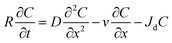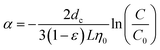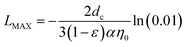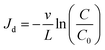DOI:
10.1039/C6RA07445F
(Paper)
RSC Adv., 2016,
6, 66672-66681
Transport and return of an oilfield scale inhibitor reverse micelle nanofluid: impact of preflush and overflush†
Received
22nd March 2016
, Accepted 6th July 2016
First published on 6th July 2016
Abstract
In this study, oilfield scale inhibitor reverse micelle nanomaterials and a nanomaterial fluid (nanofluid) were investigated to expand their use in the delivery of a scale inhibitor into reservoir formation for oilfield mineral scale control. The prepared inhibitor nanomaterials are in a non-aqueous medium designed for application in low water cut or water sensitive production wells. The transport behavior of the inhibitor nanofluid was evaluated with a focus on examining the impacts of flow rate and preflush fluid. It shows that an increase in flow rate can improve the transportability of the nanomaterials in a calcite medium. The increase in flow rate can also lead to a reduced tendency of nanomaterials to be collected by a calcite medium. An isooctane preflush can enhance the transport of the nanomaterial compared with an aqueous brine preflush. Inhibitor return performance of the nanofluid was evaluated by laboratory squeeze simulation tests. It shows that the prepared inhibitor reverse micelle nanomaterials demonstrated an extended squeeze lifetime compared with the conventional pill solution. Furthermore, it is evident that an aqueous brine overflush can improve the return performance of the nanomaterials, compared with an isooctane overflush. This can be explained by the adsorption–desorption dynamics of the previously attached nanomaterials. Laboratory transport and squeeze simulation studies suggest that the inhibitor nanofluid has the potential to serve as a delivery vehicle to improve placement and return of inhibitors inside formation for oilfield scale control.
Introduction
Solubility is the chemical property of a chemical substance, the solute, to dissolve in a solvent.1 A mineral salt normally has a fixed solubility in an aqueous solution under certain conditions, including pressure, temperature, salinity, etc. Exceeding the solubility can lead to mineral scale precipitation out of an aqueous phase and eventually scale deposition onto the surface of the surrounding environment. Mineral scale blockage is one of the most problematic production chemistry threats in the oil and gas industry, particularly for offshore productions.2 Due to the significant financial outlay and the complex well configuration, scale control and management for an offshore production well is even more critical to avoid expensive remediation and major production deferral.3 Scale inhibitors are the chemicals designed to apply to oil production facilities to control scale deposition. Since scale formation is an aqueous phenomenon, scale inhibitors need to dissolve into aqueous phase and then react with the mineral nuclei. Scale inhibitors function by inhibiting the mineral crystal growth or interfering with the mineral nucleation process, depending on the type of the inhibitors.4 One of the most commonly used scale inhibitors is aminophosphonate (hereafter referred to as “phosphonate”). Scale blockage can occur at any location of the oil production system from the bottom of the well to gathering facilities or processing facilities. Scale inhibitors should be applied at a location upstream of the onset of scale deposition in order to inhibit scale formation. Normally, scale inhibitors are injected in a continuous mode via an injection mandrel located on the production system so that the injected inhibitor can mix with the produced water to inhibit scale formation. However, if the onset of scale deposition is at or near the perforations or in the wellbore formation, continuous scale inhibitor injection will no longer be a viable option since the injection mandrel cannot be placed at the bottom of the well. In this case, scale inhibitor squeeze treatment probably should be the engineering solution to control scale deposition from wellbore to processing facilities.4,5 During a squeeze job, inhibitor is delivered from an oil-producing well into formation pore space, so that the injected inhibitor can flow back together with the production fluids to inhibit scale formation. Typically, squeeze treatment is initiated by injecting a volume of preflush solution into the formation, to displace wellbore fluids and clean up the formation. Next, a volume of scale inhibitor solution (pill solution) will be injected into the formation. Following the pill injection, another volume of fluid (typically a brine solution) will be injected to push the previously injected inhibitor deeper into the reservoir. This step is called injection of overflush fluid. After the completion of overflush, the production well will be shut-in for a number of hours to allow the delivered inhibitor to be able to affix to the surfaces of formation materials. Subsequently, the production will be resumed by putting the well back online. The reservoir fluid will once again flow into the well. At the same time, the retained scale inhibitor will gradually partition from formation surfaces into aqueous phase by dissolving into the produced water to inhibit scale deposition, which process is also called scale inhibitor return.2,4,5 It is commonly believed that inhibitor return is a result of inhibitor desorption into the water phase.6 It is also speculated that phosphonate inhibitors are retained inside formation by forming metal–phosphonate precipitates and the release of phosphonates is controlled by the dissolution of the precipitates into the produced water.7 Sorbie et al.8 described the mathematical solution to calculate the inhibitor placement and presented the case study of modeling viscosified inhibitor placement with and without crossflow between formation layers.
