Retracted Article: Cell orientation, proliferation, and differentiation on poly(L-lactide) spherulites
F. Zoua,
F. Z. Lua,
X. S. Maa,
D. W. Heb,
T. T. Tangc,
X. L. Xiaa,
J. Y. Jiang*a and
Y. F. Niu*b
aDepartment of Orthopaedics, Huashan Hospital, Fudan University, Shanghai, 200040, China. E-mail: zillion-faculty@126.com
bDepartment of Orthopaedics, Changhai Hospital, The Second Military Medical University, Shanghai 200433, China. E-mail: nyflying126@126.com
cShanghai Key Laboratory of Orthopedic Implants, Department of Orthopedic Surgery, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
First published on 1st June 2016
Abstract
The ringless spherulites of poly(L-lactide) (PLLA), fabricated via isothermal crystallization at temperatures of 100 °C and 140 °C (PLLA100 and PLLA140, respectively), were used to evaluate the effect of spherulites on MC3T3-E1 cell functions, including adhesion, proliferation, and differentiation. The atomic force microscopy (AFM) images indicated that PLLA100 presented a ridge width of 50–100 μm and a ride depth of 50–100 nm, whereas the values of PLLA140 were 25–50 μm and 100–350 nm, respectively. The cytoskeleton and nuclear alignment were observed on PLLA140, while no alignment was found on PLLA100. More interestingly, MC3T3-E1 cell proliferation and differentiation were also promoted significantly on PLLA140 compared with PLLA100. Our results, undoubtedly, provide a fundamental understanding regarding the effects of polymer spherulites on cell functions.
Introduction
In tissue engineering, a variety of materials have been investigated and developed to meet the clinical needs of tissue substitutes or regeneration materials. Unfortunately, to date, even though they might show some great properties to partially meet the requirement of a perfect candidate used for the tissue engineering, there is no single material, natural or synthetic, good enough to address the unmet needs in tissue engineering.1 The major reason is that many physicochemical factors, exemplified as chemistry, topography, mechanics, and charges,2 associate with the bio-performance of tissue implants, which makes the application of biomaterials in tissue engineering challenging.3 Among of these factors, a wide category of topographical surfaces have been fabricated and studied, to understand the impact of topography on cellular functions, such as cell orientation, mobility, activation, adhesion, and propagation. The mechanism of cell interaction with topography, popularly agreed on, is called “contact guidance”.1,4 The dimensions, including depth and width, of surface topography influence cellular events via integrin-based focal adhesions, since the cells can feel and react to the grooves and ridges sensitively.2,5As a semi-crystalline polymer, the biodegradable PLLA has been gaining increasing attraction in tissue engineering. Recently, investigations on the influence of PLLA materials with different surface topographies, such as microgrooves fabricated with a silicon template, hemispherical islands fabricated with hemispherical pits as template, and spherulites, on cellular responses have been carried out.6–8 However, the cell alignment on an individual ringless spherulite has not been reported yet. Even though the MC3T3-E1 cell was seeded on a single PLLA ringless spherulite,8 the cell did not show morphological orientation. However, these accumulated evidences, still, could not rule out the influence of the ridges on polymer spherulites on cell alignment due to the fact that, to date, the effect of ringless spherulites on cell alignment was only studied on those spherulites with shallow ridges, namely, small ridge depth,8 the distance between the ridge top and the groove valley. Previously, it was demonstrated that thermal treatment played a great role in governing both crystallinity and crystal morphology of semi-crystalline polymers,9–11 for example, the procedure for thermally treating PLLA polymer during crystallization influences the topography of ringless spherulites, such as ridge size formed by lamellar twisting along the radial direction, the spherulite diameter, and spherulite roughness.9,12–16
As investigated in the past decades, many natural and synthetic polymers, such as polycaprolactone (PCL), polyether ether ketone (PEEK), and PLLA, have the potential to gain application in bone tissue repair, as substitutes or filling materials. The remaining challenges are how to improve the osteoblast functions on those materials.17,18 In this study, we aim to prepare the PLLA ringless spherulites with proper ridge width, the distance between two neighbouring ridges, and ridge depth, to explore if the ridges with appropriate dimension can induce cytoskeletal alignment and subsequent cellular responses of MC3T3-E1 cells, which are typical cell line for studying bone tissue-related biomaterials. This work would be the first report of controlling cell fate on a single polymer ringless spherulite, which might provide a promising modality to improve the surface topography of semi-crystalline polymers for bone tissue repair.
