Thermal activation process of Au/TiO2 system: a molecular spectroscopy study†
S. R. Islas,
R. Zanella and
J. M. Saniger *
*
Centro de Ciencias Aplicadas y Desarrollo Tecnológico (CCADET), Universidad Nacional Autónoma de México, A. P. 70-186, 04510 México D. F., Mexico. E-mail: jose.saniger@ccadet.unam.mx; Tel: +52 56228602 ext. 1133
First published on 25th April 2016
Abstract
The activation process of Au nanoparticles deposited on TiO2 (Au/TiO2) was studied by in situ diffuse reflectance UV-VIS and IR spectroscopy. The deposition–precipitation method with urea was used to support the gold nanoparticle precursor (AuPrs) on TiO2 Degussa P25 and the activation process was carried out in H2–Ar atmosphere. The results shows the existence of strong correlations during the different stages of thermal activation which include the decomposition of the Au complex delivering molecular reactive species, the formation of metallic AuNPs and the change in the effective optical bandgap of the TiO2 support as a result of charge transfer processes.
Introduction
Gold nanoparticles (AuNPs) supported on TiO2 (Au/TiO2) constitute a system widely studied in the last few decades because of their outstanding performance as redox catalysts1 in processes such as low-temperature CO oxidation,2 the selective oxidation of propane and NOx conversion.3 More recently Au/TiO2 have also been studied as very effective systems for photocatalytic mineralization of organic pollutants4 and H2 production by photoelectrochemical water splitting.5A method widely used to synthesize Au/TiO2 is deposition–precipitation with urea (DPU),6 the deposition of the Au(III) complex onto TiO2 in the form of a yellowish powder is reached because of coulombic interactions between the complex and the positively charged TiO2 surfaces site in the first hour of reaction. The reaction continues for 15 hours more and the decomposition of urea causes an increase of the solution pH and a homogenous and quantitative deposition of the Au complex.7
The size, dispersion and final catalytic performance on the supported AuNPs are highly dependent on the experimental conditions of the activation process. The optimization of these experimental parameters has been widely studied6–8 and they are well established at present. For instance, it is well known that the use of a hydrogen atmosphere in the activation process results in the decrease of the activation time because of the reductive character of hydrogen that leads to the formation of oxygen vacancies in the TiO2 support surface9 which behaves as active sites where the AuNP seeds are anchored.10–15 However, the details of the different reaction processes taking place during the thermal activation of the Au/TiO2 system and the specific role played by each of its components, such as the Au complex, thermal decomposition products, TiO2 support, AuNP seeds, and the reactive atmosphere, is not well understood at the present time, despite the important clues that this knowledge will provide in connection with the design of improved performance catalysts based on the Au/TiO2 system.
In this paper a molecular spectrometric study by infrared (DRIFTS) and UV-VIS diffuse reflectance spectroscopies were used to in situ investigate the reaction paths involved in the catalytic activation process and the interactions among the components of the reactive system. The analysis of the overall results highlights the existence of strong correlations during the different stages of catalytic activation which includes the decomposition of the Au complex delivering molecular reactive species, the onset of AuNPs formation on TiO2, the adsorption/reaction of the delivered molecular species on gold nanoparticle seeds, and the change in the effective optical band gap of the TiO2 support as a result of the charge transfer processes.
Experimental section
Preparation of supported and unsupported Au complexes
The supported Au complex was prepared by deposition–precipitation with urea (DPU). TiO2 (Degussa P25 submicrometric particles, BET surface area 45 m2 g−1, 70% anatase and 30% rutile, purity > 99.5%) was used as support, and HAuCl4·3H2O (Aldrich, ≥ 99.9%) as gold precursor, following a previously reported procedure.4 The nominal metal loading in the sample was fixed at 4 wt%. In a typical process, 0.16 g of the gold precursor HAuCl4 (4.2 × 10−3 M), and 2.54 g of urea (0.42 M) were dissolved in distilled water. Then, 2 g of TiO2 was added to this solution forming a suspension. The suspension temperature was kept constant at 80 °C for 16 h under continuous stirring, in absence of light. Once the reaction was completed, the solid phase of the suspension was separated by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm), washed with distilled water four times and finally dried under vacuum at 80 °C for 2 h. After drying, the samples were stored at room temperature in a desiccator under vacuum, away from light in order to prevent any alteration. The obtained powder was pale yellow in color indicating the deposition of the gold precursor on TiO2 particles. On the other hand, unsupported Au complex were synthesized replicating the conditions of the DPU method, but without the presence of TiO2. The obtained powder was now orange in color.