Scale squeeze treatment is a common approach in oilfield to control mineral scale deposition. It is important to design the scale squeeze with a desirable squeeze lifetime, which is the duration time of a squeeze job before another squeeze treatment is required. Laboratory squeeze simulation test is the experimental approach to evaluate the performance of a squeeze design and also to rank the performance of different inhibitors in terms of squeeze lifetime. Laboratory squeeze simulation is purposely designed to simulate the field squeeze job in a laboratory setting using a column packed with formation materials. The selected formation materials are typically either calcium carbonate (calcite) or sandstone acquired from the field to represent the characteristics of the formation of interest. Similar to the field squeeze job, squeeze simulation test comprises of consecutive steps of preflush, inhibitor injection, overflush, shut-in and inhibitor return.7,9 Typically, oilfield squeeze treatment employs an aqueous inhibitor solution as the pill solution. In the past few years, the idea of utilizing inhibitor-containing nanomaterial fluid (nanofluid) as an inhibitor delivery vehicle has been reported and tested via a number of laboratory column transport and squeeze simulation tests.10–18 The objective of these investigations is to expand the use of nanomaterials in the delivery of phosphonate inhibitors into reservoir formation for scale control. By virtue of the observed transport behavior in formation media and the extended squeeze life compared with conventional inhibitor pill solution, these authors claim that these prepared inhibitor nanomaterials have the potential to be applied in a field squeeze treatment. More recently, we reported a non-aqueous scale inhibitor nanofluid for scale control for low water cut or water sensitive wells.18 Water cut is basically the percentage of produced water volume to the total liquids produced.2 Water sensitive wells are the production wells sensitive to water induced formation damage. Similar to the reported non-aqueous based inhibitor package,19,20 non-aqueous inhibitor nanofluid has the advantage of reducing formation damage over aqueous inhibitor product for low water cut or water sensitive wells. This non-aqueous inhibitor nanofluid was prepared by mixing reverse micelle (RM) solution of calcium with RM solution of DTPMP, a common phosphonate inhibitor. The prepared Ca–DTPMP reverse micelle nanofluid (RMNF) was a stable and translucent solution at room temperature and 70 °C. Transport experiment at calcite medium suggested that the RMNF was transportable through the medium and isooctane preflush showed an enhanced transportability compared with the scenario of preflushing with aqueous brine solution. Furthermore, the laboratory squeeze simulation test of this nanomaterial indicated that the nanomaterial performed comparably with the conventional pill solution in terms of squeeze lifetime.18
The present study focuses on the investigation of the effects of preflush and overflush treatments on RMNF transport and squeeze lifetime, respectively. Preflush is designed to displace the fluids at bottom of the well and wellbore back into formation. The preflush fluid serves as a spacer fluid between the production fluids coming from the reservoir and the inhibitor pill solution.20,21 On the other hand, overflush is designed to displace the injected inhibitor away from near wellbore region into formation. Jordan et al.22 summarized the benefits of overflush from the perspectives of better chemical adsorption, reduction of flow impairment risk, enhancement of inhibitor retention and also benefits from final tubing volume displacement. As such, both preflush and overflush treatments can effectively impact the interaction of the RM nanomaterials with formation medium as well as the placement of nanomaterials inside formation: preflush treatment can change the formation surface chemistry; while overflush can push the inhibitor deeper into formation. The previous study has elaborated the impact of organic preflush versus aqueous preflush on RMNF transport.18 This study will further evaluate the effects of preflush solution and flow rate on RMNF transport. The investigation of preflush and overflush effects detailed in this study will enhance the understanding of transport and return behavior of the non-aqueous inhibitor nanomaterials to expand their use in the delivery of phosphonate inhibitors in reservoir formation for enhanced scale control performance.
