Plant pathogenic fungus F. solani mediated biosynthesis of nanoceria: antibacterial and antibiofilm activity†
K. S. Venkatesha,
K. Gopinathb,
N. S. Palanib,
A. Arumugamb,
Sujin P. Josec,
S. Asath Bahadura and
R. Ilangovan*d
aMultifunctional Materials Laboratory, Department of Physics, International Research Centre, Kalasalingam University, Krishnankoil-626 126, Tamil Nadu, India
bDepartment of Nanoscience and Technology, Alagappa University, Karaikudi-630 003, Tamil Nadu, India
cDepartment of Computational Physics, School of Physics, Madurai Kamaraj University, Madurai-625 021, Tamil Nadu, India
dNational Centre for Nanoscience and Nanotechnology, Guindy Campus, University of Madras, Chennai-600 025, Tamilnadu, India. E-mail: rajangamilangovan@gmail.com; Tel: +91 9442043403
First published on 15th April 2016
Abstract
The aim of the present study was to synthesize CeO2 nanoparticles using plant pathogenic fungus F. solani and also to study the antibacterial activity as well as the influence on the inhibition of biofilm formation against biomedically important bacterial strains namely Staphylococcus aureus, Psedomonas aeriginosa, Escherichia coli and Klebsiella pneumoniae. Thermogravimetric/differential thermal analysis (TG/DTA) suggested a crystallization temperature of the as-synthesized CeO2 nanopowder at 400 °C. Powder X-ray diffraction analysis and Raman spectroscopy substantiated the presence of CeO2 nanoparticles with a cubic fluorite structure. The contribution of functional groups corresponding to the F. solani fungal supernatant for the synthesis of CeO2 nanoparticles was studied by Fourier transform infrared (FTIR) spectroscopy. The room temperature photoluminescence spectrum of calcined CeO2 nanopowder was recorded. Field emission scanning electron microscopy (FESEM) equipped with energy dispersive X-ray spectroscopy (EDAX) ascertained the formation of homogeneously distributed spherically shaped CeO2 nanoparticles. Furthermore, transmission electron microscopy (TEM) demonstrated the spherical morphology of the CeO2 nanoparticles having sizes ranging from 20 to 30 nm and also the selected area electron diffraction (SAED) pattern revealed the polycrystalline nature of the CeO2 nanoparticles, which is consistent with the XRD results. The presence of surface oxidation states Ce (3d) and O (1s) of the CeO2 nanoparticles was confirmed by X-ray Phoelectron Spectroscopy (XPS) analysis. The antibacterial activity of CeO2 nanoparticles was evaluated by the disc diffusion method and it showed the highest activity against P. aeruginosa as well as K. pneumoniae. In addition, the inhibition on biofilm formation by CeO2 nanoparticles has also been examined by confocal laser scanning microscopy (CLSM). Furthermore, the electrochemical property of the biosynthesized CeO2 nanoparticles was studied by the cyclic voltammetry technique.
1. Introduction
Nowadays, nanomaterials are being used extensively in many applications. Research towards the synthesis of nanoparticles is becoming an inevitable activity for applications and environmental concerns. In this context, biosynthesis of metal and metal oxide nanoparticles has recently been realized in the field of nanotechnology for biomedical applications. Biosynthesis has elicited the keen attention of researchers to explore the naturally available resources for the synthesis of nanoparticles as a safer alternative to conventional physical and chemical methods. Therefore, natural resources such as fungi, yeast, actinomycete, bacteria and medicinal plants have been exploited for nanoparticles synthesis. The large diversity of microorganism including fungi and bacteria contains an innate potential for the synthesis of nanoparticles. It can be notice that synthesis of inorganic nanoparticles by biological systems occurs through a remarkable process at ambient temperature as well as atmospheric pressure and at neutral pH. Moreover, biosynthesis offers great advantages such as low cost, biocompatibility, easiest experimental protocol etc.Furthermore, microorganisms have variety of organic biomolecules with high prosperity and they can simultaneously play different role such as capping, reducing and surfactive agent thereby it ultimately paves the way to achieve nanoparticles easily at one pot synthesis. Cerium oxide or ceria (CeO2) is a rare earth compound semiconductor material and it has wide band gap and large excitation binding energy.1 It is being used as an insulating material in Si based device technology owing to its unique properties such as high refractive index, high dielectric constant.2–4 Also, cerium oxide nanoparticles have been potentially used in many industrial and commercial applications, which includes polishing agent,5 UV absorbent in sunscreen,6 solid electrolyte in solid oxide fuel cell,7 additives as a catalyst in automobile exhaust,8 electrochromic devices,9 biomedical applications10 etc. CeO2 has been synthesized and studied in the form of thin films, nanoparticles.2,11–13 Fungus mediated biosynthesis of metal oxide nanoparticles such as TiO2,14 SnO2,15 BaTiO3,16 ZrO2,17 ZnO,18 Magnetite,19 CeO2 (ref. 20) have been reported. A. Ingle et al. reported the extracellular biosynthesis of silver nanoparticles using Fusarium solani as a novel biological agent.21
Due to the presence of toxic chemicals over the sheath of nanoparticles synthesized by conventional methods restricts their usage for biomedical applications. But, biosynthesized nanoparticles involve with environmentally benign materials for their synthesis and highly suitable for the biomedical and pharmaceutical applications as they do not involved with the toxic chemicals during the synthesis.22 Proteins can be exploited for the synthesis of nanoparticles since it possess characteristics such as metal binding site, positively and negatively charged interfaces, and unique geometries23 thereby it leads to the formation of nanoparticles. Among the different microorganisms, fungi are highly exciting due to its huge secretion of proteins and easy handling in the laboratory.24 Moreover, fungus extraction contains rich quantity of active metabolites which can be exploited for the synthesis of nanoparticles. Based on this perception, fungus mediated biosynthesis was adopted for the synthesis of CeO2 nanoparticles.