500 rpm), washed with distilled water four times and finally dried under vacuum at 80 °C for 2 h. After drying, the samples were stored at room temperature in a desiccator under vacuum, away from light in order to prevent any alteration. The obtained powder was pale yellow in color indicating the deposition of the gold precursor on TiO2 particles. On the other hand, unsupported Au complex were synthesized replicating the conditions of the DPU method, but without the presence of TiO2. The obtained powder was now orange in color.
Growth of the AuNPs on the TiO2 support
In order to obtain the metallic AuNPs, the gold complex deposited on TiO2 was thermally treated in situ in a UV-VIS spectrometer equipped with a diffuse reflection accessory (Praying Mantis™, Harrick) and a high temperature reaction chamber (Harrick). A heating rate of 2 °C min−1 was initially applied from RT until 300 °C followed by an isothermal plateau at 300 °C for 1 hour, under a constant flow of 40 mL min−1 of H2/Ar (10% H2 balance Ar) mixture. After the thermal treatment the sample was left to reach RT. All measurements were carried out at atmospheric pressure.Characterization techniques
Diffuse reflectance UV-visible spectra of the samples were obtained using a CARY 5000 (UV-VIS-NIR) spectrophotometer. The spectra were recorded in situ using the experimental parameters mentioned above. Diffuse reflectance spectrum of white PTFE (polytetrafluoroethylene) was used as reference. The optical bandgap energy (Eg) was determined from Tauc plot (hνα)1/n = A(hν − Eg) obtained from the diffuse reflectance spectra of the samples and the support. All the diffuse reflectance spectra were acquired using the Kubelka–Munk function (F(R)) included in the spectrophotometer data processing software. Thereby we consider that the absorption coefficient (α), is proportional to the (F(R)) and it was substituted in the Tauc plot.16After the thermal treatment, the samples were examined by High Resolution TEM with a Jeol-2010 FasTem analytical microscope equipped with a Z-contrast annular detector, operated at 200 keV (See ESI Fig. 1S†).
ATR-IR spectroscopy was carried out using an iD5 ATR Nicolet iS5-IR spectrophotometer. The spectra were recorded at the 600–4000 cm−1 range, with a resolution of 4 cm−1 and accumulating up to 32 scans. DRIFT spectroscopy was carried out using a Thermo-Nicolet 670 FT-IR spectrophotometer. The spectra were recorded at the 1400–4000 cm−1 range, with a resolution of 4 cm−1 and accumulating up to 128 scans. The DRIFTS analyses were carried out with a high temperature reaction chamber (Harrick).
Thermo-gravimetric analysis (TGA) was recorded with a TGA Q5000 IR TA Instruments analyzer, using a heating rate of 2 °C min−1 in H2/N2 (10% H2 balance N2).