Experimental section
Chemicals
Commercial grade diethylenetriamine pentakis(methylenephosphonic acid) (DTPMP) with 50% activity was used as the scale inhibitor. Chemicals including sodium chloride, calcium chloride, sodium hydroxide and ethanol were reagent grade and purchased from Fisher Scientific. Organic solvent isooctane, anionic surfactant bis(2-ethylhexyl)sulfosuccinate sodium salt (AOT, 95%), nonionic surfactant nonaethylene glycol monododecyl ether (C12EO9) and tritiated water were purchased from Sigma Aldrich. Deionized water (DI water) was prepared by reverse osmosis and ion exchange water purification process.
Calcium–DTPMP RM microemulsion synthesis
As reported previously,18 the RMNF was prepared by combining microemulsion solutions containing CaCl2 RM and DTPMP RM in the presence of anionic surfactant AOT and nonionic surfactant C12EO9 (details in the ESI†). The reverse micelle solution was prepared by mixing anionic surfactant AOT, nonionic surfactant C12EO9 into an isooctane medium. The obtained Ca–DTPMP RM solution was further filtered through a 1 μm filter (Merck Millipore Corp.) to remove undissolved particles. The resultant Ca–DTPMP RMNF was a translucent solution with a pH of 5.8.
Reverse micelle microemulsion column breakthrough experiments
Similar to the previous studies,11–14,18 the transport behavior of the RMNF was evaluated via laboratory column transport studies. A glass column (Omnifit, Bio-Chem Fluidics, Boonton, NJ) was packed with calcite (Iceland spar, Creel Chihuahua, Mexico) and the dimension of the column was 1.02 cm inner diameter (ID) and 8 cm length. The calcite materials had a grain size distribution of 106 to 180 μm. Initially, a tracer test (tritiated water, 3H2O) was conducted to measure the dispersion coefficient, porosity and pore volume (PV) of the packed column. PV is basically the column volume unoccupied by the formation materials and can be calculated as the total column volume subtracted by the formation material volume. RMNF transport study was carried out by injecting a number of PV of the nanofluid through the packed column. The effluent was collected by a sampler and measured for DTPMP concentrations. RM effluent concentration can be obtained by measuring DTPMP concentrations. RM breakthrough curve can be established by plotting the RM effluent concentration vs. PV of RMNF flushed into the column. In this study, the impacts of preflush solution and flow rate were investigated: the preflush solution impact was examined by flushing the calcite column with 4 PV of either 2 M NaCl or isooctane prior to the injection of RMNF; the impact of flow rate was studied by varying the RMNF flow rates (mL h−1) to achieve different linear pore velocities (cm min−1) of RMNF flow inside the calcite column. The impact of flow rate was examined by pumping RMNF at three different flow rates of approximately 120, 60, and 15 mL h−1, corresponding to linear pore velocities of 5.7, 2.9, and 0.7 cm min−1, respectively.