On account of considering the advantages and features offered by the fungus mediated biosynthesis, a plant pathogenic fungus Fusarium solani has been employed to synthesize CeO2 nanoparticles. Thus, synthesized nanoparticles have been subjected for respective characterizations to study the thermal, structural and morphological properties. FT-IR spectroscopy was also employed to study the contribution of various functional group vibrations of F. solani fungal extract towards the synthesis of CeO2 nanoparticles. Furthermore, antibacterial and antibiofilm activity of the biogenic CeO2 nanoparticles were examined against gram positive and gram negative bacterial strains. The electrochemical property was also studied by cyclic voltammetry technique.
2. Experimental details
2.1. Preparation of Fusarium solani supernatant
Fusarium solani (MTCC-2671) was obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India. The Czapek-Dox-Broth (CDB) media was prepared as per the standard procedure and sterilized at 121 °C with 15 lbs for 1 hour. After the sterilization process, species from Fusarium solani culture were inoculated in the CDB media and incubated at 120 rpm for 120 hours in an orbital shaking incubator.25 The fungi grown CDB media was filtered using Whatman No. 1 filter paper and the filtered fungal extraction was centrifuged at 8000 rpm for 20 minutes. After the centrifugation, the supernatant was collected and used for the synthesis of CeO2 nanoparticles. The pH of the collected supernatant was 6.8 and it was not adjusted further.2.2. Preparation of CeO2 nanopowder
5.589 g of cerium(III) chloride heptahydrate (CeCl3·7H2O) (Alfa Aesar) was added in 250 mL of fungal supernatant under vigorous stirring. The stirring was continued for 5 hours to get homogeneous mixing. Then the CeO2 precursor solution was centrifuged at 8000 rpm for 10 minutes. Thereafter, the precipitated powder was collected and washed for 5 times in deionized water for the removal of chlorine ions. Thus obtained powder was dried at 100 °C in hot air oven and gently grinded using agate mortar and pestle to avoid any structural and morphological changes in the prepared final fine powder (CeO2 nanopowder). Finally, the grinded powder was calcined for one hour in tubular furnace (Carbolite, UK).2.3. Characterization of CeO2 nanopowder
Before calcination, the thermal behavior of the as-synthesized CeO2 nanopowder was analyzed by means of thermogravimetric/differential thermal analysis (TGA/DTA) (PA Instruments, model: Q 500 Hi-Res TGA) from 30 to 900 °C with the heating rate of 10 °C per minute in an air atmosphere. Powder XRD pattern was recorded by Cu Kα radiation with the wavelength of 1.54060 Å using PANalytical X-PERT PRO diffractometer. The functional group vibrations of the F. solani supernatant (drop coated on the glass substrate), as-synthesized and calcined CeO2 nanopowder were analyzed using Thermo Nicolet 380 FT-IR spectrometer by the KBr pellet technique in the range of 400–4000 cm−1. Raman spectrum was recorded using imaging spectrograph STR 500 mm focal length laser Raman spectrometer (SEKI Japan). Photoluminescence spectra were recorded in the wavelength range of 350 to 600 nm under the excitation wavelength of 400 nm using photoluminescence spectrometer (Varian Cary Eclipse). The morphology of the calcined CeO2 nanopowder was examined by field emission scanning electron microscopy (FE-SEM, Model: Hitachi S-4500) equipped with the energy dispersive X-ray spectroscopy (EDAX). The size, shape and crystalline nature of CeO2 nanoparticles were demonstrated by transmission electron microscopy (TEM) and selected area electron diffraction (SAED) (Tecnai Instruments). XPS analysis was performed with XPS instrument (Carl Zeiss). The spectra were recorded with Al Kα excitation at 250 W under ultra high vacuum condition. Electrochemical analysis has been carried out using three electrode system equipped with Ag/AgCl as reference electrode, platinum wire as counter electrode and glassy carbon electrode (GCE) as working electrode. The cyclic voltammogram was recorded using electrochemical workstation (Model CHI 6005D series).2.4. Antibacterial assay
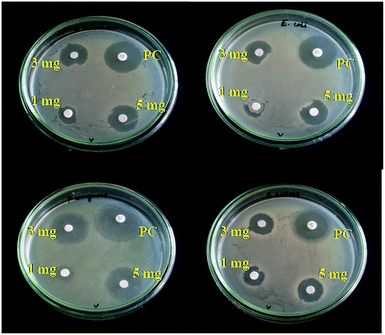
The antibacterial activity of the biosynthesized CeO2 nanoparticles has been evaluated against Staphylococcus aureus (G+), Pseudomonas aeruginosa (G−), Escherichia coli (G−), and Klebsiella pneumoniae (G−) by disc diffusion method. These four bacterial strains were grown in nutrient broth at 37 °C until the bacterial suspension was reached to 1.5 × 108 CFU mL−1. 20 mL of molten nutrient agar was poured into the petri dishes and allowed to cool, and then the bacterial suspension was inoculated on the nutrient broth media using sterilized cotton swab. The Whatman No. 1 filter discs have been separately loaded with the colloidal suspension of CeO2 nanoparticles (1, 3 and 5 mg per disc, respectively) and placed over the media using sterilized forceps. A standard antibiotics (gentamicin) was used as a positive control. The bacteria suspended plates were incubated at 37 °C for 24 hours. The zone of inhibition (ZOI) formed around each disc were measured. Thus the antibacterial activity test of the biogenic CeO2 nanoparticles was performed in triplicates.2.5. Antibiofilm assay