Results and discussion
Au(III) complex
Until now, the nature of the gold complex deposited on TiO2 by the DPU method is not completely elucidated.7 In order to gain insight on this subject, unsupported and supported gold complex precursors, AuPrs and AuPrs/TiO2, respectively, were studied by IR spectroscopy.Fig. 1 shows for comparative purposes, the ATR-IR spectra of urea (CO(NH2)2) and the gold complex obtained by the DPU method but in the absence of the TiO2 support (Fig. 1a). The urea spectrum displays between 3500 and 3000 cm−1, a set of bands corresponding to the ν(N–H) vibrations. Regarding, the spectral interval between 1800 and 700 cm−1 it is composed of the bending NH2 band (1677 cm−1) with a shoulder at 1625 cm−1; the stretching carbonyl band C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1588 cm−1); the CN stretching vibrations (1460 and 1006 cm−1), and the NH2 rocking vibrations (1150 and 1055 cm−1). The weak band associated with the πCO vibration (786 cm−1); and the antisymmetrical NH2 torsional mode (727 cm−1).17
O (1588 cm−1); the CN stretching vibrations (1460 and 1006 cm−1), and the NH2 rocking vibrations (1150 and 1055 cm−1). The weak band associated with the πCO vibration (786 cm−1); and the antisymmetrical NH2 torsional mode (727 cm−1).17
On the other hand, the spectrum of the gold complex obtained by the DPU method in the absence of the TiO2 support (Fig. 1a), shows in the 3700–2800 cm−1 region an intense and broadband indicative of the presence of water, whereas in the 1800–700 cm−1 spectral interval displays a band profile similar to that of urea but shifted when compare with the free urea molecule.
A comparison of both spectra indicates that the unsupported Au complex spectrum is dominated by the presence of water and urea species that are part of the coordination sphere of the Au complex. Indeed, the broad and very strong band at 3700–2800 cm−1 corresponds principally to unbound humidity water non linked to the complex but hiding the stretching vibration of the NH groups; the shift of the main urea bands, between 1800 and 700 cm−1, is a strong indication that urea molecules are not free but they are linked to the metallic centre of the Au complex. The urea molecule may coordinate with metal ions through the nitrogen as well as the oxygen atoms,18 but when the nitrogen metal bond is present in the complex, the corresponding spectrum differs significantly from that of the free urea molecule.19 It can be observed that in the unsupported gold complex, the coordination mode takes place via the oxygen atoms of the amide group because of the increases of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond character bond and the decreases of the C
N double bond character bond and the decreases of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond character, resulting in an increase of the C
O double bond character, resulting in an increase of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency (1445 cm−1) with a simultaneous decrease in the C
N stretching frequency (1445 cm−1) with a simultaneous decrease in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency (1552 cm−1).20
O stretching frequency (1552 cm−1).20
In order to get additional information on the molecular species present on the supported gold complex, the IR spectra of the AuPrs, AuPrs/TiO2, the Au nanoparticles supported on TiO2 (Au/TiO2) and the pristine TiO2 are shown in the Fig. 2 for comparison purposes. The comparison of this set of spectra facilitates the assignment of the bands in the AuPrs/TiO2 sample. For all the samples, a broad complex band between 3700 and 2800 cm−1 is observed, which contains the convolution of the stretching vibrations of different species such as uncoordinated adsorbed water, hydrogen bonded OH groups and N–H groups. Although the deconvolution of the individual bands subjacent under the whole band is difficult to obtain, some general remarks can be inferred from the comparison of this broad complex band for each sample. For the pristine TiO2, the band is centered around 3300 cm−1 and it is dominated by the presence of adsorbed water, showing a small shoulder around 3700 cm−1 which indicates the presence of residual surface OH groups.21,22 Similar considerations apply for the Au/TiO2 sample, since both Au/TiO2 and TiO2 only exhibited the bands related to titania and molecular species adsorbed on its surface. Notably, for the AuPrs/TiO2 and AuPrs samples the corresponding broad bands are centered around 3200 cm−1, being this shift consistent with the expected higher contribution of the N–H stretching vibration due to the presence of NH species derived from the urea decomposition occurring during the DPU process.
Around 2900 cm−1, the spectra of the pristine TiO2, Au/TiO2 and AuPrs/TiO2 exhibit a set of very weak bands assigned to C–H stretching modes of organic compounds usually observed on TiO2 surfaces;23,27 note that these bands are not observed at the unsupported Au precursor (AuPrs) sample.
The spectrum of the unsupported AuPr samples shows a different profile in this region, a dominant band at ∼2170 cm−1 (assigned to N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching vibration) corresponds to the isocyanate ion, the presence of this functional group in the sample is confirmed with the bands ν(C–O) and δ(NCO−) at 1443 and 664 cm−1, respectively.
C stretching vibration) corresponds to the isocyanate ion, the presence of this functional group in the sample is confirmed with the bands ν(C–O) and δ(NCO−) at 1443 and 664 cm−1, respectively.
