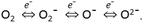Three-dimensional In2O3–CuO inverse opals: synthesis and improved gas sensing properties towards acetone†
Ruiqing Xing,
Kuang Sheng,
Lin Xu*,
Wei Liu,
Jian Song and
Hongwei Song*
State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun, 130012, People’s Republic of China. E-mail: linxu@jlu.edu.cn; songhw@jlu.edu.cn
First published on 8th June 2016
Abstract
Specific three-dimensional inverse opal (3DIO) In2O3–CuO architecture with additional via-holes was first prepared by a simple sacrificial template method. Such specific nanostructures enable fast transport of gas molecules to the entire thin-walled sensing layers, which is very helpful for improving the sensing performance. Moreover, the mole ratio of Cu/In was controlled, ranging from 0–38.1% to adjust the hetero-contact amounts in the In2O3–CuO composites. The gas sensing properties of the as-prepared 3DIO In2O3–CuO samples were evaluated toward trace acetone, which is an important biomarker of diabetes in exhaled breath. The response of the 3DIO In2O3–CuO gas sensor with the best performance (with a mole ratio of Cu/In = 16.4%) was ∼14 to 5 ppm acetone, and had a calculated low detection limit of ∼30 ppb at 370 °C when Ra/Rg ≥ 1.2 was used as the criterion for reliable gas sensing. Besides, it also showed good selectivity, fast response (τres) and recovery (τrec) times, and stability. The enhanced gas sensing performance could be attributed to the hetero-contact effects between the different components and the specific 3DIO structure with the via-holes which provided a larger effective surface area for gas adsorption. It is believed that the as-prepared 3DIO sensor can be a promising ppb-level acetone sensor in various areas.
1. Introduction
Clinical data indicated that the volatile organic compound (VOC) concentrations in exhaled gas from healthy people are different from those of specific patients, such as those with lung cancer, liver cancer, diabetes, etc.1,2 Inspired by this, convenient, non-invasive, and fast disease diagnosis could be achieved through confirming the VOC concentrations in exhaled breath.2–5 However, the VOC concentration in exhaled gas is generally at a trace level; for instance, acetone is a specific biomarker of diabetes, and the concentration of exhaled acetone from diabetes patients only exceeds 1.8 ppm compared to the lower value of 0.9 ppm for healthy people.3 Besides, the complex environment in exhaled breath, such as high humidity and complicated components, also makes the detection of a specific VOC gas more difficult.6,7 Thus, an excellent detection technique with super sensing performance is urgently needed.To date, many methods have been explored for gas detection, such as electrochemical methods, solid-state electrolyte methods, infrared absorption methods, chemiluminescence methods, surface acoustic wave sensors, gas chromatography, quartz crystal microbalances, and semiconductor gas sensors, etc.8–15 Among these methods, semiconductor oxide gas sensors have attracted much interest in exhaled biomarker recognition,1,13,16 due to their high sensitivity, fast response and recovery time, lower cost, and simplicity of use. As is known, the gas sensing process of oxide sensors generally involves a catalytic reaction which mainly takes place on the surface of the sensor. Thus, through changing the nanostructure of the oxide gas sensor, the surface micro-environment will also change, and the corresponding sensing performance could be effectively controlled.17,18 Recently, the 3DIO structure has been considered to be a promising chemo-resistive candidate to effectively detect low concentration target gases because of its excellent permeability and large surface-to-volume ratio, which is accessible readily for the analyte molecules, and makes it become an efficient transducer of gas concentration into electrical signal. Up to now, there has been little work focused on p–n composites with 3DIO gas sensing performances, although the composition of the oxide gas sensor is also an important factor that could affect the sensing properties.
Recently, we have successfully synthesized macroporous specific 3DIO In2O3 with additional via-hole architectures, which can enhance the electrical responses effectively.19 Herein, we first report a macroporous In2O3–CuO-based 3DIO with additional via-hole architectures by a simple and controllable sacrificial template method, and the gas sensing properties of 3DIO In2O3–CuO were carefully investigated. The experiment results indicated that 3DIO In2O3–CuO shows excellent sensing permanence to trace acetone gas, and so can be a highly promising candidate for diabetes diagnoses in the future.