Reverse micelle microemulsion laboratory squeeze simulation tests
Laboratory squeeze simulation tests were carried out to investigate the return behaviour of Ca–DTPMP RM in calcite medium using totally contained squeeze simulation apparatus.7,14,18 A glass column was packed with calcite materials and the dimension of the column was 1.02 cm in ID and 8 cm in length. Initially, the column was preflushed with 1 M NaCl solution for a number of PV. To simulate the field inhibitor injection and subsequent overflush treatment, the calcite-packed column was then loaded with half of a PV of the prepared RMNF, followed by half a PV of 1 M NaCl as overflush solution. Then the column was shut-in for 24 h at 70 °C condition. This shut-in procedure is to allow the injected RM to attach to the surfaces of calcite. Subsequently, the column was flushed with a synthetic brine solution from an opposite direction at 75 psia back pressure, to simulate the field reservoir fluid flowing back after a squeeze treatment. The composition of the synthetic brine is 0.025 M CaCl2, 0.015 M NaHCO3 and 1 M NaCl, sparged with 100% CO2, which composition is in equilibrium with respect to calcite at 70 °C and 75 psia. The flow rate of the synthetic brine return was 60 mL h−1, corresponding to a linear pore velocity of 2.8 cm min−1. The effluent solutions were collected and analyzed for DTPMP concentrations to establish a relation of DTPMP flow back concentration vs. flushing time (pore volumes). In order to examine the impact of overflush fluid on Ca–DTPMP RM return, a similar squeeze simulation test was repeated in a calcite medium packed column (0.66 cm ID and 7 cm length) at 70 °C and 75 psia pressure. The main difference from the previous squeeze test was that the overflush fluid was isooctane instead of 1 M NaCl. The flow rate was 25 mL h−1, corresponding to the same linear pore velocity of 2.8 cm min−1.
Analytical methods
Calcium and DTPMP concentrations were analyzed by inductively coupled plasma-optical emission spectrometer (ICP-OES) (Optima 4300 Dv, Perkin Elmer). Low concentrations of DTPMP were analyzed spectrophotometrically at 890 nm (Hach Co., Loveland, CO.). Spectrophotometric method is able to measure DTPMP as low as 0.12 mg L−1 (ref. 23) (details of phosphonate measurement in the ESI†).
Results and discussion
Transport of reverse micelle in calcite porous medium
As elaborated previously, calcite is the most active mineral in terms of reacting with phosphonate inhibitors in reservoir formation.11,14 Thus, it is expected that Ca–DTPMP RM particles inside RMNF might experience the most hindrance and retardation effect in calcite than in other formation mineral media. In the previous study investigating RMNF transport,18 it was shown that preflush solutions have a considerable impact on inhibitor RM particle transport. An organic preflush solution (isooctane) was determined to be able to enhance the breakthrough level as well as reducing RM particle deposition towards calcite medium surface than preflushing the calcite medium with NaCl aqueous solution. In this study, we further evaluate the factor of flow rate coupled with preflush solution and their impact on RMNF transport behaviour. The objective is to explore the conditions to enhance the transport of RMNF in formation medium so as to improve the efficiency and effectiveness of oilfield scale squeeze by use of the non-aqueous inhibitor nanomaterials for low water cut or water sensitive production wells.
Theories to elucidate particle transport
Similar to the previous study,12–14,18 the breakthrough curve of RMNF in calcite medium was fitted into a one-dimensional advection dispersive equation (1-D ADE) in order to obtain the retardation factor (R) as well as the removal coefficient (Jd). These two factors (R and Jd) are the key to describing the characteristics of submicron scaled particles migration in formation medium.24,25 1-D ADE is in the form of24,25| |
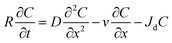 | (1) |
where C (mg L−1) is the effluent RM concentration at a given time; t (min) is the time; x (cm) is the travel distance; D (cm2 min−1) is hydrodynamic dispersion coefficient; and v (cm min−1) is the linear pore velocity. R denotes the retardation factor and Jd (min−1) represents the first order deposition rate coefficient of RM particles to calcite medium surfaces. D value is determined by the calculated dispersivity and pore velocity (details of 1-D ADE and associated parameters explained in the ESI†). R describes the sorption of RM particles to calcite medium surfaces during flow and Jd signifies the deposition of RM particles to calcite surfaces. Furthermore, the mathematical solution to eqn (1) can be obtained for a clean bed filtration model (details in the ESI†). As discussed in the below section, the calculated breakthrough levels of RM particles based on 1-D ADE are very close to the experimentally determined ones for all the transport scenarios considered in this study.