The spectral interval between 1750 and 1000 cm−1, shows a clear difference between the unsupported Au precursor (AuPrs) and the other samples. The AuPrs exhibit a complex spectral profile, starting with the prominent bands at 1443 and 1281 cm−1, assigned to ν(C–O) and δ(Au–OH), respectively and additional bands at 1103 and 1028 cm−1 assigned to wagging and twisting deformation of the NH2 groups, δw(NH2) and δT(NH2). As it was previously discussed, this set of bands in the AuPrs is consistent with the presence of urea and urea decomposition products.
Moreover, the spectrum corresponding to the Au/TiO2 formed during the thermal activation treatment does not exhibit the bands associated with the NH2 and C–O groups indicating the total decomposition of the Au complex (AuPrs/TiO2). Finally, below 1000 cm−1, the spectra of the AuPrs/TiO2, Au/TiO2 and pristine TiO2 are clearly dominated by the intense broad band between 800 and 650 cm−1 assigned to the Ti–O–Ti vibration.
Based on the former discussion, it is possible to assume that the unsupported AuPrs and the supported AuPrs on TiO2 have a similar chemical composition;8,24 additionally, from the assignment of these bands it should be assumed that the Au species resulting from the DPU process are in the form of [Au(CO(NH2)2)x(NCO)y(OH)z] complex. This study confirms that the gold complex deposited on the TiO2 surface arising from the reaction among the gold precursor, the urea and the decomposition products of urea.
Thermogravimetric analysis of the Au(III) complex
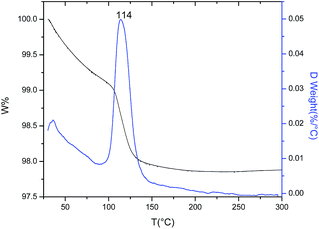
In the former section, the nature of different molecular ligands present in the supported Au precursor (AuPrs/TiO2) was discussed; in addition, the elimination of most of these ligands was evidenced as result of the thermal treatment leading to the formation of supported AuNPs. In order to study in more detail this treatment, a thermogravimetric analysis (TGA) of AuPrs/TiO2 was carried out under H2/Ar atmosphere from RT to 300 °C with a heating ramp of 2 °C min−1.The thermogram (TG and DTG curves) of the supported Au precursors depicted in Fig. 3 shows a continuous mass loss occurring throughout the whole heating process but with evident changes in the decomposition rate as function of temperature. Initially, an almost constant rate is observed until 80 °C followed of an abrupt increase of the decomposition rate up to 150 °C, at which a quasi-plateau is reached.
The mass loss taking place between RT and 90 °C is assigned principally to the removing of the water,19 and some remainder organic volatiles related to the Au complex. The inflexion point of the TG curve at about 90 °C would represent the onset of the supported Au complex decomposition, which reaches the maximum decomposition rate at around 114 °C and stabilizes after reaching 150 °C. This interpretation is in agreement with the results of Temperature Programmed Reduction (TPR) of the AuPrs/TiO2 sample where the maximum of the Au reduction peak occurs at around 120 °C.25,26
It is important to note here that the onset point of the Au complex decomposition heated under H2/Ar atmosphere is different for the case of supported and unsupported AuPrs. The thermal decomposition of the Au complex occurs at significantly lower temperature in the case of supported AuPrs, indicating the critical role of the TiO2 support on the mechanism of the Au complex decomposition (see Fig. 2S†).