Experimental section
Preparation and characterization of PLLA100 and PLLA140
PLLA (Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, PDI ≤ 1.1) was purchased from Sigma-Aldrich. PLLA, dissolved in dichloromethane (0.5 g mL−1), was spin-coated onto round glass coverslips (12 mm, Fisher Scientific) using a WS-400-6NPP-LITE spin processor (Laurell Technologies Corporation, USA) at 2000 rpm for 30 s at room temperature. After left in chemical hood overnight, the films were dried at 37 °C. Then, the films were melted at 230 °C for 10 min and transferred to a hot stage for isothermal crystallization at 100 °C and 140 °C to obtain PLLA100 and PLLA140, respectively.
000 g mol−1, PDI ≤ 1.1) was purchased from Sigma-Aldrich. PLLA, dissolved in dichloromethane (0.5 g mL−1), was spin-coated onto round glass coverslips (12 mm, Fisher Scientific) using a WS-400-6NPP-LITE spin processor (Laurell Technologies Corporation, USA) at 2000 rpm for 30 s at room temperature. After left in chemical hood overnight, the films were dried at 37 °C. Then, the films were melted at 230 °C for 10 min and transferred to a hot stage for isothermal crystallization at 100 °C and 140 °C to obtain PLLA100 and PLLA140, respectively.
PLLA100 and PLLA140 morphologies
The PLLA100 and PLLA140 morphologies were observed using a polarized optical microscopy (POM, Olympus Model BHSP, Japan). The topographical features of PLLA100 and PLLA140 were examined by the AFM with a Nanoscope IIIa controller (Digital Instruments, Santa Barbara, USA) using the tapping mode over a scanning area of 200 μm × 200 μm. The height profile and the root mean square (rms) roughness (Rrms) were obtained from the AFM height images using the WSxM 4.0 (Nanotec).Hydrophilicity and protein adsorption
The hydrophilicities of PLLA100 and PLLA140 were measured by water contact angle measurements (contact angle system, OCA15, Dataphysics Co., Germany) at room temperature. Protein adsorptions on PLLA100 and PLLA140 were measured using fibronectin (FN)/phosphate buffered saline (PBS) solution (10 μg mL−1) and Dulbecco's Modified Eagle Medium (DMEM) used for culturing MC3T3-E1 cells. PLLA100 or PLLA140 samples (Φ 12 mm) were immersed in fibronectin (FN)/PBS solution or DMEM for 4 h at 37 °C. Then, the samples were rinsed with PBS and soaked in sodium dodecyl sulfate (SDS) solution (300 μL) for 30 min (×3) to collect proteins. An ELISA plate reader (Bio-Rad) and a MicroBCA protein assay kit (Pierce, Rockford, IL, USA) were employed to measure the optical density (OD) values of the SD solution and calculate the protein concentration according to the manufacturer's instruction.Cell maintenance
The PLLA100 or PLLA140 were rinsed, mouse MC3T3-E1 preosteoblasts were purchased from ATCC and cultured in DMEM (Hyclone, Thermo Fisher Scientific Inc., MA, USA) supplemented with 10% fetal bovine serum (FBS; GibcoBRL, Grand Island, NY, USA), 1% penicillin (100 U mL−1), and streptomycin sulphate (100 mg mL−1) (GibcoBRL, Grand Island, NY, USA). Then the cells were cultured in the cell incubator with 95% relative humidity (RH) and 5% CO2 at 37 °C. DMEM was refreshed every 3 days and confluent cells were sub-cultured through trypsinisation.Cell adhesion and morphology
