Photo-thermal oxidation of single layer graphene†
Abstract
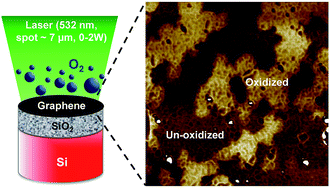
Controlled oxidation of graphene is important for electronic and sensing applications as it offers routes for hole doping and metal–insulator transition. Current methods of oxidation, however, disrupt the graphene lattice and yield pores having diameter > 20 nm for any oxidizing species. In this work, a method for photo-thermal oxidation of graphene is presented for the first time that suggests absence of pores in the graphene layer during atomic force microscopy. The mechanism of oxidation is studied on CVD (chemical vapor deposition)-grown graphene using in situ Raman spectroscopy. Analysis of the temporal evolution of Raman spectra in different oxidizing environments enabled extraction of the reaction energy of oxidation – providing fundamental insight into the oxidation process. Additionally, atomic force microscopy revealed clear phase contrast between the oxidized and un-oxidized domains which were randomly distributed across the graphene layer. This work will enable engineering of oxygen-related defects in graphene for electronic and sensing applications.


 Please wait while we load your content...
Please wait while we load your content...