2. Experimental section
2.1. Fabrication of 3DIO In2O3–CuO films with via-hole structure
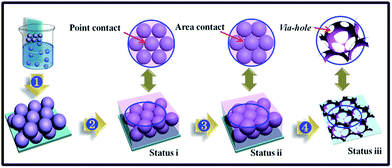
3DIO In2O3–CuO films with a via-hole structure were prepared by the sacrificial template method with some improvements.19,20 Polymethylmethacrylate (PMMA) latex spheres were synthesized through emulsion polymerization of methylmethacrylate.21 The glass substrates were initially immersed into sulphuric acid for 2 h and then washed with deionized water in order to clean up the dirt on the surface. Then, 3DIO In2O3–CuO films were prepared according to the illustration in Fig. 1. Firstly, the pre-treated glass substrates were inserted into the aqueous PMMA colloid suspension vertically, and then placed in a 32 °C oven for 2 days (process 1). In this process, thin opal templates would self-assemble on the glass substrates with the evaporation of water solvent. In order to enhance their physical strength, the obtained thin opal templates were first sintered for 40 min at 120 °C. Then, the lacunas of the as-prepared thin opal templates were filled by different precursor solutions containing different amounts of In(NO)3 and Cu(NO)2 (process 2). In a typical procedure, a mixed solution (in ethanol) with 0.26 M citric acid, 0.76 M In(NO)3, and different amounts of Cu(NO)2 was first prepared with magnetic stirring for 4 h at room temperature. The Cu/In atom ratios in the precursor solutions were set as 0% (pure In2O3), 5.5%, 16.4%, 27.3%, and 38.1%, which were marked as S1–S5 for convenience. The as-prepared thin opal templates were placed on a table with a slight angle of 5 degrees to a horizontal plane. Then, the as-prepared precursor solutions were penetrated into the thin opal templates from top to bottom slowly and the extra solvent was allowed to evaporate in air atmosphere at room temperature. Note that the adjacent PMMA sphere contact was in a point-to-point way (status i) during this process. The key way to obtain the via-hole structure was to change the point contact of the adjacent PMMA spheres into area contact. Here, the filled thin opal templates were placed in a 115 °C oven for 2.5 h to ensure the formation of area contact among the PMMA spheres (process 3, status ii). Then, the obtained films were annealed in a tube furnace with a rising speed of 1°C min−1 to 600 °C and kept for 3 h to completely remove the polymer spheres (process 4). Finally, the 3DIO In2O3–CuO films with a via-holes structure were obtained (status iii).2.2. Characterization
The morphology of the 3DIO In2O3–CuO films was inspected using JEOL JSM-7500F field emission scanning electron microscopy (SEM) (Japan) at an accelerating voltage of 15 kV with gold sputtered on the surface. Transmission electron microscopy (TEM) images, high resolution TEM (HRTEM) images, and the selected area electron diffraction (SAED) pattern were recorded on a JEM–2010 transmission electron microscope under a working voltage of 200 kV. The X-ray diffraction (XRD) patterns were conducted on Rigaku D/max 2550 using a monochromatized Cu target radiation resource (λ = 1.5045 Å) and the corresponding lattice constants of the samples were calculated by MDI Jade 5.0 software based on the XRD data.2.3. Fabrication and measurement of gas sensing properties
The fabrication of the sensors was similar to that in our previous report.19 The 3DIO In2O3–CuO films were first scraped down from the substrates by a metal blade, and then the corresponding powder was mixed with ethanol in a weight ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form pastes. Then, the pastes were coated on ceramic tubes on which a pair of gold electrodes was previously printed. A small spring-like Ni–Cr alloy was inserted into the ceramic tube to provide the operating temperature. After the solvent was evaporated, the ceramic tube with the S1–S5 thin layer was sintered in an oven for 2 h at 350 °C. Subsequently, the gas sensors were thermally aged with a heating voltage of 5 V (∼368 °C) for 5 days before the first measurement. The thermal ageing process was conducted on a TS60 desktop thermal ageing gas sensitive instrument (Weisheng Instruments Co., Zhengzhou, China).