Pure and CeO2 nanoparticles treated biofilms of Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli and Staphylococcus aureus bacterial strains were prepared on glass slides to study the inhibition effect of CeO2 nanoparticles. The biofilms were prepared as follows: bacterial strains were grown at 37 °C in Luria Bertaini (LB) broth. The sterilized glass slides with the dimension of 1 cm × 1 cm were placed in the 24 well polystyrene plates of volume 2.5 mL. Then 2.3 mL of sterilized LB broth, 50 μL (100 μg mL−1) of CeO2 nanoparticles (sonicated) and 150 μL of bacterial strains (1.5 × 108 CFU mL−1) were placed in the each well of polystyrene plates. The eight different biofilms were incubated at 37 °C for 24 h in the 24 well polystyrene plates. The strains without CeO2 nanoparticles were considered as a control. After 24 h of incubation, the glass slides (i.e. eight biofilms of four treated and four untreated) were washed well with the PBS (phosphate buffer solution) to remove the non-attached bacterial cells and then stained with 0.1% of acridine orange. Finally, they have been subjected to the CLSM analysis for examining the antibiofilm activity.3. Results and discussion
3.1. TGA/DTA analysis
The simultaneous thermogravimetric/differential thermal analysis (TGA/DTA) curves of biogenic as-synthesized CeO2 nanopowder are shown in Fig. 1. The TGA curve shows the continuous weight loss till 400 °C and thereafter no weight loss is observed. Accordingly in DTA curve, the peak appeared at 100 °C is attributed to the elimination of physically and chemically absorbed water molecules whereas in the region from 160 to 350 °C is related to the decomposition and combustion process of the bio-organic matrix. A broad exothermic peak at ∼400 °C in DTA curve is indicate the starting of oxidation process of the material, where the formation of metal oxide (CeO2) can takes place and it is consistent with the TGA result. Another exothermic peak observed at ∼520 °C may be attributed to the phase change or valence variation of cerium and this is consistent with the previous work.26 Hence, based on the TGA/DTA results, the as-synthesized CeO2 nanopowder was subjected to calcination at 400 °C for one hour.3.2. Powder X-ray diffraction analysis
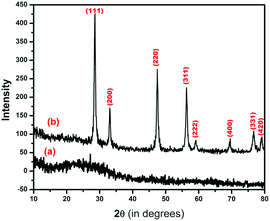
As shown in Fig. 2, there is no any peaks in the X-ray diffraction pattern of the as-synthesized CeO2 nanopowder, which indicates the amorphous nature, whereas the calcined CeO2 nanopowder exhibits a predominant X-ray diffraction peak at 2θ = 28.5° with (111) plane, and the other diffraction peaks corresponding to (200), (311), (222), (400), (331) and (420) planes confirm the presence of CeO2 with cubic fluorite structure. The observed values are closely agreed with the standard diffraction data (JCPDS File No. 89-8436) and this is consistent with the TG/DTA result. The sharp diffraction peaks reveal the well crystallinity of CeO2 nanoparticles. The crystallite size of the calcined CeO2 nanopowder was estimated using Scherrer's equation27 and it was found to be 11 nm.3.3. FT-IR spectroscopy analysis
The peaks located at 3459, 1657, 1229, 1348, 1073 cm−1 (curve 1) in the FTIR spectra (Fig. 3) are attributed to the –OH stretching, amide groups, C–N stretching vibrations of aromatic amines and –C–O–C group vibration, respectively21 and they have also been given in Table S1 in ESI.† This represents the presence of various kinds of bio-organic molecules such as proteins, heterocyclic compounds, amines in the fungal supernatant. Furthermore, the presence of heterocyclic compound derivatives can simultaneously and effectively act as capping ligands for the nanoparticles.28,29 The FT-IR spectrum of as-synthesized CeO2 nanopowder (curve 2) showed a number of peaks and the shift in peaks position as well as the appearance of new peaks clearly indicates the interaction between bio-organic molecules and CeO2 nanoparticles. | ||
| Fig. 3 FT-IR spectra of CeO2 nanopowder (a) fungal supernatant (b) as-synthesized CeO2 nanopowder (c) CeO2 nanopowder calcined at 400 °C. | ||
The appearance of peak at 2370 cm−1 is due to the trapped CO2 in an air ambience. The shift observed in amide group vibration (i.e.) the characteristic peak of proteins from 1671 to 1654 cm−1 manifests the interaction of CeO2 nuclei with protein linkages thereby the stabilization of CeO2 nanoparticles can takes place. The disappearance of –OH stretching vibration as well as some other peaks and the huge decrease in the intensity of the characteristic peak of proteins (curve 3) suggested the removal of biological entities and formation of CeO2 nanoparticles upon calcination at 400 °C.