DRIFTS study of the supported gold complex
In order to get additional experimental support for the interpretation of the former TG analysis, the DRIFTS spectra of the supported Au complex (AuPrs/TiO2) during the catalytic activation process were obtained with the purpose of identifying the molecular species produced as result of the thermal treatment. Fig. 4 presents the evolution of bands from RT until 300 °C in the 2700–1500 cm−1 spectral interval. Three important bands are present in this region, namely, 2118 cm−1, which is assigned to CO adsorbed on Auδ+;22 2177 cm−1, which is assigned to the isocyanate ion (NCO)− considered as an urea thermal decomposition product;8 and finally, 2330 cm−1, which is assigned to CO2.Fig. 5 shows a graph of the temporal evolution of the intensities of these bands, in which a consecutive evolution for the appearance and disappearance of isocyanate and adsorbed CO bands is observed. The isocyanate band (2177 cm−1) starts to increase at around 50 °C, reaching its maximum value at 110 °C and rapidly decreasing from this point until 150 °C. Note that around 110–120 °C the maximum of the mass loss (DTG), as well as the maximum of the isocyanate concentration and the Au reduction occurs, which is a clear indication that the Au complex decomposition, releasing isocyanate, defines the starting point for the formation of metallic gold.
 | ||
| Fig. 5 Thermal evolution of the isocyanate, CO and CO2 bands for the AuPrs/TiO2 treated under H2 atmosphere. | ||
Interestingly, the band corresponding to the Auδ+–CO (2118 cm−1) appears when the isocyanate band starts to decrease, strongly suggesting that this CO arises from the thermal decomposition of the isocyanate. This band reaches its maximum intensity around 180 °C and practically disappears at 230 °C.
On the other hand, the CO2 band (2330 cm−1), which is present from the beginning of the DRIFTS measurement (probably because of environmental contributions), undergoes a first increase following the profile of the NCO− band, and then a much higher increase, in coincidence with the appearance of the CO band.
AuPrs/AuNPs transformation: formation and evolution of the LSPR and evolution of the optical band gap of the TiO2 support
Continuing with the study of the thermal activation process, the next point of interest in this work is to follow the transformation of the supported Au complex into supported AuNPs (see ESI Fig. 2S†).To get the necessary information on this subject, now the thermal activation of the AuPrs/TiO2 was carried out in situ in a UV-VIS spectrometer using a temperature controlled cell under H2/Ar atmosphere. This experimental approach was considered in order to follow, with a single experiment, the onset and evolution of the Localized Surface Plasmon Resonance (LSPR) associated with the growth of the AuNPs,25 as well as the changes of the optical band gap of the support.28 The diffuse reflectance spectra were acquired using the Kubelka–Munk function, which is proportional to the absorption coefficient.
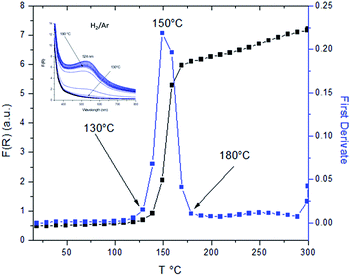
The evolution of LSPR is shown in Fig. 6 (black curve) in which three different regions are observed. In the first one, between RT and approximately 130 °C, the plasmonic band is not detected; in the second region, a linear growth of the LSPR band occurs which defines the onset and stabilization temperatures; finally, in the third step, a much lower intensity increase in the LSPR is observed. The first derivative graph (blue curve) allows the definition of the onset and the stabilization temperature (130 °C, 180 °C) and the maximum growth rate (150 °C) for the formation of the AuNPs.
Moreover, to completely understand the thermal activation process of the Au/TiO2 system, it is necessary to study also the evolution of the electronic properties of the support. For this purpose, the same diffuse reflectance UV-VIS spectra previously presented (Fig. 6 inset) were used to follow the evolution of the optical bandgap. To obtain the effective optical band gap values of the system during the thermal treatment, the changes of the adsorption edge of the diffuse reflectance spectra in the 300–400 nm spectral interval were studied.30 These values were determined by extrapolating the linear portion of Tauc plots16 (square root of absorption coefficient vs. photon energy) to the energy axis (Fig. 7).