1 to form pastes. Then, the pastes were coated on ceramic tubes on which a pair of gold electrodes was previously printed. A small spring-like Ni–Cr alloy was inserted into the ceramic tube to provide the operating temperature. After the solvent was evaporated, the ceramic tube with the S1–S5 thin layer was sintered in an oven for 2 h at 350 °C. Subsequently, the gas sensors were thermally aged with a heating voltage of 5 V (∼368 °C) for 5 days before the first measurement. The thermal ageing process was conducted on a TS60 desktop thermal ageing gas sensitive instrument (Weisheng Instruments Co., Zhengzhou, China).
The gas sensing properties were measured on a WS-30A system (Weisheng Instruments Co., Zhengzhou, China). A static process was used to achieve a different gas concentration of the target gases. More specifically, the gas sources are 1000 ppm standard gas mixed with N2. Then a certain amount of standard gas was injected into the glass chamber with a small mouth (about 2.5 L in volume) which had been pumped in a vacuum state and then mixed with the atmosphere gas. Both the inner wall of the mouth and the corresponding rubber stopper were sealed by petroleum jelly in order to maintain the stability of the gas concentration. When testing, the sensor, with an extended line passing through the rubber stopper, was put into the chamber; when the response reached a constant value, the sensor was taken out to return to the initial position in ambient air. The response is defined as Ra/Rg for an n-type sensor and Rg/Ra for a p-type sensor (Ra and Rg are the resistances of the sensors in air and in the target gas, respectively). The response and recovery times are defined as the time required to reach 90% of the final equilibrium value. Here, the working temperature is obtained by voltage regulation of the WS-30A gas sensing measurement system.
3. Results and discussion
3.1. Morphological and structural characteristics
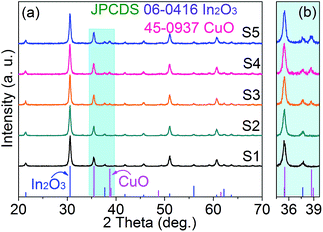
The phase composition and crystallinity of the obtained 3DIO In2O3–CuO films were investigated by XRD (Fig. 2). As is shown in Fig. 2a, the XRD peaks of the 3DIO S1 sample (pure In2O3) can be well indexed to body-centered cubic phase In2O3 (JCPDS card 06-0416). For 3DIO In2O3–CuO composites, with an increasing amount of CuO, the base-centered monoclinic phase CuO (JCPDS card 45-0937) also gradually appears (Fig. 2b), indicating the formation of 3DIO In2O3–CuO composites. Note that due to the small amount of CuO introduction in the 3DIO S2 films (5.5%), the XRD peaks of CuO are not obvious.Fig. 3 displays the morphology of the as-prepared special 3DIO In2O3–CuO films with via-holes, as marked in each corresponding SEM image in Fig. 3a–e. As can be clearly seen, all the samples yield a long-range ordered 3DIO nanostructure with additional via-holes and no pore occlusion is detected. Together considering status iii in Fig. 1 (one single pore chamber), the larger macropores are gained by the face contact of the adjacent PMMA spheres in the upper or lower floor, which could be obtained in a typical sacrificial template method.19 The additional via-holes are obtained by adjusting the point contact of the adjacent PMMA spheres into area contact in the same plane. To further illustrate the specific structure of the as-obtained 3DIO films, the morphology of the 3DIO S3 film is enlarged and is revealed in Fig. 3f. As shown, 3DIO with a via-holes architecture can be seen clearly, as the arrows point out. Besides, the average macropore diameters of the 3DIO S1–S5 films are calculated to be 410 ± 5 nm, indicating that the shrinkage of sphere diameters is nearly the same during calcination in S1–S5.