The PLLA100 or PLLA140 were rinsed with alcohol solution (70%, ×3) and sterilized via exposure to UV light for 60 min. At a cell density of ∼104 cm−2, MC3T3-E1 cells were seeded on PLLA100 or PLLA140 in 24-well plates with the DMEM supplemented with 10% FBS, 1% penicillin (100 U mL−1), and streptomycin sulphate (100 mg mL−1). Then the cells were cultured in the cell incubator.After cultured for 1 day, the cells were fixed with a paraformaldehyde (PFA, 4%) solution for 15 min. Afterwards, cells were rinsed with PBS (×5) and permeabilized with 0.2% (v/v) Triton X-100 for 10 s, rinsed with PBS (×3) again, stained with rhodamine-phalloidin (RP) and subsequently stained with 4,6-diamidino-2-phenylindole (DAPI) according to the protocols. The cytoskeleton and nuclei were observed with a confocal laser scanning microscopy (CLSM, Zeiss, Oberkochen, Germany). The cell area was quantified from 100 individual cells at day 1. Focal adhesions were stained with mouse monoclonal anti-vinculin antibody and anti-mouse IgG-FITC antibody according to the protocol provided by the manufacturer, followed by CLSM observation.
The cell or nuclear alignment were also analysed in terms of alignment and circularity using ImageJ software. The cells or nuclei with the long axis of the approximated ellipse deviating from the radial direction by smaller than 15° were considered aligned. The circularity value of 1.0 indicated a perfect circle and smaller value showed stronger alignment.
Cell attachment and proliferation
The cell attachment at 4 h and proliferation at 1, 4, and 7 days were investigated using a cell counting kit-8 (CCK-8) assay according to a standard procedure. Briefly, the specimens (Φ 12 mm) were sonicated in ethanol and sterilized under UV light. Cells at a density of ∼104 cells per cm2 were seeded on the samples in 24-well tissue culture plates. At time points, the samples were rinsed with PBS (×3) and then transferred to a new 24-well plate. 50 μL of CCK-8 solution (Dojindo Molecular Technologies Inc., Kumamoto, Japan) was added to each well and empty wells containing DMEM as a negative control. After 3 h, 10 μL of the supernatant was transferred into a 96-well plate and read at 450 nm using a microplate reader (Synergy HT, Bio-tek, Winooski, Vermont, USA) with 620 nm as a reference wavelength.Cell differentiation
After incubation in 24-well plates for 24 h as described above, the culture medium was changed to the osteogenic induction medium, the Minimum Essential Medium Alpha Modified (α-MEM) culture medium, supplemented with 10% FBS, 0.1 μM dexamethasone (Sigma), 50 μM ascorbic acid (Sigma), and 10 μM β-glycerophosphate sodium (Sigma). The α-MEM medium was refreshed every other day during 2 weeks. At 14 days, the cell differentiation was examined using the alkaline phosphatase (ALP) activity and calcification measurements. The α-MEM was extracted and washed with PBS (×3). Afterwards, 200 μL of Nonidet P-40 (NP-40, 1%) was added to each well and the samples were incubated for 60 min to obtain cell lysates. According to the manufacturer's protocols, the QuantiChrom calcium assay kit (BioAssay Systems, Hayward, CA) and the ALP detection kit (Beyotime Institute of Biotechnology, Shanghai, China) were used to determine the calcium content and ALP activity on samples, respectively.The calcium deposition and ALP activity on the samples were also characterized by cell staining using an alizarin red S solution (Ricca Chemical, Arlington, TX) and a leukocyte Alkaline Phosphatase Kit (Beyotime Institute of Biotechnology, Shanghai, China), respectively. The staining images were taken using a digital camera after the samples were rinsed with pure water (5 × 15 min).