Alternatively, RM particle transport in calcite medium can be elaborated from the standpoint of particle filtration and attachment. In other words, while migrating through the calcite medium, RM particles were continuously removed by the calcite medium as a result of collection and attachment of RM particles to calcite medium.25,26 The parameters of collection efficiency (η0) and an attachment efficiency (α) were provided to characterize the collection and attachment of RM particles, respectively. η0 considers the removal of RM particle by calcite medium via Brownian diffusion, interception and sedimentation. Thus, η0 can be expressed in the form of25,26
where
ηD,
ηI and
ηG are the single collector efficiency components due to Brownian diffusion, interception and sedimentation, respectively.
α can be calculated by
25–27| |
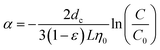 | (3) |
The meaning of each term is that dc is the diameter of the calcite medium (143 μm in this study); L (cm) is the length of the column (8 cm in this study), ε is the calcite medium porosity (0.43 in this study) and C/C0 is the final breakthrough level measured in each transport study. Generally speaking, the higher the η0 value (or α value), the higher the likelihood for the RM particles to be removed by the calcite medium surfaces. The theoretical details of particle filtration and attachment are available in the ESI.† Rearranging eqn (3) can yield the expression of L as a function of η0, α and C/C0. By setting the breakthrough level to 0.01 (i.e., C/C0 = 0.01), a maximum travel distance (LMAX) for RM particles can be defined.25 Therefore, according to eqn (3), LMAX can be expressed as:
| |
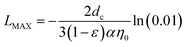 | (4) |
In this study, a number of column flow-through experiments were conducted to evaluate the transport of the Ca–DTPMP RM particles in calcite. The focus on these experiments is to investigate the impacts of flow rate and preflush fluid on RM particle transport. Table 1 lists the conditions of the RMNF transport experiments in calcite medium considered in this study. Tables 2 and 3 show the experimental results. Transport experiments (TE) #1 to #3 were carried out using isooctane as the preflush fluid; where TE #4 to #6 preflushed the column with 2 M NaCl. Among these tests, TE #2 and #5 were reported previously.18 All the transport tests were conducted at 70 °C in calcite medium with a porosity of 0.43.
Table 1 Conditions for column transport experiment
| Transport experiment (TE) |
Preflush fluid |
Flow rate (mL h−1) |
Pore velocity (cm min−1) |
Formation medium |
Formation porosity |
Experimental temperature (°C) |
Dispersion coefficient, Da (cm2 min−1) |
| Note D value is calculated based on the obtained dispersivity and pore velocity (details of D value calculation in the ESI). |
| TE – 1 |
Isooctane |
121.3 |
5.73 |
Calcite for all cases |
0.43 for all cases |
70 for all cases |
0.131 |
| TE – 2 (ref. 18) |
Isooctane |
60.0 |
2.85 |
0.065 |
| TE – 3 |
Isooctane |
15.0 |
0.71 |
0.016 |
| TE – 4 |
2 M NaCl |
122.5 |
5.78 |
0.132 |
| TE – 5 (ref. 18) |
2 M NaCl |
63.4 |
2.99 |
0.068 |
| TE – 6 |
2 M NaCl |
15.2 |
0.72 |
0.016 |
Table 2 Calculation results of RM transport experiments from the standpoint of advection and diffusion