 | ||
| Fig. 7 Diffuse reflectance UV-visible spectra and Tauc graph obtained of (a) pristine TiO2, (b) the Au/TiO2 system treated under H2/Ar atmosphere. | ||
The effective band gap energies of the AuPrs/TiO2 and the pristine TiO2 were estimated during the thermal treatment procedure as outlined above; the results are presented in Fig. 8. It is important to note that, at the initial point, before the thermal treatment starts, the band gap value for the AuPrs/TiO2 system is approximately 0.2 eV lower than that for the pristine TiO2,29,30 indicating that the mere deposition of AuPr molecules on the surface of the support results in a red shift of the adsorption edges. Moreover, during the activation process, a clearly different behaviour in the band gap evolution of the pristine TiO2 and that of the AuPrs/TiO2 system was found. In both cases, when the temperature starts to increase, the band gap values undergo a slight initial decrease, but while the changes of the pristine TiO2 are small and reversible, those of the AuPrs/TiO2 system are significantly larger and irreversible. Furthermore, in the AuPrs/TiO2 system, there is a clear inflexion point of the band gap evolution close to 140 °C, at the same thermal interval where the onset points of the isocyanate decomposition and the growth of the plasmonic band intensity occur (Fig. 5 and 6).
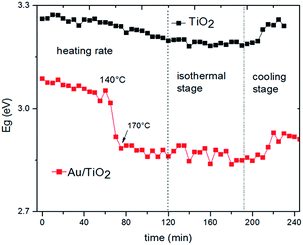 | ||
| Fig. 8 Band gap values during the thermal treatment obtained from the Tauc graphs of Fig. 7. | ||
Discussion
The preceding results indicate that the thermal activation of the supported Au precursor proceeds through a set of concatenated reactions involving not only the main components of the system (Au complex and TiO2 support), but also all the intermediate species as well as the H2/Ar treatment atmosphere.The set of data resulting from the present work is depicted in Fig. 9; it represents the major steps involved in the activation process of the Au/TiO2 system.
As can be seeing in Fig. 9, isocyanate band (red line) starts to growth around 60–70 °C, it reaches its highest decomposition rate at around 110 °C and gradually decreases until 130 °C. It is worth noting that isocyanate was previously identified in this work as a ligand present in the Au complex and then its evolution will indicates the thermal decomposition of the Au complex.
The declining of the isocyanate band coincides, firstly (120 °C), with the growth of the AuNPs LSPR (blue line), and subsequently (130 °C) with the appearance of the band of CO (green line), which is considered as a decomposition product of isocyanate. It is important to note that the frequency of the observed CO stretching band, when compared to free CO, exhibits a red shift corresponding to the CO adsorbed on Auδ+.
Following these results, it seems clear that the decomposition of isocyanate and the growth of LSPR are coupled processes resulting in the onset of the AuNPs formation (AuNP seeds). Additionally, once the AuNP seeds start to form the CO adsorbed on them is observed. CO is a decomposition product of isocyanate and its detection, adsorbed on Auδ+, just when the AuNP seeds are under formation suggests that the decomposition of the isocyanate could be promoted by AuNP seeds. Nevertheless, formally, this promotion effect it is not evident from the present experimental results.
The decomposition of isocyanate species and the raising of the AuNP seeds, where CO became adsorbed, are correlative with an abrupt decrease of the effective optical band gap of the Au/TiO2 system (purple line). In fact, because of the strong interaction between the supported AuNPs and the TiO2 support, this decrease in the band gap values should be associated with a change of the electronic state not of the TiO2 support itself, but of the Au/TiO2 system.
Finally, it is important to discuss the origin and evolution of the CO2 band (black line) present in the DRIFTS spectra. The intensity of this band initially follows the profile of the isocyanate curve, then experiments an increase in coincidence with the appearing of the CO, and reaches its higher values when CO starts to decrease (Fig. 8). This last fact is interpreted as a signal of the oxidation of the CO adsorbed on the AuNPs. This oxidation process requires the presence of oxidizing species such as those generated from the oxygen vacancies that are known to form on the surface of TiO2, especially when it is thermally treated in the presence of reducing agents such as H2.
The formation of oxygen vacancies on the surface of an oxide semiconductor such as TiO2, involves the liberation of atomic oxygen and 2e− (center F) or 1e− (center F+),31 providing the atomic oxygen necessary to oxidize CO to CO2, and the electrons to reduce Au(III) to Au(0). It is then important to note the central role played by oxygen vacancies as the sites where the Au(III) species (coming from the decomposition of the Au complex) are reduced to metallic Au to form the AuNP seeds, where the CO becomes adsorbed and then oxidized to CO2. All these processes occurs at the interface between the TiO2 surface and the AuNPs. Under this framework, the electron migration from the surface TiO2 oxygen vacancy to the Au ions will be the mechanism associated with the charge transfer process, which results in the observed changes of the band gap values.