Gene expression
Trizol reagent (Takara, Tokyo, Japan) and PrimeScript RT reagent Kit (Takara, Tokyo, Japan) were used to extract the RNA from the cells and synthesize the cDNA, respectively, according to the manufacturers' protocols. The synthesized cDNA was diluted in sterile distilled water and 4 μL of the diluted cDNA was used for real-time PCR using SYBR Premix Ex Taq™ (Takara, Tokyo, Japan). Gene-level expressions of integrin β1, integrin α2, integrin α1, ALP, osteocalcin (OCN), osteopontin (OPN), and runt-related transcription factor 2 (Run × 2), with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the house keeping gene for normalization, which were performed using a real-time polymerase chain reaction (PCR). The oligonucleotide primer sequences used in this study were referred to previous studies.19,20Statistical analysis
All quantitative data were expressed as the mean ± standard deviation (SD) and analyzed with the Origin 8.0 software (Origin Lab Corporation, USA). Statistical comparisons were carried out using analyses of variance (ANOVA). The difference greater than 95% or 99% confidence level (p < 0.05 or p < 0.01, respectively) was considered significant.Results and discussion
Characterizations
In this study, the polymers were melted and then isothermal crystallized at 100 °C or 140 °C until the glass slides were fully covered with spherulites. The surface morphologies of PLLA100 and PLLA140 were analyzed with POM, as depicted in Fig. 1A and B. As reported earlier, the isothermal crystallization temperature played a great role in regulating the spherulite size, in a way that higher isothermal crystallization temperature induced fewer nucleation sites, resulting in bigger spherulites.9,10,21,22 In a good agreement, Fig. 1A showed that PLLA140 spherulites crystallized at 140 °C expanded up to ∼800 μm in radius, whereas PLLA100 spherulites at lower temperature of 100 °C were ∼150 μm in radius. Here, the radius was quantified using ImageJ software based on ∼100 spherulites. The more detailed topographies of PLLA100 and PLLA140 were observed using the tapping mode AFM scanning as shown in Fig. 1C and D. Clearly, the PLLA100 displayed irregular lamellar twisting, conversely, PLLA140 showed highly aligned lamellar twisting along the radial direction, with visibly sharp micro-ridges. Consistently, the height profiles in Fig. 1E and F demonstrated that PLLA100 presented a ridge width of 50–100 μm, and a ridge depth of 50–100 nm, whereas these values of PLLA140 were 25–50 μm and 100–350 nm, respectively. As a consequence, the Rrms increased from ∼35 nm on PLLA100 to ∼88 nm on PLLA140, consistent with previous findings that higher isothermal crystallization temperature generated bigger roughness.9,23Hydrophilicity and protein adsorption
The CAs, representing hydrophilicity, and protein adsorption, including FN and DMEM, were determined on PLLA100 and PLLA140 as shown in Fig. 2. The CA measurements indicated that, without difference, both PLLA100 and PLLA140 had a water contact angle of ∼77°, undeviating with previous findings.24 Apparently, the topographical and roughness differences between PLLA100 and PLLA140 did not influence the CAs significantly. However, the protein adsorption on PLLA140 in both FN solution and DMEM medium was significantly enhanced than that on PLLA100 as indicted in Fig. 2B. As discussed earlier, the roughness of PLLA140 was measured to be ∼88 nm, much higher than that of PLLA100, ∼35 nm. The enhanced protein adsorption on PLLA140 might be ascribed to the higher roughness, as earlier study suggested that rougher spherulites induced higher protein adsorption.25 | ||
| Fig. 2 Water contact angles (A) and protein adsorptions (B) of PLLA100 and PLLA140. *: p < 0.05 compared with PLLA100. Error bars indicate the SD of the mean values (n = 4). | ||
Cell adhesion