| Transport experiment (TE) |
Preflush fluid |
Flow rate (mL h−1) |
Pore velocity (cm min−1) |
Observed final C/C0 (%) |
Calculated final C/C0 (%) |
Removal coefficient, Jda (min−1) |
Removal coefficient, Jdb (min−1) |
Retardation factor, R |
| The Jd values shown in this column are calculated based on the mathematical solution to eqn (1) with consideration of 1-D ADE.24,25 The Jd values shown in this column are calculated based on eqn (5) with consideration of filtration theory.28 |
| TE – 1 |
Isooctane |
121.3 |
5.73 |
62 |
60 |
0.37 |
0.35 |
2.41 |
| TE – 2 (ref. 18) |
Isooctane |
60.0 |
2.85 |
52 |
48 |
0.26 |
0.24 |
2.34 |
| TE – 3 |
Isooctane |
15.0 |
0.71 |
39 |
37 |
0.09 |
0.08 |
2.32 |
| TE – 4 |
2 M NaCl |
122.5 |
5.78 |
48 |
48 |
0.53 |
0.53 |
2.53 |
| TE – 5 (ref. 18) |
2 M NaCl |
63.4 |
2.99 |
34 |
32 |
0.42 |
0.40 |
2.44 |
| TE – 6 |
2 M NaCl |
15.2 |
0.72 |
30 |
29 |
0.11 |
0.10 |
2.61 |
Table 3 Calculation results of RM transport experiments from the standpoint of filtration and attachment
| Transport experiment (TE) |
Preflush fluid |
Pore velocity (cm min−1) |
Observed C/C0 (%) |
ηD (×10−4) |
ηI (×10−4) |
ηG (×10−4) |
η0 (×10−4) (=ηD + ηI + ηG) |
α (×10−2) |
LMAX (m) |
| TE – 1 |
Isooctane |
5.73 |
62 |
60.9 |
5.5 |
2.0 |
68.5 |
14.9 |
0.76 |
| TE – 2 (ref. 18) |
Isooctane |
2.85 |
52 |
100.5 |
6.1 |
4.3 |
110.9 |
12.3 |
0.56 |
| TE – 3 |
Isooctane |
0.71 |
39 |
271.6 |
7.2 |
20.2 |
299.1 |
6.6 |
0.39 |
| TE – 4 |
2 M NaCl |
5.78 |
48 |
60.6 |
5.5 |
2.0 |
68.1 |
22.3 |
0.51 |
| TE – 5 (ref. 18) |
2 M NaCl |
2.99 |
34 |
96.9 |
6.0 |
4.1 |
107.0 |
21.0 |
0.34 |
| TE – 6 |
2 M NaCl |
0.72 |
30 |
271.6 |
7.2 |
20.2 |
299.2 |
8.3 |
0.31 |
Impact of flow rates on RM particle transport
The flow rate impact on RM transport can be revealed by examining the RM breakthrough curves with the same preflush fluid (Fig. 1 and 2). Table 2 shows that with isooctane as the preflush fluid (TE #1 to #3), an increase in flow rate from 0.7 to 5.7 cm min−1 leads to an increase in the final breakthrough levels from 39% to 62%. This suggests that the RMNF breakthrough level can be elevated by increasing the nanomaterial flow rates inside the calcite column. By evaluating the calculated R values among TE #1 to #3, R varied insignificantly from 2.32 to 2.41. Since R value is a reflection of the sorption of RM particles to calcite surfaces, change in flow rate is not expected to impact the sorption nature of RM particles or the R value considerably. On the other hand, the calculated Jd value varied more substantially from 0.08 to 0.35 with the increase in flow rates. The calculation of Jd value can be derived from eqn (1) and also from the filtration theory via:28| |
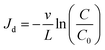 | (5) |
 |
| | Fig. 1 Breakthrough curves of reverse micelle inhibitor nanomaterials at two different pore velocities of 5.73 and 2.85 cm min−1 in calcite medium with isooctane preflush (TE #1 and TE #2). The triangle and square markers represent the experimentally obtained breakthrough levels; while the dashed lines denote the calculated breakthrough levels based on the mathematical solution to eqn (1). | |
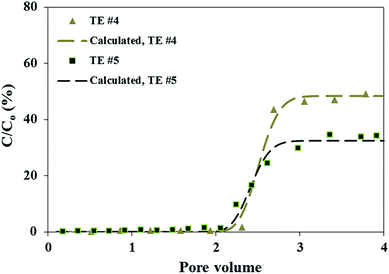 |