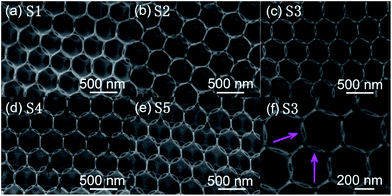 | ||
| Fig. 3 (a–e) SEM images of the 3DIO S1–S5 samples on the glass substrate, and (f) enlarged SEM image of the 3DIO S3 sample. | ||
Detailed information about the microstructure and morphology of the as-synthesized 3DIO In2O3–CuO samples is further obtained by TEM. Herein, the 3DIO S3 sample is chosen as an example since the structures of all the other samples are similar. As given in Fig. 4a, the 3DIO structure with via-holes can be well maintained even after the steps are scraped down from the glass subtracts and then with ultrasonic treatment for a short period of time. The solid line area is further enlarged in Fig. 4b. The skeleton of the 3DIO structure is firm and slim, and the corresponding via-holes also can be clearly seen. Fig. 4c and d exhibit the HRTEM image of the 3DIO S3 sample and the corresponding SAED pattern, respectively. In Fig. 4c, the different crystals with clear lattice fringe distances can be observed in the entire HRTEM images, demonstrating that the sample is polycrystalline and highly crystalline. The lattice distances in Fig. 4c are calculated to be ∼0.29 nm and 0.25 nm, which could be assigned to the (222) plane of cubic phase In2O3 and (002) plane of monoclinic phase CuO, respectively. The corresponding SAED pattern in Fig. 4d is just consistent with the above description.
The STEM image is enlarged from the dotted line area in Fig. 4a, which provides similar information to the TEM image (Fig. 4b). Then, to further investigate the specific distribution of In, Cu, and O elements in the 3DIO S3 sample, the EDX mappings are conducted in this area. A homogeneous distribution of In, Cu, and O elements can be clearly seen, and exhibits the same structure with that in STEM, indicating that uniform 3DIO In2O3–CuO samples are obtained.
3.2. Gas sensing properties of 3DIO In2O3–CuO sensors with via-holes
The gas sensing properties of the 3DIO In2O3–CuO sensors are carefully studied and compared. The operating temperature is an important factor in determining the specific interaction between gases and the sensing material.22 Thus, Fig. 5a first depicts the response curves of the 3DIO S1–S5 sensors toward 50 ppm acetone gas as a function of the working temperature. In our case, all the 3DIO sensors show n-type sensing characteristics due to the smaller amount of introduced CuO. As is shown, at a lower temperature, the responses are poor which could be ascribed to the gas molecules not having enough thermal energy to react with the surface-adsorbed oxygen species. Then, at higher temperatures, the responses improved due to the thermal energy obtained being high enough for the corresponding chemical reaction. However, the response values will decrease when the temperature further increases and this process may be because of the increase of the speed of diffusion of gas molecules. Thus, the gas sensor response reaches its maximum at an intermediate temperature. The optimal working temperature and corresponding response value of the 3DIO S1 (pure In2O3) sensor are 400 °C and 19.5. After being composited with CuO, the optimal working temperatures all obviously decrease to 370 °C, while the response values increase. Among these sensors, the 3DIO S3 sensor always shows the highest response, which can reach 39.1 at the optimal working temperature. | ||
| Fig. 5 (a) Responses of the 3DIO S1–S5 sensors to 50 ppm of acetone as a function of the operating temperature. (b) Selectivity of 3DIO S1–S5 on exposure to 5 ppm of various gases. | ||
The gas responses of the 3DIO S1–S5 sensors to 5 ppm various testing gases are tested at the working temperature of 370 °C, including ethanol for drink driving, toluene and methanol for lung cancer, etc.1,23 As shown in Fig. 5b, the 3DIO sensors always exhibit the maximum response to acetone gas, especially for the 3DIO S3 sensor, where its response to acetone is 5.4–9.5 times higher compared to the other gases studied in our work. Then, the anti-interference performance of the 3DIO S3 sensor was further tested to confirm the sensing properties of the as-prepared sensor. In this test, the response value to 5 ppm acetone was compared to the responses to some bi-mixtures that also contained 5 ppm acetone. As listed in Table 1, the sensor responses show a little difference after mixing with the other interference gas, indicating that the anti-interference performance of the 3DIO S3 sensor to acetone is acceptable.
