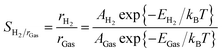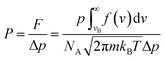Heptazine-based graphitic carbon nitride as an effective hydrogen purification membrane†
Yujin Ji,
Huilong Dong,
Haiping Lin*,
Liling Zhang,
Tingjun Hou and
Youyong Li*
Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou, Jiangsu 215123, China. E-mail: yyli@suda.edu.cn; hplin@suda.edu.cn
First published on 25th May 2016
Abstract
The purification of H2 from other gases (CH4, CO, CO2, N2, and H2O) is a vital step for its safe usage. By using first-principles calculations and molecular dynamics simulations, we find that the porous graphitic carbon nitride (g-C3N4) monolayer works as an efficient and highly selective hydrogen purification membrane. In the DFT calculations, the transition state theory is used to search the lowest diffusion barrier (0.55 eV) for H2 to go through the well-ordered intrinsic holes. Meanwhile, the excellent selectivity between H2 and other gases shows that the g-C3N4 nanosheet is specific for diffusion of H2. The MD simulations exhibit the whole dynamic purification process and confirm our previous DFT results. Our results indicate that the g-C3N4 nanosheet has great potential in separating H2 from undesirable gases.
1. Introduction
Hydrogen, as an environment-friendly, sustainable and high-energy-density resource, is regarded as one of the most efficient substitutes of fossil fuels to address the issue of the shortage of energy in the future.1,2 However, a technical challenge in hydrogen production, storage, transportation and usage is the existence of undesirable gases, which may result in security risks. For example, a mixture of H2, CO, and CH4 is produced through the steam-methane reforming in current hydrogen production methods.3 The purification of H2 from undesirable gases (CH4, CO, CO2, N2, and H2O) is, therefore, regarded as an important step in the hydrogen production process. Compared with traditional gas separation techniques (e.g. pressure swing adsorption or cryogenic distillation), two-dimensional (2D) materials with single-atom thickness seem more promising because of their distinct advantages in low energy consumption and recycling through physical interactions at the atomic scale.4,5The search and design of appropriate porous 2D materials have attracted wide attention both by theoretical calculations and experimental investigations.6–9 In general, a suitable H2 purification membrane should possess two characteristics: (a) no/little interaction between H2 and membrane materials; (b) a certain diffusion barrier to differentiate migration between H2 and other gases migration. Correspondingly, the permeance and selectivity are two important factors to evaluate the performance of purification membrane.10 Organic 2D materials including carbon-based,11,12 polymer13 and h-BN membranes14,15 and inorganic materials16 containing metallic, silica17 and zeolite18 membranes are current well-known membrane materials. Notably, Jiang et al.19 demonstrated that porous graphene with hydrogenated and nitrogen-functionalized at the edge of pores can facilitate H2/CH4 separation and reduce the barrier for H2 diffusion. Meanwhile, the doping of nitrogen atom will not modify the hydrogen adsorption ability. Thus, it is straightforward for us to consider 2D carbon nitride material as a natural membrane in the H2 purification and filtering.
Graphitic carbon nitride (g-C3N4) is similar to graphene with a sp2 π-conjugated system through van der Waals interaction between layers, which has been applied in the field of energy and materials science.2,20–24 Free-standing g-C3N4 monolayer is chemically and thermally stable. It can be synthesized by the thermal polycondensation process2 and has shown huge potentials in the spintronic devices25 and photocatalysis fields.26,27 The single layer g-C3N4 is a direct band gap of about 2.4 eV at the gamma point, rendering them promising as metal-free water-splitting materials. At the same time, the intrinsic vacancies surrounded with nitrogen atoms in g-C3N4 nanosheet provide natural pathways for gases to diffuse compared to the extra/man-made pores or defects in other 2D materials, which makes them promising for H2 purification.
In this context, we report that the g-C3N4 nanosheet may serve as an effective and highly selective H2 separation membrane via the first-principles calculations. The target mixtures include H2/(CH4, CO) from the steam-methane reforming, H2/H2O from water splitting. The size of the intrinsic hole in g-C3N4 is appropriate, well-ordered and exhibits a higher permeability and selectivity for H2 rather than H2O, N2, CO, CO2, and CH4. The combination of g-C3N4 in catalytic water splitting and H2 purification will greatly extend the practical applications of g-C3N4 materials.