The exposed findings of the present work are reinforced by previously published results indicating that, in the presence of H2, Au catalyzes the formation of oxygen vacancies on TiO2 surface, generating crystalline defects that work as pinning centers for the gold particles.32 In addition, it was also reported the role of Au/TiO2 perimeter interface in the activation of O2 molecules,33,34 as well as that the surface lattice oxygen at this Au/TiO2 perimeter interface is easily removed in a CO atmosphere.35
Conclusions
The different stages involved in the thermal activation process of AuPrs supported on TiO2 were evidenced by a set of in situ molecular spectroscopic studies.In general terms, new strong experimental evidences were obtained indicating the existence of a set of concatenated reactions resulting in the final deposition of AuNPs on the TiO2 support involving numerous interactions among all the components of the system: gold complex and gaseous molecular species originated from their thermal decomposition; reactive H2/Ar work atmosphere; and TiO2 support surface.
To the best of our knowledge, this detailed sequence of correlated reactions and their corresponding molecules/support interactions taking place during the thermal activation process have not been previously reported in the scientific literature. They suppose a significant advance in the better understanding of the Au/TiO2 system interactions and clarified some of the clues governing the design of catalytic systems formed by metallic nanoparticles supported on semiconductor supports.
Acknowledgements
The authors acknowledge the financial support given by CONACYT 133316 project, Mexico. Selene Islas gratefully acknowledges CONACYT for her PhD fellowship. We also acknowledge LUCE and LUNA laboratories of CCADET, UNAM, Mexico, for the experiments performed for this study. Finally, the authors wish to acknowledge Dr Heriberto Pfeiffer, from IIM-UNAM, for allowing access to TGA measurements.References
- G. C. Bond, C. Louis and D. Thompson, Catalysis by Gold, Imperial College Press, London, 2006, vol. 6 Search PubMed.
- M. Haruta, S. Tsubota, T. Kobayashi, H. Kageyama, M. J. Genet and B. Delmon, J. Catal., 1993, 144, 175–192 CrossRef CAS.
- C. W. Corti and R. J. Holliday, Gold Bull., 2004, 37(1–2), 20–26 CrossRef CAS.
- S. Oros-Ruiz, R. Zanella and B. Prado, J. Hazard. Mater., 2013, 263, 28–35 CrossRef CAS PubMed.
- P. A. DeSario, J. J. Pietron, D. E. DeVantier, T. H. Brintlinger, R. M. Stroud and D. R. Rolison, Nanoscale, 2013, 5(17), 8073–8083 RSC.
- R. Zanella, S. Giorgio, C. R. Henry and C. Louis, J. Phys. Chem. B, 2002, 106, 7634–7642 CrossRef CAS.
- R. Zanella and C. Louis, Catal. Today, 2005, 107–108, 768–777 CrossRef CAS.
- R. Zanella, L. Delannoy and C. Louis, Appl. Catal., A, 2005, 291, 62–72 CrossRef CAS.
- X. Bokhimi, R. Zanella, A. Morales, V. Maturano and C. Ángeles-Chavez, J. Phys. Chem. C, 2011, 115, 5856–5862 CAS.
- A. Sanchez, S. Abbet, U. Heiz, W.-D. Schneider, H. Häkkinen, R. N. Barnett and U. Landman, J. Phys. Chem. A, 1999, 103, 9573–9578 CrossRef CAS.
- E. Wahlström, N. Lopez, R. Schaub, P. Thostrup, A. Rønnau, C. Africh, E. Lægsgaard, J. Nørskov and F. Besenbacher, Phys. Rev. Lett., 2003, 90, 026101–026104 CrossRef PubMed.
- R. Zanella, V. Rodríguezz, Y. Arzola and A. Moreno, ACS Catal., 2012, 2, 1–11 CrossRef CAS.