| Gas species (ppm) | Response of S3 sensor |
|---|---|
| 5 acetone | 14.000 |
| 5 acetone + 5 toluene | 15.438 |
| 5 acetone + 5 ethanol | 16.496 |
| 5 acetone + 5 methanol | 15.654 |
To further investigate the performance indicators of the as-fabricated 3DIO sensors, the one circle dynamic process when exposed to 50 ppm acetone gas at the working temperature of 370 °C is shown in Fig. 6a. The responses of all the sensors rapidly increase when contacted with acetone gas, and then reach their stable response levels. After exposure in ambient air, the sensor responses decrease quickly to their original states. The stable response value of the 3DIO S1–S5 sensor shows a similar trend with that in Fig. 5a, that is to say, the 3DIO S3 sensor (Cu/In atom ratio = 16.4%) has the highest response value. Based on Fig. 6a, the corresponding τres and τrec of the 3DIO S1–S5 sensor are calculated and depicted in Fig. 6b. The results demonstrate that τres and τrec exhibit a decreased trend with the increasing amount of CuO, indicating that the introduction of p-type CuO appropriately is helpful of shortening the dynamic process. Moreover, τres and τrec of the 3DIO S3 sensor are ∼8 and 20 s, respectively, which is very close to the lowest values that were obtained in our case (∼7 and 19 s for the 3DIO S5 sensor, respectively).
Since the 3DIO S3 sensor has the best performance, further sensing tests are carried out. Note that the concentration of the acetone biomarker in exhaled breath is very low; detailed tests are conducted focusing on trace gas concentrations (0.5–5 ppm) at 370 °C which can meet the requirements of exhaled gas detection. It is apparent that the 3DIO S3 sensor has a clear response to such a low acetone concentration, and the response increases rapidly with the increase of acetone concentration. Besides, the corresponding τres and τrec are also exhibited in Fig. 6d. As can be seen, the τres and τrec curves slightly decrease with the increase of acetone concentration; this may be attributed to a higher gas concentration needing a shorter time to diffuse to the sensor surface.
The detailed response curves of the 3DIO S3 sensor compared with the 3DIO S1 (pure In2O3) sensor to different acetone concentrations at the working temperature of 370 °C are shown in Fig. 7a. The inset shows the gas sensor response to 0.05–100 ppm acetone gas. It can be seen that the response of the 3DIO S3 sensor increases linearly with the increase of acetone concentration in the studied range at first, and the response values are systematically higher than that of the 3DIO S1 sensor, indicating the advantage of the In2O3–CuO composites. In order to obtain more information about the properties of the 3DIO S3 sensor toward exhaled breath, the corresponding responses in low gas concentrations (0.05–20 ppm) are enlarged in Fig. 7. As can be clearly seen, the 3DIO S3 sensor shows high resolution even to trace acetone concentrations, which could be attributed to the abundant active sites at the 3DIO structure surface and the interactive effect between p-type CuO and n-type In2O3. Besides, the corresponding responses to acetone are below 6.2 for healthy humans (<0.9 ppm) and above 8.7 for diabetics (>1.8 ppm). This 40% response increase may allow reliable diagnosis of diabetic patients by breath acetone monitoring. Moreover, the low detection limit of the 3DIO S3 sensor to acetone is calculated to be ∼30 ppb, when Ra/Rg ≥ 1.2 is used as the criterion for reliable gas sensing; this detection limit is much lower than the exhaled acetone from clinical data of diabetics, which indicates that the sensor could be a promising candidate for the detection of diabetes in the future. Besides, Table 2 lists the response, actual detection limit, and τres and τrec of the 3DIO S3 sensor to acetone obtained in this study and those of other metal oxide semiconductor gas sensors.24–30 As is listed, the sensing performance of the 3DIO S3 sensor is satisfactory, especially as the response is higher than that of the other oxide semiconductor gas sensors listed in the literature, indicating that the as-prepared 3DIO sensor has a good sensing ability for acetone detection.