2. Computational details
First, all of the first principles calculations were carried out by the Vienna Ab-initio Simulation Package (VASP).28 The Perdew–Burke–Ernzerhof (PBE)29 functional with van der Waals correction (DFT-D2)30 was chosen to consider the interaction between the gas molecules and g-C3N4. The climbing image nudged elastic band (CI-NEB) method31 was used to search the minimum energy pathway (MEP) when gases diffuse through the porous g-C3N4. The cut-off energy was set to 500 eV and the surface Brillouin zone was sampled with 5 × 5 × 1 Monkhorst–Pack k-point grids. A vacuum thickness of 20 Å with periodic boundary conditions was set to avoid the interactions between periodically-repeated layers. All of the structural relaxations are accomplished until the energy change is less than 10−4 eV and force change is less than 0.001 eV Å−1. The adsorption energy (Ea) between gases and g-C3N4 is defined as| Ea = Egas@g-C3N4 − (Egas + Eg-C3N4) |
Then the MD simulations of H2, N2, H2O, CO, CO2 and CH4 permeation were implemented by Forcite module available in Materials Studio software package. Similar as previous work,12,14,35 gas molecules were interspersed between the g-C3N4 membranes and the initial conditions were 300 K with a total time of 1000 ps. The constant-volume and constant-temperature (NVT) ensemble and COMPASS36 forcefield were employed during the simulation with a time step of 1 fs. The electrostatic interaction and van der Waals interaction were calculated using the atom based method.
3. Results and discussion
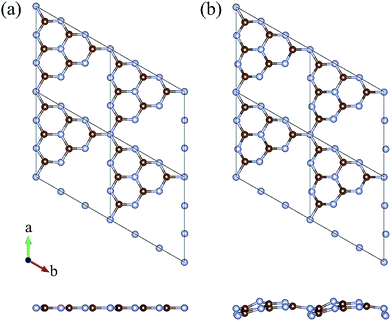
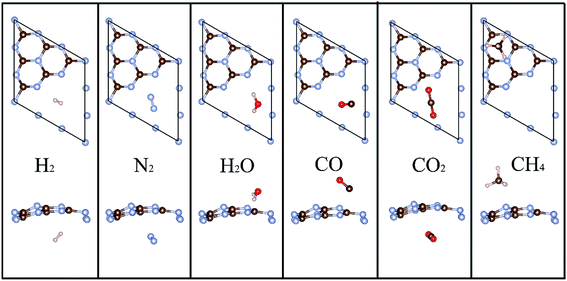
The geometric structure of g-C3N4 is determined first. Unlike the planar graphene, the single layered g-C3N4 forms two configurations – planar and buckled structures, containing 6 carbon and 8 nitrogen atoms in the primitive unit cell, as shown in Fig. 1. The optimized lattice constant of the planar g-C3N4 is 7.13 Å, in good agreement with the previous investigation2 and the lattice constant of the buckled one is calculated to be 7.05 Å. When gas molecules adsorb on the surface of planar g-C3N4, the planar one transforms into the buckled one and this transformation is irreversible. Moreover, the buckled unit cell is 0.50 eV per unit cell more stable than the planar one, indicating a higher stability. This agrees well with previous reports.37 The more stable buckled structure is, therefore, adopted for the following studies. Our buckled configuration is more stable than the reported planar graphitic carbon nitride,38 which has a better H2 purification performance.Then various gas molecules (containing H2, H2O, N2, CO, CO2, and CH4) adsorbed on the g-C3N4 with different adsorption configurations are investigated. Our simulations demonstrate that the adsorption sites of H2, H2O, N2, CO, and CO2 are at the intrinsic vacancy of g-C3N4 while the CH4 prefers to adsorb above the hexagonal ring (the equilibrium adsorption configurations are presented in Fig. 2). The equilibrium adsorption heights and energies are summarized in Table 1. As listed in Table 1, the calculated equilibrium distance between molecules and substrate is mostly within 2–3 Å. The adsorption energies of most gas molecules are smaller than 0.3 eV, which indicates that the common adsorption mode is physisorption through weak van der Waals (vdW) interaction. H2O molecule is an exception because of the orbital hybridization between H2O and g-C3N4 nanosheet, which results in a much higher adsorption energy (the comparison between the density of state of H2O absorbed g-C3N4 and pure buckled g-C3N4 is shown in Fig. S1 of ESI data†). It is worth noting that the adsorption configuration of H2 here is similar with the pristine and nitrogen-doped graphdiyne,9,39 confirming that the nitrogen atoms negligibly affect the adsorption properties.