3.4. Morphological and elemental analysis
FE-SEM images of the calcined CeO2 nanopowder are apparently exhibit the formation of spherical shaped nanoparticles Fig. 4(a) and (b).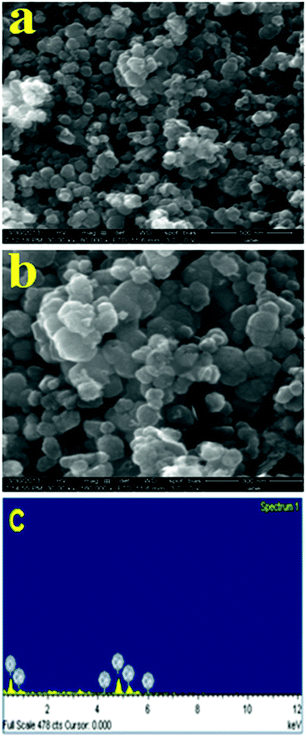 | ||
| Fig. 4 (a and b) FE-SEM images at different magnifications (c) EDAX spectrum of CeO2 nanopowder calcined at 400 °C. | ||
FE-SEM images noticed the homogeneously distributed CeO2 nanoparticles, which indicates the good interaction between the biomolecules of F. solani fungal supernatant and CeO2 nanoparticles during the synthesis process. In addition, EDAX study showed the presence of Ce and O elements only and no other elements are detected, which manifests the purity of the resultant CeO2 nanopowder. The set of data for the elemental percentage is given in Table S2 in ESI† Fig. 4(c). Further, TEM analysis was employed to ascertain the particles size of the resultant CeO2 nanoparticles.
3.5. TEM analysis
The morphology and particles size of the calcined CeO2 nanoparticles are vividly demonstrated by Transmission Electron Microscopy (TEM) and shown in Fig. 5(a)–(c). It can be clearly seen from the TEM images that the particles are homogeneously distributed having the sizes are in the range between 20 and 30 nm. The average size was found to be 24.5 nm with the standard deviation of 8%. The selected area electron diffraction (SAED) pattern shows well defined spotty rings, which implies the good polycrystalline nature of the nanoparticles. In accordance with the SAED pattern, the d-spacing values can be calculated using the following equation:30| Lλ = dR | (1) |
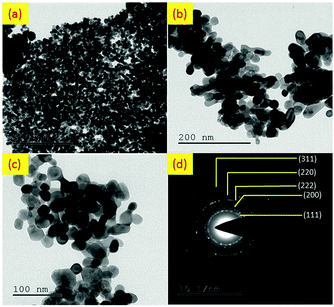 | ||
| Fig. 5 (a–c) TEM images at different magnifications (d) SAED pattern of CeO2 nanoparticles calcined at 400 °C. | ||
3.6. Raman spectroscopy analysis
The presence of cubic structure in the calcined CeO2 nanopowder is ascertained by Raman spectroscopy and shown in Fig. 6. The strong Raman band observed at 465 cm−1 confirms the presence of CeO2 with cubic structure. This is attributed to the symmetric vibrations of Ce–O (F2g) Raman active mode. This is coincided with the previous report31 and also consistent with the XRD results of this present work. There is no any other peak detected in the Raman spectrum, which indicates the purity of the material.3.7. Photoluminescence spectroscopy analysis
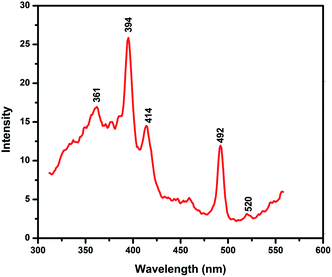
Room temperature photoluminescence (PL) spectrum of the calcined CeO2 nanoparticles was recorded and it showed UV emission bands at 361 and 394 nm, a blue band at 414 nm, a strong blue green band at 492 nm and a weak green band at 520 nm (Fig. 7). The high intensity peak in the UV region (394 nm) is associated to the defects localized between Ce 4f and O 2p valance band.32,33 A blue and blue green emission bands are possibly due to the surface defects of CeO2 nanoparticles and a low intensity of the green band manifests the low density of the oxygen vacancies during the synthesis process.263.8. Growth mechanism of CeO2 nanoparticles
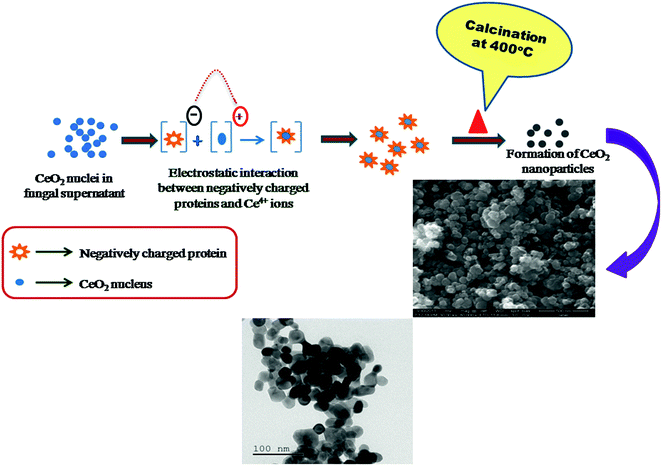
Based on the observed results, the possible growth mechanism for the resultant CeO2 nanoparticles is understood as follows (Fig. 8). The obtained fungal supernatant was clear and became to white colour upon the addition of CeCl3·7H2O, thereafter it became to yellowish under continuous stirring. This indicates the reaction took place between CeCl3·7H2O and the fungal supernatant. In this stage, the nucleation process of CeO2 nanoparticles i.e. Ce(OH)4 can takes place by leaving chlorine ions.Since, the microorganism based nanoparticles synthesis is an enzymatic process, the growth mechanism may occur as follows. First, protein/enzyme–nanoparticle interaction could takes place (i.e.) the protein linkages of fungal supernatant can bind with the surface of CeO2 nanoparticles via electrostatic interaction. This is supported by the peak shift from 1671 to 1654 cm−1 in the FT-IR spectrum.21 Secondly, the presence of carbonyl compounds and other such bio-constituents may also be involved in the capping process of CeO2 nanoparticles. Thus CeO2 nanoparticles can be stabilized thereby agglomeration can be impeded during the growth process. The removal of biological constituents in the as-synthesized CeO2 nanopowder took place upon the calcination and finally results to the CeO2 nanoparticles. Hence, it can be note that the formation of CeO2 nanoparticles is due to the synergistic effect of various bio organic compounds present in the F. solani fungal supernatant.
3.9. XPS analysis
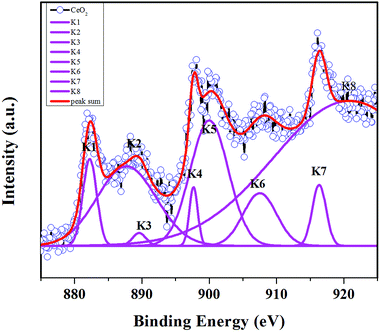
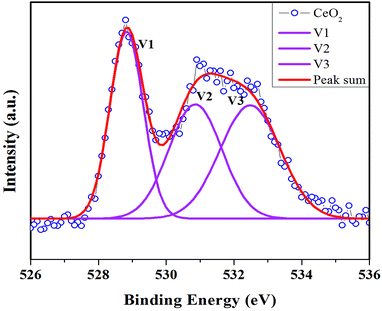
The surface oxidation state of the biosynthesized CeO2 nanoparticles has been understood by XPS spectra which showed signals, belongs to Ce (3d) and O (1s). The signals of Ce (3d) are divided as K1, K2, K3, K4, K5, K6 and K7 in the Gaussian fitting as shown in Fig. 9. The peaks appeared at binding energies of 882.26 (3d5/2), 887.72 (3d5/2), 889.61 (3d5/2), 897.67 (3d5/2), 900.01 (3d3/2), 907.52 (3d3/2) and 916.33 (3d3/2) eV indicate the presence of CeO2 with Ce4+ oxidation state. The XPS spectrum of O (1s) is divided to three signals namely V1, V2 and V3 in the Gaussian fitting as shown in Fig. 10. The energy levels (V1 and V2) of O (1s) signals at 528.83 and 530.86 eV are ascribed to O2− ions surrounded by Ce4+ ions, which is due to the Ce–O bond in CeO2 nanoparticles.The signal corresponding to another energy level at 532.47 eV may be associated with loosely bounded oxygen such as adsorbed O2 or moisture on the surface of CeO2 nanoparticles. The observed results are consistent with the previous work.34,35
3.10. Antibacterial activity of CeO2 nanoparticles
The antibacterial activity of calcined CeO2 nanoparticles has been evaluated with three different concentrations (1, 3 and 5 mg per disc) against K. pneumoniae (G−), E. coli (G−), P. aeruginosa (G−) and S. aureus (G+) (Fig. 11 and 12). A maximum zone of Inhibition (ZOI) was noticed towards P. aeruginosa and the others were observed with the least difference, which indicates that P. aeruginosa has more susceptibility towards CeO2 nanoparticles. A direct proportionality between ZOI and the concentration indicates the proficient antibacterial activity of CeO2 nanoparticles. The difference in antibacterial activity is due to the structural differences of cell membrane among the bacteria. In this work, CeO2 nanoparticles showed a good antibacterial activity nearly on par to the standard antibiotics i.e. gentamicin which was used as a positive control. The mechanism of antibacterial activity can be explained as follows: the adsorption of metal oxide nanoparticles onto the bacterial cell wall can takes place due to the electrostatic attraction between the negatively charged biological entities of bacteria and CeO2 (Ce4+) nanoparticles.This interaction is not only limited to significantly affects the bacterial growth but also induces to the generation of reactive oxygen species (ROS) such as hydroxyl and oxy radicals (˙OH, O−, O2−, O2−), which causes an oxidative stress thereby leads to cell death.36 Furthermore, CeO2 nanoparticles can disturbs the cellular respiration, DNA replication, cell division through binding with mesosome present in the cell membrane and ultimately leads to the disassociation of cell. Thus the interaction of nanoparticles and generation of oxidative stress by ROS causes to the cell death.
The obtained results of antibacterial activity are good compared to some of the previous work and presented in (Table S3 in ESI†). Hence, nanoceria synthesized using plant pathogenic fungus F. solani showed good antibacterial activity and can be used as novel materials for various biomedical applications.