| Sensing materials | Response (Ra/Rg)/corresponding concentration | Corresponding response in this work | Actual detection limit | Temp. | Res./Rec | Ref. |
|---|---|---|---|---|---|---|
| 3DIO S3 | 4.8/0.5 ppm | 4.8 | 50 ppb | 370 °C | ∼13/20 s | This work |
| ZnFe2O4 microspheres | 1.4/1 ppm | 6.5 | 1 ppm | 215 °C | ∼7.5/200 s | 24 |
| Hierarchical ZnO | 1.16/0.25 ppm | 3.3 | 0.25 ppm | 230 °C | ∼3/— s | 25 |
| Cr2O3 doped WO3 films | 9/20 ppm | 28.3 | 0.5 ppm | 320 °C | ∼20/40 s | 26 |
| Y-doped SnO2 nanofibers | 12/50 ppm | 39.1 | 20 ppm | 260 °C | 9–30/6–9 s | 27 |
| WO3 nanorods | 3.1/0.5 ppm | 4.8 | 0.25 ppm | 230 °C | ∼9/14 s | 28 |
| Pt-WO3 nanober | 1.27/0.3 ppm | 3.7 | 0.3 ppm | 350 °C | — | 29 |
| ZnFe2O4 nanospheres | 12/30 ppm | 31.8 | 800 ppb | 200 °C | ∼9/272 s | 30 |
Taking account of the high humidity environment in exhaled breath, the response of 3DIO S3 is further investigated and compared in a 94 ± 1% relative humidity (RH) environment. The resistance of the sensor at a RH = 94 ± 1% environment is defined as Rh for convenience. As shown in Fig. 7b, the response values of 3DIO S3 in a high RH environment are higher compared to those in air, which may be due to the background response caused by higher humidity. Moreover, the linear slope in RH is nearly the same with that in air, indicating that the 3DIO S3 sensor can detect a low concentration of acetone biomarkers in high RH precisely. The inset in Fig. 7b shows the curve of long-term stability of the 3DIO S3 sensor to 50 ppm acetone at 370 °C in air. As can be seen, only a little variation (no more than ∼7%) can be observed during the whole test process, demonstrating that the 3DIO S3 sensor possesses significant stability. All in all, taking consideration of the advantages of the sensor, the sensor could be a promising candidate for the detection of diabetes in the future.
3.3. Sensing mechanism of the 3DIO In2O3–CuO sensor with via-holes
For In2O3-based sensing materials, the sensing performance could be explained by the space charge layer mode.31 The surface of In2O3 could adsorb the oxygen molecules and ionize them to O2−, O−, or O2− readily by capturing free electrons from the conduction band, which caused a narrow conduction channel and a formation of depletion layers in the near surface region of In2O3. This process could lead to a decrease in the carrier concentration and an increase in resistance, which could be simply expressed by the following equations:32Upon exposure to reducing gas, the gas molecules can react with the adsorbed ions, and this process could release the trapped electrons back to the conduction band, and then lead to an increase of the conduction channel and carrier concentration of In2O3.
In the case of the 3DIO n-type In2O3–CuO composite, the acetone gas sensing improvement may be attributed to the hetero-contact between the p-type CuO and n-type In2O3. When in the atmosphere, a thicker depletion layer will form near the grain surface of In2O3 and CuO as a p–n hetero-contact. As shown in Fig. S1,† the electrons transfer from CuO to In2O3 and the holes transfer from In2O3 to CuO until an equilibrium state is acquired, due to the disparity of the conduction band and the valence band between In2O3 and CuO. Therefore, a much wider depletion layer is formed, and the numbers of p–n hetero-contacts lead to a remarkable increase in resistance.
As acetone gas is introduced, reactions between acetone and the sensor increase the number of electrons in In2O3 and decrease the concentration of holes in CuO,33 resulting in a significant decrease of sensor resistance. Additionally, the distribution and extent of electrically active contacts of the p–n hetero-contact are also the key parameters in describing the active junction where sensing can take place. In our case, the active junction of the 3DIO S3 sensor may be in the optimal amounts, leading to the enhanced acetone sensing properties. Besides that, the excellent performance of the 3DIO In2O3–CuO sensor could also be attributed to the unique 3DIO via-holes structure. The 3DIO via-holes structure is surface-accessible and could provide more active sites, which are beneficial for the sensing properties. Thus, acetone gas could diffuse not only on the surface but also in the inner regions, thus the gas sensing reaction occurs both on the surface and in the inside, leading to the gas reacting more completely.
In addition, after introducing CuO, the sensors responded in a higher sensitivity to acetone than to the other gases tested in our case. Actually, the detailed mechanism of the selective performance is still not very clear and it can be influenced by many factors, including working temperature, the physical preparation of the sensor material, the speed of the chemical reaction on the surface, the speed of diffusion of the gas to the surface, the charge carrier concentration, and the Debye length in the semiconductor.34–37 In our case, all the gas sensing tests were performed at the optimum working temperature toward acetone gas. However, the optimum working temperatures of the other gases are not at 370 °C (data not shown); this means that the reactions of the sensors with the other types of gases weren’t in the most effective status. This may be the reason why the sensors resulted in a higher sensitivity to acetone than to the other gases tested, because the activation energy of the related reactions is not enough at this temperature.
4. Conclusions
In summary, different 3DIO In2O3–CuO with additional via-hole architectures were prepared by a simple sacrificial template method and their gas sensing properties were carefully investigated. The specific 3DIO composites were composed of body-centered cubic phase In2O3 and end-centered monoclinic phase CuO. The sensing results indicated that the sensor performance was improved by controlling the mole ratio of Cu/In, which could be attributed to the formation of an appropriate amount of p–n hetero-contact and the specific 3DIO structure with excellent permeability and large surface-to-volume ratio. Moreover, the optimal 3DIO S3 sensor exhibited a lower working temperature, higher response, shorter τres and τrec, and better selectivity than 3DIO S1 (pure In2O3) did, and it also demonstrated a detection limit as low as 30 ppb and good stability. These advantages make the as-prepared 3DIO sensor a promising ppb-level acetone sensor in various areas, especially in exhaled breath.Acknowledgements
This work was supported by NSFC (Grant no. 61204015, 81301289, 61177042) Program for Chang Jiang Scholars and Innovative Research Team in University (No. IRT13018), the Jilin Province Natural Science Foundation of China (No. 20140101171JC, 20150520090JH), and Project 2015021 Supported by Graduate Innovation Fund of Jilin University.References
- H. Haick, Y. Y. Broza, P. Mochalski, V. Ruzsanyi and A. Amann, Chem. Soc. Rev., 2014, 43, 1423–1449 RSC.
- A. Amann, B. d. L. Costello, W. Miekisch, J. Schubert, B. Buszewski, J. Pleil, N. Ratcliffe and T. Risby, J. Breath Res., 2014, 8, 1–17 Search PubMed.
- M. Righettoni, A. Tricoli and S. E. Pratsinis, Anal. Chem., 2010, 82, 3581–3587 CrossRef CAS PubMed.
- B. Ibrahim, M. Basanta, P. Cadden, D. Singh, D. Douce, A. Woodcock and S. J. Fowler, Thorax, 2011, 66, 804–809 CrossRef PubMed.
- Y. Y. Broza and H. Haick, Nanomedicine, 2013, 8, 785–806 CrossRef CAS PubMed.
- S.-J. Choi, F. Fuchs, R. Demadrille, B. Grevin, B.-H. Jang, S.-J. Lee, J.-H. Lee, H. L. Tuller and I.-D. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 9061–9070 CAS.
- B. Buszewski, A. Ulanowska, T. Ligor, N. Denderz and A. Amann, Biomed. Chromatogr., 2009, 23, 551–556 CrossRef CAS PubMed.
- V. V. Plashnitsa, P. Elumalai, Y. Fujio and N. Miura, Electrochim. Acta, 2009, 54, 6099–6106 CrossRef CAS.
- Y. Zhu, V. Thangadurai and W. Weppner, Sens. Actuators, B, 2013, 176, 284–289 CrossRef CAS.
- W. Hong, Y. Chen, X. Feng, Y. Yan, X. Hu, B. Zhao, F. Zhang, D. Zhang, Z. Xu and Y. Lai, Chem. Commun., 2013, 49, 8229–8231 RSC.
- G. Xi, S. Ouyang, P. Li, J. Ye, Q. Ma, N. Su, H. Bai and C. Wang, Angew. Chem., Int. Ed., 2012, 51, 2395–2399 CrossRef CAS PubMed.
- E. T. Zellers, S. A. Batterman, M. Han and S. J. Patrash, Anal. Chem., 1995, 67, 1092–1106 CrossRef CAS PubMed.
- Y. Masuda, T. Itoh, W. Shin and K. Kato, Sci. Rep., 2015, 5, 1–7 Search PubMed.