| | Fig. 2 Breakthrough curves of reverse micelle inhibitor nanomaterials at two different pore velocities of 5.78 and 2.99 cm min−1 in calcite medium with 2 M NaCl preflush (TE #4 and TE #5). The triangle and square markers represent the experimentally obtained breakthrough levels; while the dashed lines denote the calculated breakthrough levels based on the mathematical solution to eqn (1). | |
The calculated Jd values for TE #1 to #6 based on eqn (1) as well as eqn (5) were list in Table 2. Evidently, these Jd values derived from eqn (1) and (5) are very similar (Table 2), suggesting the agreement on Jd values from the perspectives of ADE and filtration theory and also the validity of these two modeling approaches. According to eqn (5), Jd value is a function of flow velocity (v) and the final breakthrough level (C/C0). Among TE #1 to #3, the increase in the calculated Jd values at a higher flow velocity (Table 2) indicates a higher tendency of RM particles to be removed by the calcite medium surfaces.
From the standpoint of particle collection and attachment, η0 and α values (Table 3) can be calculated based upon the breakthrough levels. For the scenario of isooctane preflushing (TE #1 to #3), ηD is calculated to be much larger than ηI and ηG, thus dominates the overall η0 value. This suggests that Brownian diffusion is the predominate mechanism for RM particles to be collected by calcite surfaces, which observation is in accordance with the previous investigations on inhibitor nanomaterials.13,14 Furthermore, the increase in flow velocity in TE #1 to #3 leads to a reduction in η0 (mainly ηD) value from 299.1 to 68.5 and an increase in α value from 6.6 to 14.9 (Table 3). The reduction in ηD suggests a decrease in the tendency of RM particles to be collected by calcite surfaces. This is because ηD value is a function of the flow rate and the increase in flow rate will result in a lower ηD value (details in the ESI†). As for the α value, the increase in α suggests an increase in the tendency of RM to attach to calcite surfaces. Based on eqn (3), α value is jointly impacted by η0 and breakthrough level. An increase in η0 value or a decrease in the breakthrough level will result in a higher α value. In addition, following the discussion in the previous study18 α value can be impacted by the variation in the flow rate. This suggests that the attachment of RM particles with the calcite medium surfaces is expected to be weak at secondary energy minimum.29
In the same manner as evaluating the experimental results of TE #1 to #3, the flow rate impact can be understood for the scenarios of preflushing with 2 M NaCl (TE #4 to #6). Similar to TE #1 to #3, the increase in flow rates can result in a higher final breakthrough level, even though the increase was less pronounced (from 30% to 48%). Additionally, the increase in flow rates for 2 M NaCl preflush cases leads to an increase in the Jd values from 0.11 to 0.53 and the calculated R values remain relatively constant. From the standpoint of particle collection and attachment, similar to TE #1 to #3, the increase in flow rate leads to a reduction in η0 and an increase in α. Again, ηD dominates the overall calculated η0 value.
