves as an efficient indicator by reacting with MA rapidly and then generating a powerful fluorescent product. Based on the ionic liquid combined with DNC, a low-cost sensing paper chip is developed, which could be used for rapid and accurate in-site screening of MA abusers. Furthermore, considering the number of challenges that are posed to forensic toxicology laboratories by new designer drugs, such as dimethoxyphenyl ethanamines (2C, 2C-T) and dimethoxy-phenylpropanamine (DO) series, which are difficult to be detected,10 the excellent extraction ability of ionic liquids would provide ample opportunities for the detection of these new designer drugs in future.
Support from National Natural Science Foundation of China (No. 21305146 and 21403259).
All experiments were performed in compliance with relevant laws and institutional guidelines and were approved by the Lanzhou Institute of Chemical Physics and Public Security Bureau of Ordos. For the experimentation with human urine, the informed consents were obtained from the volunteers.
Notes and references
- United Nations Division for Policy Analysis and Public Affairs, World Drug Report 2015, Vienna, Austria, 2015 CrossRef CAS PubMed; Drug Enforcement Agency Domestic Drug Seizures Statistics & Facts, http://www.dea.gov/resource-center/statistics.shtml CrossRef CAS PubMed; P. M. Mach, E. M. McBride, Z. J. Sasiene, K. R. Brigance, S. K. Kennard, K. C. Wright and G. F. Verbeck, Anal. Chem., 2015, 87, 11501 CrossRef CAS PubMed.
- T. Guinan, C. D. Vedova, H. Kobusc and N. H. Voelcker, Chem. Commun., 2015, 51, 6088 RSC; A. Liau, J. Liu, L. Lin, Y. Chiu, Y. Shu, C. Tsai and C. Lin, Forensic Sci. Int., 2003, 134, 17 CrossRef CAS PubMed; M. Concheiro, S. M. S. S. Simões, Ó. Quintela, A. de Castro, M. J. R. Dias, A. Cruz and M. López-Rivadulla, Forensic Sci. Int., 2007, 171, 44 CrossRef PubMed; J. A. Levisky, D. L. Bowerman, W. W. Jenkins, D. G. Johnson, J. S. Levisky and S. B. Karch, Forensic Sci. Int., 2001, 122, 65 CrossRef PubMed.
- P. R. Paetsch, G. B. Baker, L. E. Caffaro, A. J. Greenshaw, G. A. Rauw and R. T. Coutts, J. Chromatogr. B: Biomed. Sci. Appl., 1992, 573, 313 CrossRef CAS; T. Kraemer and H. H. Maurer, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 713, 163 CrossRef; M. S. Fuh and K. Lu, Talanta, 1999, 48, 415 CrossRef PubMed; I. Bokor, V. C. Trenerry and P. Scheelings, Forensic Sci. Int., 1997, 85, 177 CrossRef; W. Matapatara, P. Thongnopnua and V. Lipipun, Asian Biomed., 2007, 1, 161 Search PubMed.
- S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef PubMed.
- F. Maffei, P. Betti, D. Genovese, M. Montalti, L. Prodi, R. D. Zorzi, S. Geremia and E. Dalcanale, Angew. Chem., Int. Ed., 2011, 50, 4654 CrossRef CAS PubMed; S. W. Thomas III, J. P. Amara, R. E. Bjork and T. M. Swager, Chem. Commun., 2005, 36, 4572 RSC; C. He, Q. G. He, C. M. Deng, L. Q. Shi, D. F. Zhu, Y. Y. Fu, H. M. Cao and J. G. Cheng, Chem. Commun., 2010, 46, 7536 RSC; J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef.
- T. Welton, Chem. Rev., 1999, 8, 2071 CrossRef.
- M. Rezaee, Y. Yamini and M. Faraji, J. Chromatogr. A, 2010, 1217, 2342 CrossRef CAS PubMed; P. Viñas, N. Campillo, I. López-García and M. Hernández-Córdoba, Anal. Bioanal. Chem., 2014, 406, 2067 CrossRef PubMed; R. Jain and R. Singh, TrAC, Trends Anal. Chem., 2016, 75, 227 CrossRef; Q. Zhou, Y. Gao, J. Xiao and G. Xie, Anal. Methods, 2011, 3, 653 RSC.
- Y. Shu, L. Han, X. Wang, X. Chen and J. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12156 Search PubMed; M. Ali, V. Kumar and S. Pandey, Chem. Commun., 2010, 46, 5112 RSC; H. Wu, L. Zhang and L. Du, Talanta, 2011, 85, 787 CrossRef CAS PubMed.
- H. Yamada, A. Yamahara, S. Yasuda, M. Abe and K. Oguri, J. Anal. Toxicol., 2002, 26, 17 CrossRef CAS PubMed; H. Yamada, S. Ikeda-Wada and K. Oguri, Biol. Pharm. Bull., 1998, 21, 718 Search PubMed.
- S. Kerrigan, M. B. Mellon, S. Banuelos and C. Arndt, J. Anal. Toxicol., 2011, 35, 444 CrossRef CAS PubMed.
- G. Tang and D. Li, Modern clinical pharmacology, Chemical Industry Press, Beijing, 2nd edn, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Extraction details and comparison experiment. See DOI: 10.1039/c6ra08193b |
| This journal is © The Royal Society of Chemistry 2016 |