The topographical discrepancy between PLLA100 and PLLA140, remarkably, influenced cell adhesion in a different way as shown in Fig. 3. Cells were randomly distributed on PLLA100 without showing preferential alignment on PLLA100 (Fig. 3A–C). In contrast, they were strongly aligned along the radial direction on PLLA140 (Fig. 3D–F). Previous study reported the MC3T3-E1 cell adhesion on PLLA ringless spherulites, however, the cell alignment was not observed.8 The cell alignment was analyzed using ImageJ software in terms of alignment, represented by percentage, and circularity, as shown in Fig. 3G and H. Notably, ∼85% cells on PLLA140 were aligned, significantly higher than ∼8% on PLLA100, the average value of random cell distribution, meaning that cells on PLLA100 were not aligned. Consistently, the circularity of cells on PLLA140 was prominently lower than that on PLLA100, indicating strong cell alignment on PLLA140. The micro-environmental influence on cytoskeleton could be transferred to intermediate filaments and then further to nuclei because of the mechanical integration in the entire cell via mechanotransduction.1 In a good agreement, the nuclear alignment was also found on PLLA140 as shown in Fig. 3E, whereas the nuclei did not show alignment on PLLA100, which was further confirmed by the quantitative alignment and circularity results in Fig. 3I and J.The cell adhesion on PLLA100 and PLLA140 was further examined using focal adhesion staining as shown in Fig. 4. The focal adhesions on PLLA100 exhibited random extension, while they were evidently aligned along radial direction on PLLA140 (Fig. 4A and B). The number per cell body and the circularity of focal adhesions were also quantified using ImageJ software as shown in Fig. 4C and D. Both factors, again, suggested that focal adhesions on PLLA140 were significantly aligned compared with those on PLLA100.
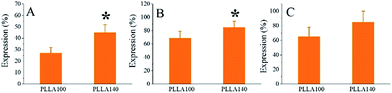
In this study, the cell responses on PLLA140, including the alignment of focal adhesions and associated cytoskeleton should be ascribed to the specific topography on spherulites through “contact guidance”.26–28 Engineering surface features, such as micro- or nanopatterns through chemical or topographic cues, are demonstrated to regulate cell functions and the sensitivity of cells to nanoscale signals can be as small as ∼10 nm.29 Previous findings suggested that corresponding to the alignment of focal adhesions, the actin filaments or stress fibers would be aligned accordingly.30,31 In our present study, consistently, the focal adhesions were aligned along the radial direction, subsequently resulting in the cytoskeleton orientation, as well as the nuclear alignment through mechanotransduction,32,33 indicating that the cells sensed the topography on PLLA140 significantly compared with PLLA100. Besides, the area of junction or discontinuity was evidenced to promote cell spreading earlier.2 Apparently, PLLA140 with higher roughness than PLLA100 provided more junction or discontinuity, which might provide subtle micro-circumstance for cell adhesion. As demonstrated earlier, integrins played a great role in osteoblast-extracellular matrix (ECM) dialogue, also the principle mediators of the molecular interaction.30 The transmembrane receptors, integrins, transfer information from the ECM to the intracellular compartment via signal transduction pathways through focal adhesion kinase and mitogen-activated protein kinases (MAPK).31,34 The combinations of integrin subunits dominate the recognition of ECM proteins by a cell. The combinations of α3β1, α4β1, and α5β1 specifically bind fibronectin, whereas type I collagen reacts with α1β1, α2β1, and α3β1.30,35,36 When cells were cultured on PLLA100 and PLLA140 in DMEM medium, the ECM proteins from the DMEM medium absorbed on samples might quickly provide attachment sites between the cells and the ECM proteins via integrins. Hence, the gene expressions of integrin subunits, α1, α2, and β1, closely associated with osteoblastic adhesion, attachment, proliferation, and differentiation,18,30,37,38 at day 1 were examined as shown in Fig. 5. Unsurprisingly, the expression levels of α1 and α2 subunits on PLLA140 were much higher than on PLLA100, which provided another solid evidence that cell adhesion was enhanced on PLLA140 compared with PLLA100. The expression of β1, even though not significantly higher on PLLA140, still showed greater value than on PLLA100. The rougher surface of PLLA140 might lead to more formation of focal adhesions and generated higher gene expressions of integrin subunits α1, α2, and β1.
