Potential continuous removal of toluene by ZnO nanorods grown on permeable alumina tube filters
Abstract
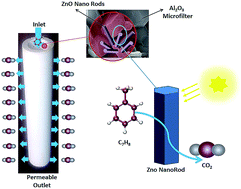
Vertical ZnO nanorods were successfully grown on the crystalline surface of an Al2O3 microfilter by the simple technique of evaporation of prepared solution at atmospheric pressure (ESAP) for photocatalytic degradation of toluene. The nanorods were grown from evaporated solution at low temperatures of 300, 400, and 500 °C. The crystalline structure and morphology of the deposited films were evaluated by X-ray diffraction (XRD) and Field Emission Scanning Electron Microscopy (FE-SEM), respectively. The aqueous photocatalytic activity of the samples was measured by degradation of Methylene Blue (MB) dye. Toluene photodegradation was studied using a tubular photoreactor under various gas concentrations, flow rates (FR), relative humidities (RH), reaction temperatures (RT), and using various light sources. The grown ZnO nanorods were highly uniform and hexagonal wurtzite crystals with mono-sized nanorods. For the nanorods grown at 300 °C, the photocatalytic investigation showed approximately 98% degradation of MB for the first 20 minutes of UV light irradiation. The toluene degradation results indicated that alterations in gas concentration and flow rate affect the toluene degradation ability by direct and indirect oxidation and reduction mechanisms and the absorption capability of the ZnO nanorods' surface. In this system, 20% relative humidity and 30 °C reaction temperature were found to be the optimum conditions for maximum toluene conversion.

 Please wait while we load your content...
Please wait while we load your content...