- S. E. Collins, J. M. Cíes, E. del Río, H. M. López, S. Trasobares, J. J. Calvino and S. Bernal, J. Phys. Chem. C, 2007, 111, 14371–14379 CAS.
- G. Pacchioni, Phys. Chem. Chem. Phys., 2013, 15(6), 1737–1757 RSC.
- Z. Yang, R. Wu and D. W. Goodman, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61(20), 66–71 CrossRef.
- R. López and R. Gómez, J. Sol-Gel Sci. Technol., 2011, 61, 1–7 CrossRef.
- A. Keuleers, H. O. Desseyn, B. Rousseau and C. Van Alsenoy, J. Phys. Chem. A, 1999, 103(24), 4621 CrossRef.
- R. B. Penland, S. Mizushima, C. Curran and J. V. Quagliano, J. Am. Chem. Soc., 1957, 79(2), 1575–1578 CrossRef CAS.
- O. B. Ibrahim, Adv. Appl. Sci. Res., 2012, 3(6), 3522–3539 CAS.
- S. A. Sadeek and M. S. Refat, J. Coord. Chem., 2005, 58, 1727–1734 CrossRef CAS.
- J. Soria, J. Sanz, I. Sobrados, J. M. Coronado, A. J. Maira, M. D. Hernández-Alonso and F. Fresno, J. Phys. Chem. C, 2007, 111, 10590–10596 CAS.
- K. Chakarova, M. Mihaylov, S. Ivanova, M. A. Centeno and K. Hadjiivanov, J. Phys. Chem. C, 2011, 115, 21273–21282 CAS.
- M. Manzoli, A. Chiorino, F. Vindigni and F. Boccuzzi, Catal. Today, 2012, 181(1), 62–67 CrossRef CAS.
- A. Aguirre and S. E. Collins, Catal. Today, 2013, 205, 34–40 CrossRef CAS.
- A. Sandoval, C. Louis and R. Zanella, Appl. Catal., B, 2013, 140–141, 363–377 CrossRef CAS.
- A. Sandoval, A. Gómez-Corté, R. Zanella, G. Díaz and J. M. Saniger, J. Mol. Catal. A: Chem., 2007, 278(1–2), 200–208 CrossRef CAS.
- K. Ataka, T. Yotsuyanagi and M. Osawa, J. Phys. Chem., 1996, 100, 10664–10672 CrossRef CAS.
- M. A. Debeila, M. C. Raphulu, E. Mokoena, M. Avalos, V. Petranovskii, N. J. Coville and M. S. Scurrell, Mater. Sci. Eng., A, 2005, 396(1–2), 70–76 CrossRef.
- C. Wang and D. Astruc, Chem. Soc. Rev., 2014, 43(20), 7188–7216 RSC.
- P. M. Kumar, S. Badrinarayanan and M. Sastry, Thin Solid Films, 2000, 358, 122–130 CrossRef CAS.
- N. Serpone, J. Phys. Chem. B, 2006, 110, 24287–24293 CrossRef CAS PubMed.
- A. Rodriguez, G. Liu, T. Jirsak, J. Hrbek, Z. Chang, J. Dvorak and A. Maiti, J. Am. Chem. Soc., 2002, 2(110), 5242–5250 CrossRef.
- P. M. Kowalski, M. F. Camellone, N. N. Nair, B. Meyer and D. Marx, Phys. Rev. Lett., 2010, 105(14), 146405 CrossRef PubMed.
- Y. Maeda, Y. Lizuka and M. Kohyama, J. Am. Chem. Soc., 2013, 135, 906–909 CrossRef CAS PubMed.
- M. Haruta and M. Daté, Appl. Catal., A, 2001, 222(1–2), 427–437 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. 1S shows TEM image of Au/TiO2 treated under H2/Ar and Fig. 2S shows the comparison of the thermal stability of supported and unsupported AuPrs thermally treated under H2/Ar atmosphere. See DOI: 10.1039/c6ra08136c |
| This journal is © The Royal Society of Chemistry 2016 |