Cell proliferation
Also, the cell proliferation was influenced by the distinct surface topography on PLLA140 as shown in Fig. 6. Cells proliferated on both PLLA100 and PLLA140, however, significantly faster on PLLA140 compared with that on PLLA100, indicated by the cell numbers measured by the CCK-8 assay as shown in Fig. 6A from day 1 to day 7, even though the difference between PLLA100 and PLL140 was not significant at 4 h. In addition, the cell spreading area measured from ∼150 individual fluorescent cytoskeletons at day 1 in Fig. 6B showed that cells on PLLA140 were slightly smaller than those on PLLA100. This can be interpreted by the fact that cell bodies were confined growing on the radial direction instead of freely extending on all directions, resulting in a smaller cell spreading area on PLLA140.Cell differentiation
Furthermore, the cell differentiation was also influenced by the distinct topography on PLLA140, as demonstrated in Fig. 7. Both calcification and ALP activity clearly were enhanced on PLLA140 compared with PLLA100 at day 14, evidenced by the significantly deeper red and purple colours on PLLA140, respectively. In accordance with the staining results, the quantitative ALP activity and calcium content were also sharply increased from PLLA100 to PLLA140 as shown in Fig. 7C and D. To better understand the osteogenesis on PLLA100 and PLLA140 at day 14, the gene expressions of bone-specific markers, ALP, OCN, OPN, and Run × 2 were performed as shown in Fig. 8. Consistently, all markers obtained significantly higher expression on PLLA140 than on PLLA100, indicating that the cell differentiation was promoted on PLLA140.The cell differentiation on PLLA100 and PLLA140 can be understood in several aspects. First, the cell adhesion and proliferation were significantly promoted on PLLA140 compared with PLLA100. Theoretically, MC3T3-E1 cells started to differentiate when the population reached confluence. As a consequence, the faster proliferation on PLLA140 would launch differentiation earlier than PLLA100. Therefore, cells at day 14 showed significantly higher differentiation level on PLLA140 than PLLA100. Different from our study, the previous static MC3T3-E1 cell culture on microgrooved silicone substrata indicated that cells were significantly aligned, however, the cell proliferation did not show difference between microgrooves and smooth surface.39 The mechanism attributed to this huge difference needs to be further investigated. On the other hand, the promoted differentiation on PLLA140 might be attributed to the morphological alignment. For instance, the human osteosarcoma-derived cell viability, proliferation, and ALP activity were also influenced by the nuclear deformation on micropillars.4 In this study, the nuclei and cytoskeleton on PLLA140, also, clearly showed alignment along the radial direction as discussed earlier. Hence, we carefully concluded that the deformed nuclei might induce the greater differentiation on PLLA140.
The PLLA140 spherulites obtained at the isothermal crystallization temperature of 140 °C displayed substantially distinct surface topography, in comparison with PLLA100 crystallized at lower temperature of 100 °C. The small ridges produced by the lamellar twisting can be considered as microgrooves. The PLLA100 showed a bigger ridge width of 50–100 μm and a smaller depth of 50–100 nm, whereas the values of PLLA140 were 25–50 μm and 100–350 nm, respectively. As a result, the Rrms increased from ∼35 nm on PLLA100 to ∼88 nm on PLLA140. This topographical difference exerted a great influence on the cellular responses, including adhesion, phenotype, proliferation, and osteogenesis.
The effects of microgrooved structures on cell functions have been studied extensively in last several decades. Cells could respond to microgrooves at nano-scale via adjustment of the cell body to the substrate texture.2,30 Both groove width and groove depth, here ridge width and depth, respectively, played great roles in aligning cells. Earlier studies demonstrated that rabbit mesenchymal stem cell (MSC)-derived osteoblast-like cells were greatly aligned along the direction of the nanogrooves with a depth of 60–70 nm and a width of ∼300 nm.1 However, other studies also indicated that the width smaller than several microns and the depth bigger than a micron could enhanced cell alignment.18 In our present study, the ridge width was 25–50 μm and the ridge depth was 100–350 nm, the MC3T3-E1 cell still strongly sensed the surface texture of PLLA140. Thus, we believe that the effect of microgrooved texture on cell alignment, not only depends on the groove dimensions, such as width and depth, but also relies on specific topography of the ridges and grooves. Here, the ridges on PLLA140 exhibited much sharper tops compared with ridges on conventional microgrooves, in which the ridge had identical width from the bottom to the top. The cells might respond to these unique tops sensitively, which induced the evident cytoskeleton and nuclear alignment. Besides, the PLLA100, although, had sharp tops, the PLLA140 showed more regular orientation of ridges along the radial direction with higher depth, which might also help the cell alignment.
