Perylene diimide derivatives as red and deep red-emitters for fully solution processable OLEDs†
Abstract
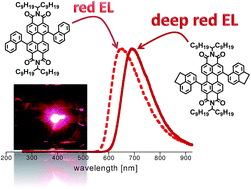
We report on the photophysical characterization of two solution-processable red-emissive perylene diimide molecules and on their use in fully solution assembled OLEDs. The two emitters contain sterically hindered aromatic naphthalene or acenaphthene substituents on the perylene core. These fused rings limits the perylene diimide intermolecular π–π interactions in the solid state due to highly sterically hindered effects, while preserving an extended conjugation between the substituents and the perylene core. Indeed, these features generate a broad absorption for both perylene derivatives along with an efficient red and deep red emission in the solid state for the PDIs with naphthalene and acenaphthene, respectively. The performances of both emitters were tested on OLEDs, where the active layer is a film of bulk PDI, fabricated by simple solution processing. The devices with acenaphthene-substituted perylene diimide provide deep red electroluminescence with emission wavelength at 690 nm, CIE coordinates of (x = 0.69, y = 0.29) and show the best efficiency reported so far for OLED based on PDI fluorescent emitters.


 Please wait while we load your content...
Please wait while we load your content...