| D0 (Å) | Ea (eV) | Eb (eV) | |
|---|---|---|---|
| H2 | 2.68 | −0.078 | 0.55 |
| H2O | 1.07 | −0.513 | 1.59 |
| N2 | 2.87 | −0.117 | 1.95 |
| CO | 2.03 | −0.155 | 1.80 |
| CO2 | 2.93 | −0.226 | 1.50 |
| CH4 | 2.31 | −0.163 | 3.36 |
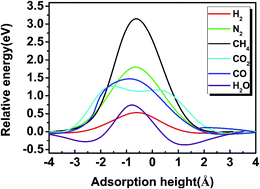
Next, in order to investigate the process of gas molecules passing through a g-C3N4 nanosheet, the initial state (IS) of the H2 molecule is set as that the H–H axis is perpendicular to the plane with a distance of nearly 5 Å between H2 and the centre of a vacancy in g-C3N4 nanosheet. The diffusion energy barrier is given by
| Eb = ETS − ESS |
Based on the MEP and diffusion energy barriers, we calculated the selectivity S and permeance P of various gas molecules to elucidate the properties of gas separation and diffusion for g-C3N4 nanosheet. For the selectivity of H2 with other gas molecules, it is calculated by using the following equation:40,41
where p, NA, m, kB and T represent the pressure, Avogadro constant, mass of molecule, Boltzmann constant and temperature, respectively. f(v) denotes the Maxwell distribution, and the kinetic energy at vB can overcome the barrier. Here we adopt an approximation that the feed gas on one side is at 1 bar and the other side of the membrane is filled with any other gas at 2 bar pressure as previous works.41 Fig. 4(b) shows that the permeance of different gas molecules increased with the temperature. The calculated permeance of H2 across the g-C3N4 nanosheet at 300 K is 10−7, which is acceptable for the industrial separation.42 Thus our DFT results provide a solid proof that the g-C3N4 with well-ordered pores is a potential H2 purification membrane at the ambient atmosphere.
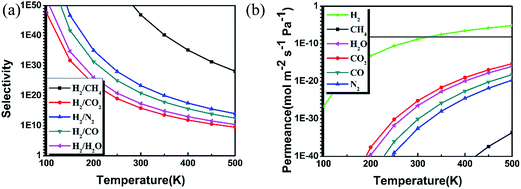 | ||
| Fig. 4 (a) Selectivity and (b) permeance for H2, H2O, N2, CO, CO2, and CH4 molecules passing through g-C3N4 nanosheet as a function of adsorption height or temperature. The horizontal line in the panel (b) is the industrial standard for gas permeation.42 | ||
| Membrane | g-C3N4 | H-graphene | Graphdiyne | Silicene |
|---|---|---|---|---|
| Dc (Å) | 1.70 | 2.5 | 2.24 | 4.7 |
| Ea (eV) | −0.078 | −0.05 | −0.07 | −0.06 |
| Eb (eV) | 0.55 | 0.22 | 0.01 | 0.34 |
| S(H2/CH4) | 1046 | 1023 | 1010 | 1022 |
The N atoms at the edge of porous graphene can increase the electrostatic interaction compared to no edge-modified graphene, resulting in a better selectivity and permeability for gas molecules.43,44 To investigate the origin of such selectivity, we plotted the electron density distribution of different gas molecules permeating through the g-C3N4 at the transitional state structures in Fig. 5. The distribution of electron density gives a way to describe the interaction between the gas molecules and the substrate. Our results show that there is little electron density overlapping at the TS of H2 diffusion compared with other TS configurations, suggesting that the electrostatic interaction plays a vital role during the permeation process. The electrostatic interaction will limit the permeation of other gas molecules due to the larger atomic sizes. Meanwhile, we plotted the vdW surface of g-C3N4 and gas molecules as shown in Fig. 6. The pore size of the g-C3N4 (unoccupied by vdW surface) as well as the diameters of cross section (Dc, vdW occupied) of different gas molecules is also evaluated (the values are listed in Table 3). The diameter of g-C3N4 pore is about 1.70 Å, significantly smaller than that of N-graphdiyne (2.24 Å), while the Dc (2.44 Å) of H2 presents more overlapping with the vdW surface of g-C3N4 pore. The comparison between the sizes of vdW surface well explains why g-C3N4 presents higher diffusion energy barrier and better selectivity for H2. Moreover, the overlapping between the vdW surface of g-C3N4 and other gases seems too much for gas molecules to permeate. Therefore, the suitable size of intrinsic vacancy in g-C3N4 is not only favourable for H2 diffusion but also helpful to hinder the permeation of other gas molecules. The N atoms at the rim of pores bring a strong electrostatic interaction and vdW interaction, resulting into excellent gas selectivity, which is consistent to previous analysis.43 Additionally, different from the traditional pores or defects caused by dangling bonds, the nitrogen atoms at the rim of intrinsic vacancy of g-C3N4 are covalent bond with the nearby carbon atoms, which provides a more stable permeation environment for the gases diffusion.19,40 The good permeation of H2 as well as the hindrance to other gases makes it feasible for application of g-C3N4 nanosheet in H2 purification.
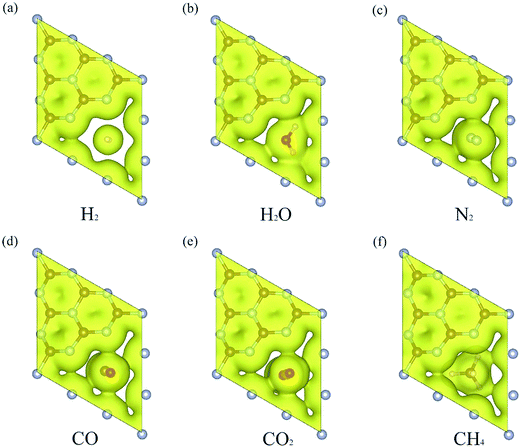 | ||
| Fig. 5 Electron density of (a) H2 (b) H2O (c) N2 (d) CO (e) CO2 and (f) CH4 at their corresponding transition states. Isosurface value is 0.002 e Å−3. | ||
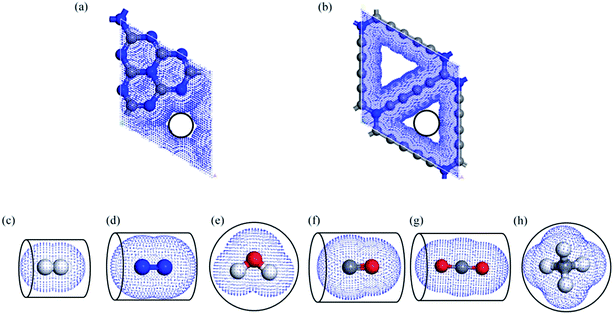 | ||
| Fig. 6 Geometric structure and van der Waals (vdW) surface of (a) g-C3N4 (b) N-graphdiyne39 (c) H2 (d) N2 (e) H2O (f) CO (g) CO2 and (h) CH4. The blue, grey, white and red beads represent the nitrogen, carbon, hydrogen, and oxygen atoms respectively. | ||
| vdW occupied volume (Å−3) | Diameter of cross section (Å) | |
|---|---|---|
| H2 | 10.87 | 2.44 |
| N2 | 23.76 | 3.20 |
| H2O | 19.48 | 3.34 |
| CO | 26.90 | 3.46 |
| CO2 | 33.71 | 3.44 |
| CH4 | 28.41 | 3.78 |
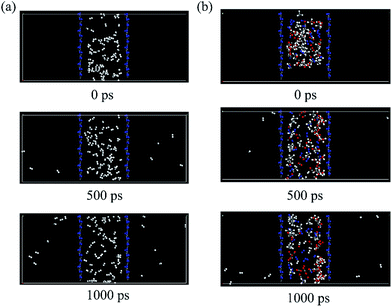
In addition, we perform molecular dynamics (MD) simulations under the realistic condition to reproduce the dynamic process of H2 and other gas molecules to diffuse through g-C3N4 nanosheet. A single component of 80 gas molecules and mixtures of 20 H2, 20 N2, 20 CO, 20 CO2, 20 CH4 and 20 H2O gas molecules are put between the g-C3N4 nanosheets with a 1000 ps MD simulation. The snapshot of pure 80 H2 molecules and mixtures of 120 gas molecules at 0 ps, 500 ps, 1000 ps are given in Fig. 7 (single components of N2, CO, CO2, CH4 and H2O gas molecules is shown in the Fig. S2†). Therefore, we found that nearly 9 H2 molecules diffuse through the g-C3N4 after 1000 ps MD simulation at the mixture system of 120 gas molecules. Meanwhile, it is difficult for other gas molecules to pass through the membrane at room temperature, which is consistent with our former DFT results and the mixtures of gas molecules do not influence the performance of H2 purification. Other gas molecules are trapped between the membranes. The MD simulations reveal that the H2 can escape from the intrinsic vacancies of g-C3N4 at the room temperature while other gases will be trapped on the one side of g-C3N4, which further confirms the reliability of our results from the first-principles calculations. Although it is difficult for the experimenters to synthesize large-scale g-C3N4 nanosheets with well-order pores, it is still promising to be applied in small-size purification devices with the development of technology.
4. Conclusions
We have performed the feasibility analysis on g-C3N4 as H2 purification membrane. The graphitic carbon nitride with well-ordered sized intrinsic vacancies offers potential diffusion pathways for hydrogen diffusion, which is superior to the graphene or silicene with extra/man-made pores and defects. The minimum energy pathways determined by the first-principles calculations suggest that H2 permeates the porous g-C3N4 with a surmountable diffusion barrier of 0.55 eV. Meanwhile, g-C3N4 exhibits a high selectivity between H2 and other gases with the order of 1046 (H2/CH4) at the room temperature. Considering the good permeation of H2 as well as the hindrance to other gases, we report that g-C3N4 may serve as a promising filtration membrane for H2 purification. Our theoretical simulations provide an extra approach for the application of g-C3N4 in addition to catalyzing water splitting into hydrogen.Acknowledgements
The authors thank Dr Yadong Zhang and Dr Ruifeng Lu for valuable discussions and helps. The work is supported by the National Basic Research Program of China (973 Program, Grant No. 2012CB932400), the National Natural Science Foundation of China (Grant No. 21273158 and 21303112), the Natural Science Foundation of Jiangsu Province (Grant BK20130291), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). This is also a project supported by the Fund for Innovative Research Teams of Jiangsu Higher Education Institutions, Collaborative Innovation Center of Suzhou Nano Science and Technology.References
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS PubMed.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- N. W. Ockwig and T. M. Nenoff, Chem. Rev., 2007, 107, 4078–4110 CrossRef CAS PubMed.
- M. Freemantle, Chem. Eng. News, 2005, 83, 49–56 Search PubMed.
- W. G. Kim and S. Nair, Chem. Eng. Sci., 2013, 104, 908–924 CrossRef CAS.
- K. Y. Kang, B. I. Lee and J. S. Lee, Carbon, 2009, 47, 1171–1180 CrossRef CAS.
- S. Giraudet, Z. H. Zhu, X. D. Yao and G. Q. Lu, J. Phys. Chem. C, 2010, 114, 8639–8645 CAS.
- S. W. Cranford and M. J. Buehler, Nanoscale, 2012, 4, 4587–4593 RSC.
- Y. Jiao, A. Du, M. Hankel, Z. Zhu, V. Rudolph and S. C. Smith, Chem. Commun., 2011, 47, 11843–11845 RSC.
- H. Li, Z. N. Song, X. J. Zhang, Y. Huang, S. G. Li, Y. T. Mao, H. J. Ploehn, Y. Bao and M. Yu, Science, 2013, 342, 95–98 CrossRef CAS PubMed.
- Y. Jiao, A. J. Du, M. Hankel and S. C. Smith, Phys. Chem. Chem. Phys., 2013, 15, 4832–4843 RSC.
- L. W. Drahushuk and M. S. Strano, Langmuir, 2012, 28, 16671–16678 CrossRef CAS PubMed.
- R. Lu, Z. Meng, D. Rao, Y. Wang, Q. Shi, Y. Zhang, E. Kan, C. Xiao and K. Deng, Nanoscale, 2014, 6, 9960–9964 RSC.
- Y. Zhang, Q. Shi, Y. Liu, Y. Wang, Z. Meng, C. Xiao, K. Deng, D. Rao and R. Lu, J. Phys. Chem. C, 2015, 119, 19826–19831 CAS.
- A. Nagashima, N. Tejima, Y. Gamou, T. Kawai and C. Oshima, Phys. Rev. Lett., 1995, 75, 3918–3921 CrossRef CAS PubMed.
- G. Q. Lu, J. C. D. da Costa, M. Duke, S. Giessler, R. Socolow, R. H. Williams and T. Kreutz, J. Colloid Interface Sci., 2007, 314, 589–603 CrossRef CAS PubMed.
- S. T. Oyama, D. Lee, P. Hacarlioglu and R. F. Saraf, J. Membr. Sci., 2004, 244, 45–53 CrossRef CAS.
- Y. Cui, H. Kita and K. Okamoto, J. Mater. Chem., 2004, 14, 924–932 RSC.
- D. E. Jiang, V. R. Cooper and S. Dai, Nano Lett., 2009, 9, 4019–4024 CrossRef CAS PubMed.
- J. Zhang, Y. Chen and X. Wang, Energy Environ. Sci., 2015, 8, 3092–3108 CAS.
- J. Liu, H. Q. Wang, Z. P. Chen, H. Moehwald, S. Fiechter, R. van de Krol, L. P. Wen, L. Jiang and M. Antonietti, Adv. Mater., 2015, 27, 712–718 CrossRef CAS PubMed.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. O. Muller, R. Schlogl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893–4908 RSC.
- J. J. Zhu, P. Xiao, H. L. Li and S. A. C. Carabineiro, ACS Appl. Mater. Interfaces, 2014, 6, 16449–16465 CAS.
- X. C. Wang, K. Maeda, X. F. Chen, K. Takanabe, K. Domen, Y. D. Hou, X. Z. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- D. Ghosh, G. Periyasamy and S. K. Pati, J. Phys. Chem. C, 2014, 118, 15487–15494 CAS.
- J. Liu, Y. Liu, N. Y. Liu, Y. Z. Han, X. Zhang, H. Huang, Y. Lifshitz, S. T. Lee, J. Zhong and Z. H. Kang, Science, 2015, 347, 970–974 CrossRef CAS PubMed.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- G. Henkelman, B. P. Uberuaga and H. Jonsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- A. Ambrosetti, A. M. Reilly, R. A. DiStasio Jr and A. Tkatchenko, J. Chem. Phys., 2014, 140, 18A508 CrossRef PubMed.
- P. L. Silvestrelli and A. Ambrosetti, J. Chem. Phys., 2014, 140, 124107 CrossRef PubMed.
- A. Ambrosetti and P. L. Silvestrelli, J. Phys. Chem. C, 2014, 118, 19172–19179 CAS.
- H. L. Du, J. Y. Li, J. Zhang, G. Su, X. Y. Li and Y. L. Zhao, J. Phys. Chem. C, 2011, 115, 23261–23266 CAS.
- H. Sun, J. Phys. Chem. B, 1998, 102, 7338–7364 CrossRef CAS.
- H. Z. Wu, L. M. Liu and S. J. Zhao, Phys. Chem. Chem. Phys., 2014, 16, 3299–3304 RSC.
- F. Li, Y. Qu and M. Zhao, Carbon, 2015, 95, 51–57 CrossRef CAS.
- Y. Jiao, A. Du, S. C. Smith, Z. Zhu and S. Z. Qiao, J. Mater. Chem. A, 2015, 3, 6767–6771 CAS.
- W. Hu, X. Wu, Z. Li and J. Yang, Phys. Chem. Chem. Phys., 2013, 15, 5753–5757 RSC.
- S. Blankenburg, M. Bieri, R. Fasel, K. Mullen, C. A. Pignedoli and D. Passerone, Small, 2010, 6, 2266–2271 CrossRef CAS PubMed.
- Z. Zhu, J. Membr. Sci., 2006, 281, 754–756 CrossRef CAS.
- M. Shan, Q. Xue, N. Jing, C. Ling, T. Zhang, Z. Yan and J. Zheng, Nanoscale, 2012, 4, 5477–5482 RSC.
- T. Wu, Q. Xue, C. Ling, M. Shan, Z. Liu, Y. Tao and X. Li, J. Phys. Chem. C, 2014, 118, 7369–7376 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra06425f |
| This journal is © The Royal Society of Chemistry 2016 |