Conclusions
The PLLA ringless spherulites, fabricated via isothermal crystallization at temperatures of 100° and 140° (PLLA100 and PLL140, respectively), were used to evaluate the effect of different topographies on MC3T3-E1 cell functions, including adhesion, proliferation, and differentiation. The results suggested that PLLA100 presented a ridge width of 50–100 μm and a ridge depth of 50–100 nm, whereas the values of PLLA140 were 25–50 μm and 100–350 nm, respectively. The cytoskeleton and nuclear alignment were observed on PLLA140, while no alignment was found on PLLA100. In addition, the MC3T3-E1 cell proliferation and differentiation were also promoted significantly on PLLA140 as compared with PLLA100. Our pioneer work, fundamentally, elucidated effect of polymer spherulites on cell orientation, proliferation, and differentiation, which might open a gate to advance the applications of semi-crystalline polymers in bone tissue repair.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 31271015) and the Shanghai Science and Technology Development Fund (No. 13DZ2294000, 13JC1403900).Notes and references
- B. Zhu, Q. Lu, J. Yin, J. Hu and Z. Wang, Tissue Eng., 2005, 11, 825–834 CrossRef CAS PubMed.
- S. Kweon, K. H. Song, H. Park, J. C. Choi and J. Doh, ACS Appl. Mater. Interfaces, 2016, 8, 4266 CAS.
- K. Matsuzaka, X. F. Walboomers, M. Yoshinari, T. Inoue and J. A. Jansen, Biomaterials, 2003, 24, 2711 CrossRef CAS PubMed.
- P. M. Davidson, H. Ozcelik, V. Hasirci, G. Reiter and K. Anselme, Adv. Mater., 2009, 21, 3586 CrossRef CAS.
- A. Curtis and C. Wilkinson, Biomaterials, 1997, 18, 1573 CrossRef CAS PubMed.
- H. Kenar, G. T. Kose and V. Hasirci, Biomaterials, 2006, 27, 885 CrossRef CAS PubMed.
- Y. Q. Wan, Y. Wang, Z. M. Liu, X. Qu, B. X. Han, J. Z. Bei and S. G. Wang, Biomaterials, 2005, 26, 4453 CrossRef CAS PubMed.
- C. G. Simon Jr, J. Microsc., 2004, 216, 153 CrossRef CAS PubMed.
- B. Crist and J. M. Schultz, Prog. Polym. Sci., 2016, 56, 1 CrossRef CAS.
- X. Cui, A. G. Shtukenberg, J. Freudenthal, S. Nichols and B. Kahr, J. Am. Chem. Soc., 2014, 136, 5481 CrossRef CAS PubMed.
- T. Miyata and T. Masuko, Polymer, 1998, 39, 5515 CrossRef CAS.
- S. Nurkhamidah and E. M. Woo, J. Appl. Polym. Sci., 2011, 122, 1976 CrossRef CAS.
- R. Androsch and M. L. Di Lorenzo, Polymer, 2013, 54, 6882 CrossRef CAS.
- J. Xu, B. H. Guo, J. J. Zhou, L. Li, J. Wu and M. Kowalczuk, Polymer, 2005, 46, 9176 CrossRef CAS.
- S. H. Kim, J. A. Kaplan, Y. Sun, A. Shieh, H. L. Sun, C. M. Croce, M. W. Grinstaff and J. R. Parquette, Chemistry, 2015, 21, 101 CrossRef CAS PubMed.