3.11. Antibiofilm activity
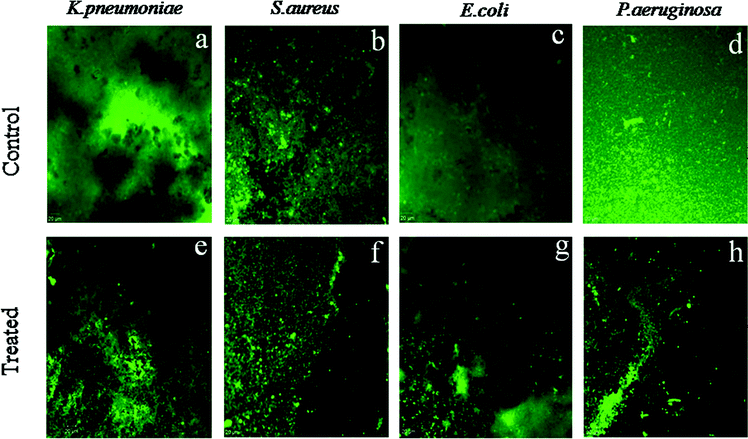
Biofilm is a complex microbial community that forms by adhesion to a solid surface and by secretion of matrix, which cover the bacterial cell community. Biofilms are known as a significant problem because biofilm protects pathogens against antibiotics and this is mainly causes the development of chronic infections.37 The biofilm formation and the effect of CeO2 nanoparticles towards the antibiofilm activity of bacterial strains such as S. aureus, P. aeruginosa, E. coli and K. pneumoniae are clearly displayed by CLSM images. Fig. 13(a)–(d) shows the homogeneous as well as strongly adhesive biofilms (control) of respective bacteria over the glass surface. The green fluorescence refers to the live cells of the biofilms. By comparing with the respective control biofilms untreated by CeO2, CeO2 nanoparticles treated biofilms show the disintegrated surface, which reveal to death of bacterial cells by the nanoparticles (Fig. 13(e)–(h)).Generally, biofilm matrix is surrounded by the bacterial cells, which include extracellular polysaccharides (EPS), proteins and DNA.37 As discussed in Section 3.9 the generation of reactive oxygen species (ROS) by CeO2 nanoparticles can took place during the incubation. This results to the generation of oxidative stress which causes to the damage of all types of organic biomolecules associated with the bacterial cell wall. Moreover, the genes responsible for the adhesion of the cell walls to the solid surface can also be affected by the generation of ROS. Thus ROS playing a major role and affects the viability of the cells thereby leads to the cell death.38 On account of this consequence, CeO2 nanoparticles treated biofilms are seen with the disruptive surfaces (Fig. 13(e)–(h)). Hence, the CLSM images suggest that the bacteriostatic effect led by the biogenic CeO2 nanoparticles impedes the biofilm formation of the respective bacterial strains.
3.12. Cyclic voltammetric (CV) analysis
Cyclic voltammetry technique was employed to understand the electrochemical behavior of biosynthesized CeO2 nanoparticles. The methodology for the fabrication of GCE modified with CeO2 nanoparticles was adopted from the literature.39 The cyclic voltammogram of bare GCE and nano CeO2 modified GCE electrodes recorded at a scan rate of 50 mV s−1 in 0.1 M KCl solution containing 1 mM of [Fe(CN)6]3−/4− as a redox couple is shown in Fig. 14. As shown in figure that the bare GCE showed a pair of well defined redox peak with a peak to peak separation of (ipa: 1.925 × 10−5 A, ΔEp: 67 mV (ΔEp = (Epa − Epc)). Deposition of the CeO2 nanoparticles onto the electrode surface increases the reversibility of [Fe(CN)6]3−/4− (ipa: 6.316 × 10−5 A and ΔEp: 150 mV) due to the barrier properties of CeO2 surface film. | ||
| Fig. 14 CV of the (a) bare GC (b) cerium oxide modified GC electrode in presence of 1 mM [Fe(CN)6]3−/4− in 0.1 M KCl at a scan rate of 50 mV s−1. | ||
Furthermore, the magnitude of current increases in nano CeO2 modified GCE than bare GCE suggest that CeO2 nanoparticles promote the electron transfer. Cyclic voltammograms of nano CeO2 modified GCE electrodes recorded at different scan rates (10–100 mV s−1) in 0.1 M KCl solution containing [Fe(CN)6]3−/4− (1 mM) show the linear increase of oxidation peak current upon the increase of scan rate and which is also shown in inset (Fig. 15). The shift of anodic peak potential towards positive side and cathodic peak potential towards reverse direction confirm the kinetic limitation in the electrochemical reaction. Also, a plot of peak current versus the square root of scan rate (inset) was observed to be linear in the range of 10–100 mV s−1, which suggested that the process is diffusion rather than surface controlled at higher potential.
Increase of electron transfer rate due to the increase of scan rate implies the perfect condition of electrode/electrolyte interface. Thus the biosynthesized CeO2 nanoparticles possess good electrochemical behavior and therefore it can be used for applications like sensors, supercapacitors, batteries, fuel cells etc.
3.13. Electrochemical impedance spectroscopy (EIS) analysis
EIS is a well known technique for studying the surface properties of modified electrodes. The charge transport process of the nano CeO2 modified GCE was studied by monitoring the charge transfer resistance (RCT) at the electrode–electrolyte interface. Fig. 16 shows the EIS curves of the bare GCE and nano CeO2 modified GCE. The value of the charge transfer resistance (RCT) for the bare GCE and nano CeO2 modified GCE were estimated to be 445.9 and 390 cm−2, respectively. It is clear that the RCT value of nano CeO2 modified GCE is lower than that of the bare GCE. The resultant lower charge transfer resistance is due to the larger anodic peak current thereby nano CeO2 modified GCE exhibited an increased conductivity. Indeed, the obtained impedance plots are in good agreement with the peak current values of cyclic voltammograms. | ||
| Fig. 16 EIS behavior of (a) bare (b) nano CeO2 modified GC electrodes recorded in the presence of 1 mM [Fe(CN)6]3−/4− in 0.1 M KCl. | ||
4. Conclusions
This report enunciated the facile, eco-friendly and cost effective fungus mediated extracellular biosynthesis of CeO2 nanoparticles using a plant pathogenic fungus F. solani. The thermal property of the as-synthesized CeO2 nanopowder was studied by TGA/DTA analysis and it revealed the crystallization temperature at 400 °C. XRD pattern showed the presence of well crystalline CeO2 with the cubic fluorite structure. FT-IR spectra clearly indicated the interaction of bio-organic molecules of the fungal supernatant such as proteins, heterocyclic compounds during the synthesis of CeO2 nanoparticles. The symmetric vibrations of Raman active mode corresponding to 465 cm−1 further confirmed the presence of CeO2 nanoparticles with the cubic fluorite structure. The room temperature photoluminescence spectrum of calcined CeO2 nanopowder showed different emission bands. FE-SEM images ascertained the spherical morphology of CeO2 nanoparticles and EDAX spectrum emphasized the existence of Ce and O elements only. The spherical shaped morphology of CeO2 nanoparticles with the sizes between 20 and 30 nm was also demonstrated by TEM analysis and SAED pattern revealed the good polycrystalline nature of the nanoparticles, which is consistent with the XRD results. XPS spectra substantiated the presence of surface oxidation states of CeO2 nanoparticles (Ce4+) with Ce (3d) and O (1s). The biogenic CeO2 nanoparticles exhibited a good antibacterial activity in the order of P. aeruginosa > K. pnemoniae > E. coli > S. aureus and they also showed the inhibition of respective bacterial biofilm formation, which is clearly evidenced by CLSM images. Finally, the electrochemical analysis suggested that the biogenic CeO2 nanoparticles can be used for other kind of applications such as sensors, batteries, supercapacitors etc.Acknowledgements
One of the authors KSV is highly grateful to thank School of Physics and Department of Animal Health and Management, Alagappa University, Karaikudi for extending the XRD and Confocal Laser Scanning Microcopy (CLSM) facilities, respectively. The authors also thank Mr M. Muthukumarasamy, Department of Nanoscience and Technology, Alagappa University, Karaikudi for providing the ATCC bacterial cultures to carry out the antibacterial and antibiofilm activity. KSV is highly thankful to Mr C. Karthikeyan, Jamal Mohamed College, Trichy, Tamil Nadu, India for helping to analyze the XPS results with Gaussian fitting.References
- H. Zhang, D. Yang, Y. Ji, X. Ma, J. Xu and D. Que, J. Phys. Chem. B, 2004, 108, 3955 CrossRef CAS.
- J. Liu, X. Huang, Y. Li, J. Duan and H. Ai, Mater. Chem. Phys., 2006, 98, 523 CrossRef CAS.
- C. D. Lokhande, P. M. Gondkar, S. M. Rajaram, V. R. Shinde and S. H. Han, J. Alloys Compd., 2009, 475, 304 CrossRef CAS.
- X. J. Wang, W. Wang and Y. L. Liu, Sens. Actuators, B, 2012, 168, 39 CrossRef CAS.
- Q. Yuan, S. Hein and R. D. K. Misra, Acta Biomater., 2010, 6, 2732 CrossRef CAS PubMed.
- S. Rani, P. Suri, P. K. Shishodia and R. M. Mehra, Sol. Energy Mater. Sol. Cells, 2008, 92, 1639 CrossRef CAS.
- C. Jayaseelan, A. Abdul Rahuman, A. Vishnu Kirthi, S. Marimuthu, T. Santhoshkumar, A. Bagavan, K. Gaurav, L. Karthik and K. V. Bhaskara Rao, Spectrochim. Acta, Part A, 2012, 90, 78 CrossRef CAS PubMed.
- J. A. Rodriguez, T. Jirsak, J. Dvorak, S. Sambasivam and D. Fischer, J. Phys. Chem. B, 2000, 104, 319 CrossRef CAS.
- T. Minami, Mater. Res. Bull., 2000, 25, 38 CrossRef CAS.
- C. J. Lee, T. J. Lee, S. C. Lyu, Y. Zhang, H. Ruh and H. J. Lee, Appl. Phys. Lett., 2002, 81, 3648 CrossRef CAS.
- J. J. Lee, Y. B. Kim and Y. S. Yoon, Appl. Surf. Sci., 2005, 244, 365 CrossRef CAS.
- J. Sawai, E. Kawada, F. Kanou, H. Igarashi, A. Hashomoto, T. Kokugan and M. Shimizu, J. Chem. Eng. Jpn., 1996, 29, 627 CrossRef CAS.
- O. Yamamoto, Int. J. Inorg. Mater., 2001, 3, 643 CrossRef CAS.
- S. P. Suriyaraj and R. Selvamumar, RSC Adv., 2014, 4, 39619 RSC.
- K. P. Gattu, K. Ghule, A. A. Kashale, V. B. Patil, D. M. Phase, R. S. Mane, S. H. Han, R. Sharma and A. V. Ghule, RSC Adv., 2015, 5, 72849 RSC.
- T. Thongtem, A. P. Grat and S. Thongtem, Ceram. Int., 2010, 36, 257 CrossRef CAS.
- S. Muthukumaran and R. Gopalakrishnan, Opt. Mater., 2012, 34, 1946 CrossRef CAS.
- R. Dobruck and J. Dlugaszewska, Saudi J. Biol. Sci., 2015 DOI:10.1016/j.sjbs.2015.05.016.
- W. J. Crookes-Goodson, J. M. Slocik and R. R. Naik, Chem. Soc. Rev., 2008, 37, 2403 RSC.
- J. Musarrat, S. Dwivedi, B. R. Singh, Q. Saquib and A. A. Al-Khedhairy, Microbes and Microbial Technology, 2011, p. 101 Search PubMed.
- A. Ingle, M. Rai, A. Gade and M. Bawaskar, J. Nanopart. Res., 2009, 11, 2079 CrossRef CAS.
- A. Ahmad, P. Mukherjee, S. Senapati, D. Mandal, M. I. Khan, R. Kumar and M. Sastry, Colloids Surf., B, 2003, 28, 313 CrossRef CAS.
- D. S. Balaji, S. Basavaraja, R. Deshpande, D. Bedre, M. B. K. Prabhakar and A. Venkataraman, Colloids Surf., B, 2009, 68, 88 CrossRef CAS PubMed.
- A. K. Gade, P. Bonde, A. Ingle, P. D. Marcato, N. Duran and M. K. Rai, J. Biobased Mater. Bioenergy, 2008, 2, 243 CrossRef.
- P. Mukherjee, A. Ahmad, D. Mandal, S. Senapati, S. R. Sainkar, M. I. Khan, R. Ramani, R. Parischa, P. V. Ajayakumar, M. Alam, M. Sastry and Rajiv Kumar, Angew. Chem., Int. Ed., 2001, 40, 3585 CrossRef CAS.
- S. Maensiri, C. Masingboon, P. Laokul, W. Jareonboon, V. Promarak, P. L. Anderson and S. Seraphin, Cryst. Growth Des., 2007, 7, 950 CAS.
- A. Ingle, M. Rai, A. Gade and M. Bawaskar, J. Nanopart. Res., 2009, 11, 2079 CrossRef CAS.
- V. Bansal, D. Rautary, A. Bharde, K. Ahire, A. Sanyal, A. Ahmad and M. Sastry, J. Mater. Chem., 2005, 15, 2583 RSC.
- G. Rajakumar, A. Abdul Rahuman, S. Mohana Roopan, V. Gopiesh Khanna, G. Elango, C. Kamaraj, A. Abduz Zahir and K. Velayutham, Spectrochim. Acta, Part A, 2012, 91, 23 CrossRef CAS PubMed.
- V. Bansal, P. Poddar, A. Ahmad and M. Sastry, J. Am. Chem. Soc., 2006, 128, 11958 CrossRef CAS PubMed.
- V. Bansal, D. Rautaray, A. Ahmad and M. Sastry, J. Mater. Chem., 2004, 14, 3303 RSC.
- K. S. Venkatesh, N. S. Palani, S. R. Krishnamoorthi, V. Thirumal and R. Ilangovan, AIP Conf. Proc., 2013, 1536, 93 CrossRef CAS.
- A. Bharde, D. Rautaray, V. Bansal, A. Ahmad, I. Sarkar, S. M. Yusuf, M. Sanyal and M. Sastry, Small, 2006, 2, 135 CrossRef CAS PubMed.
- S. M. Hirst, A. S. Karakoti, R. D. Tyler, N. Sriranganathan, S. Seal and C. M. Reilly, Small, 2009, 5, 2848 CrossRef CAS PubMed.
- A. S. Haja Hameed, C. Karthikeyan, S. Sasikumar, V. Senthil Kumar, S. Kumaresan and G. Ravi, J. Mater. Chem. B, 2013, 1, 5950 RSC.
- S. A. Khan and A. Ahmad, Mater. Res. Bull., 2013, 48, 4134 CrossRef CAS.
- R. Cusco, E. Alarcon-Liado, E. J. Ibanez, L. Artus, J. Jimenez, B. G. Wang and M. J. Callahan, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 165202 CrossRef.
- C. Ramon, L. Esther, I. Jordi, A. Luis, J. Juan, W. Buguo and J. C. Michael, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 165202 CrossRef.
- C. Sumathi, P. Muthukumaran, S. Radhakrishnan, J. Wilson and A. Umar, RSC Adv., 2014, 4, 23050 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: FTIR peaks and their respective assignment of functional groups. Table S2: EDAX data of CeO2 nanoparticles. Table S3: comparison of antibacterial activity with previous work. See DOI: 10.1039/c6ra05003d |
| This journal is © The Royal Society of Chemistry 2016 |