- H. Yamagiwa, S. Sato, T. Fukawa, T. Ikehara, R. Maeda, T. Mihara and M. Kimura, Sci. Rep., 2014, 4, 1–6 Search PubMed.
- S. Zampolli, I. Elmi, F. Mancarella, P. Betti, E. Dalcanale, G. C. Cardinali and M. Severi, Sens. Actuators, B, 2009, 141, 322–328 CrossRef CAS.
- S.-J. Choi, I. Lee, B.-H. Jang, D.-Y. Youn, W.-H. Ryu, C. O. Park and I.-D. Kim, Anal. Chem., 2013, 85, 1792–1796 CrossRef CAS PubMed.
- A. Gurlo, Nanoscale, 2011, 3, 154–165 RSC.
- L. Sun, X. Han, K. Liu, S. Yin, Q. Chen, Q. Kuang, X. Han, Z. Xie and C. Wang, Nanoscale, 2015, 7, 9416–9420 RSC.
- R. Xing, Q. Li, L. Xia, J. Song, L. Xu, J. Zhang, Y. Xie and H. Song, Nanoscale, 2015, 7, 13051–13060 RSC.
- X. Yan, T. T. Tsotsis and M. Sahimi, Microporous Mesoporous Mater., 2014, 190, 267–274 CrossRef CAS.
- J. L. Luna-Xavier, E. Bourgeat-Lami and A. Guyot, Colloid Polym. Sci., 2001, 279, 947–958 CAS.
- A. P. Lee and B. J. Reedy, Sens. Actuators, B, 1999, 60, 35–42 CrossRef CAS.
- N. Rajesh, J. C. Kannan, T. Krishnakumar, S. G. Leonardi and G. Neri, Sens. Actuators, B, 2014, 194, 96–104 CrossRef CAS.
- X. Zhou, X. Li, H. Sun, P. Sun, X. Liang, F. Liu, X. Hu and G. Lu, ACS Appl. Mater. Interfaces, 2015, 7, 15414–15421 CAS.
- Q. Jia, H. Ji, Y. Zhang, Y. Chen, X. Sun and Z. Jin, J. Hazard. Mater., 2014, 276, 262–270 CrossRef CAS PubMed.
- P. Gao, H. Ji, Y. Zhou and X. Li, Thin Solid Films, 2012, 520, 3100–3106 CrossRef CAS.
- L. Cheng, S. Y. Ma, X. B. Li, J. Luo, W. Q. Li, F. M. Li, Y. Z. Mao, T. T. Wang and Y. F. Li, Sens. Actuators, B, 2014, 200, 181–190 CrossRef CAS.
- Q.-q. Jia, H.-m. Ji, D.-h. Wang, X. Bai, X.-h. Sun and Z.-g. Jin, J. Mater. Chem. A, 2014, 2, 13602–13611 CAS.
- J. Shin, S.-J. Choi, D.-Y. Youn and I.-D. Kim, J. Electroceram., 2012, 29, 106–116 CrossRef CAS.
- X. Zhou, J. Liu, C. Wang, P. Sun, X. Hu, X. Li, K. Shimanoe, N. Yamazoe and G. Lu, Sens. Actuators, B, 2015, 206, 577–583 CrossRef CAS.
- I. Hafaiedh, S. Helali, K. Cherif, A. Abdelghani and G. Tournier, Mater. Sci. Eng., C, 2008, 28, 584–587 CrossRef CAS.
- S. Park, H. Ko, S. An, W. I. Lee, S. Lee and C. Lee, Ceram. Int., 2013, 39, 5255–5262 CrossRef CAS.
- Z. Lou, L. Wang, T. Fei and T. Zhang, New J. Chem., 2012, 36, 1003 RSC.
- H.-J. Kim and J.-H. Lee, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS.
- A. P. Lee and B. J. Reedy, Sens. Actuators, B, 1999, 60, 35–42 CrossRef CAS.
- N. Yamazoe, G. Sakai and K. Shimanoe, Catal. Surv. Asia, 2003, 7, 63–75 CrossRef CAS.
- J. Mizsei, Sens. Actuators, B, 1995, 23, 173–176 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07483a |
| This journal is © The Royal Society of Chemistry 2016 |