- S. H. Kim, Y. Sun, J. A. Kaplan, M. W. Grinstaff and J. R. Parquette, New J. Chem., 2015, 39, 3225 RSC.
- M. A. Childs, D. D. Matlock, J. R. Dorgan and T. R. Ohno, Biomacromolecules, 2001, 2, 526 CrossRef CAS PubMed.
- K. Anselme, Biomaterials, 2000, 21, 667 CrossRef CAS PubMed.
- S. Uchiyama and M. Yamaguchi, Int. J. Mol. Med., 2007, 19, 213 CAS.
- J. Zhang, H. Zhou, K. Yang, Y. Yuan and C. Liu, Biomaterials, 2013, 34, 9381 CrossRef CAS PubMed.
- Y. Sun, J. A. Kaplan, A. Shieh, H. L. Sun, C. M. Croce, M. W. Grinstaff and J. R. Parquette, Chem. Commun., 2016, 52, 5254 RSC.
- Y. Zhou, X. Xu, Y. Sun, H. Wang, H. Sun and Q. You, Bioorg. Med. Chem. Lett., 2013, 23, 2974 CrossRef CAS PubMed.
- M. L. Di Lorenzo, Eur. Polym. J., 2005, 41, 569 CrossRef CAS.
- O. Mert, E. Doganci, H. Y. Erbil and A. S. Demir, Langmuir, 2008, 24, 749 CrossRef CAS PubMed.
- K. Rechendorff, M. B. Hovgaard, M. Foss, V. P. Zhdanov and F. Besenbacher, Langmuir, 2006, 22, 10885 CrossRef CAS PubMed.
- A. I. Teixeira, G. A. Abrams, P. J. Bertics, C. J. Murphy and P. F. Nealey, J. Cell Sci., 2003, 116, 1881 CrossRef CAS PubMed.
- J. D. Lambert, D. H. Kim, R. Zheng and C. S. Yang, J. Pharm. Pharmacol., 2006, 58, 599 CrossRef CAS PubMed.
- R. Zheng, A. C. Dragomir, V. Mishin, J. R. Richardson, D. E. Heck, D. L. Laskin and J. D. Laskin, Toxicol. Appl. Pharmacol., 2014, 279, 43 CrossRef CAS PubMed.
- J. Y. Lim and H. J. Donahue, Tissue Eng., 2007, 13, 1879 CrossRef CAS PubMed.
- G. Xiao, D. Wang, M. D. Benson, G. Karsenty and R. T. Franceschi, J. Biol. Chem., 1998, 273, 32988 CrossRef CAS PubMed.
- R. Zheng, D. E. Heck, A. T. Black, A. Gow, D. L. Laskin and J. D. Laskin, Free Radical Biol. Med., 2014, 67, 1 CrossRef CAS PubMed.
- D. E. Ingber, FASEB J., 2006, 20, 811 CrossRef CAS PubMed.
- D. E. Jaalouk and J. Lammerding, Nat. Rev. Mol. Cell Biol., 2009, 10, 63 CrossRef CAS PubMed.
- E. A. Clark and J. S. Brugge, Science, 1995, 268, 233 CAS.
- R. Zheng, D. E. Heck, V. Mishin, A. T. Black, M. P. Shakarjian, A. N. Kong, D. L. Laskin and J. D. Laskin, Toxicol. Appl. Pharmacol., 2014, 275, 113 CrossRef CAS PubMed.
- R. Zheng, I. Po, V. Mishin, A. T. Black, D. E. Heck, D. L. Laskin, P. J. Sinko, D. R. Gerecke, M. K. Gordon and J. D. Laskin, Toxicol. Appl. Pharmacol., 2013, 272, 345 CrossRef CAS PubMed.
- L. Dong and C. K. Qu, Methods Mol. Biol., 2014, 1185, 79 Search PubMed.
- R. Feng and L. Dong, Int. J. Clin. Exp. Pathol., 2015, 8, 9376 Search PubMed.
- J. H. C. Wang, E. S. Grood, J. Florer and R. Wenstrup, J. Biomech., 2000, 33, 729 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |