Construction of nitrogen-doped graphene quantum dots-BiVO4/g-C3N4Z-scheme photocatalyst and enhanced photocatalytic degradation of antibiotics under visible light†
Abstract
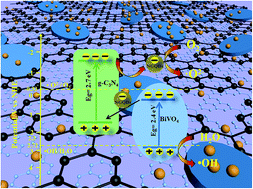
Development of high-efficiency photocatalysts for photocatalysis research has been a hotspot in recent years. In this study, an effective nitrogen-doped graphene quantum dots (NGQDs)-BiVO4/g-C3N4 Z-scheme heterojunction has been successfully prepared for environmental remediation. Antibiotics (tetracycline (TC), oxytetracycline (OTC) and ciprofloxacin (CIP)) were chosen as target pollutants to explore the photocatalytic performance. Results showed that both the BiVO4/g-C3N4 ratio (Bi/CN ratio) and NGQDs amount have an important influence on the antibiotics degradation. Under our experimental conditions, the optimum Bi/CN ratio was 1 : 2 and optimum NGQDs addition amount was 5 wt%, with the corresponding 5% NGQDs-Bi/2CN sample showing the highest photocatalytic efficiency (91.5% in 30 min with TC). By further study based on electron spin resonance (ESR) and active species trapping experiments, the enhanced photocatalytic property could be ascribed to the crucial role of NGQDs and Z-scheme photocatalytic system, which not only accelerated the separation of the charge carriers but also exhibited a strong oxidation and reduction ability for efficient degradation of organic pollutants.

 Please wait while we load your content...
Please wait while we load your content...