The significance of the flow rate impact on scale inhibitor transport in formation, either in the form of conventional aqueous pill or nanomaterials is two folds: firstly, from an operation perspective, the inhibitor flow rate during an oilfield squeeze job can be controlled by the pump injection rate set by the operator. Based on the aforementioned experimental results, it is suggested that the transport of the inhibitor RM nanomaterial can be enhanced by employing a higher inhibitor injection rate; secondly, considering the nature of the reservoir formation, different formation layers can have different intrinsic permeabilities. A higher formation permeability will allow the injected RM particles to travel longer distance with an enhanced breakthrough compared with transport in low permeable formation layers. Because of the difference in formation permeability, the injected inhibitors with the same injection rate can travel varying distances inside different layers of formation. Finally, the impact of flow rate can be delineated from the calculated LMAX values. As defined previously, LMAX corresponds to the travel distance when 99% of the RM particles are attached to the calcite medium surfaces. Therefore, a prolonged LMAX signifies an enhanced migration of the RM particles inside the formation. As expected, the increase in flow rate can result in an increase in the calculated LMAX value. This statement holds true for both scenarios of isooctane and NaCl preflushing. As shown in Table 3, the calculated LMAX in this study (TE #1 to #6) varied from 0.31 m to 0.76 m (or ca. 1 to 2 ft). The significance of the calculated LMAX can be elaborated from the viewpoint of surface coating of near wellbore formation by RMNF. The longer the travel distance, the larger wellbore formation surfaces can be surface coated by inhibitor RM particles. During resumed production, the retained inhibitor can gradually dissolve into the incoming production brine flowing towards the production well to control scale formation. In other words, the prepared Ca–DTPMP RM can serve as a delivery vehicle to effectively deliver the DTPMP inhibitor into low water cut or water sensitive formation for mineral scale control.
Impact of preflush solution
Preflush the calcite medium can remove the aqueous brine previously stored in the pore space of the calcite column and also coat the calcite medium surfaces with the selected preflush solution.21 As discussed in greater details previously,18 the preflush solution can substantially impact the RM particle transport, particularly comparing organic versus aqueous preflush solutions. The conclusion is that enhancement of RM transport in calcite medium by preflushing calcite with isooctane compared with NaCl aqueous brine is believed to be attributed to the destabilizing effect of aqueous preflush brine on the micellar structure of the RM particles. Such argument can be expanded to explain the preflush effect at different flow rates considered in this study. In each flow rate (pore velocity) of interest, the measured final breakthrough level is higher for the case with isooctane preflush compared with NaCl preflush (Table 2). This can be observed from Fig. 3 and also from comparing Fig. 1 and 2. Correspondingly, the calculated Jd values are lower for isooctane preflush cases than their counterparts with NaCl preflush (Table 2). This verifies that the enhancement of RM transport with isooctane preflush is applicable for conditions with varying pore velocities evaluated in this study.
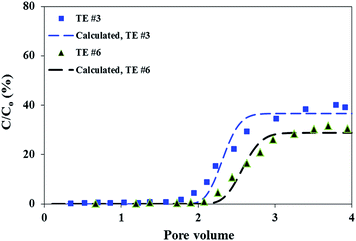 |
| | Fig. 3 Breakthrough curves of reverse micelle inhibitor nanomaterials at a similar pore velocity of ca. 0.7 cm min−1 in calcite medium with preflush solution of either isooctane or 2 M NaCl (TE #3 and TE #6). The triangle and square markers represent the experimentally obtained breakthrough levels; while the dashed lines denote the calculated breakthrough levels based on the mathematical solution to eqn (1). | |
Laboratory inhibitor reverse micelle nanofluid squeeze simulation
As for an oilfield squeeze operation, it is important to understand the release of the injected inhibitor post the squeeze treatment (inhibitor return behavior). Evaluation of inhibitor return behavior involves monitoring the variation of inhibitor return concentration vs. time and also the total elapsed time before inhibitor return concentration becomes too low (squeeze lifetime). Once the inhibitor return concentration drops below the threshold level, another squeeze campaign needs to be undertaken to maintain the inhibitor return concentration. The frequency of the squeeze job is directly related to the squeeze lifetime. A longer squeeze time is always desirable since less frequent squeeze treatment suggests of an improved project economics and a reduced project threat associated with performing squeeze jobs.1,5 Laboratory scale squeeze simulation test is designed to simulate the oilfield scale squeeze operation by including different stages of preflush, inhibitor injection, overflush, shut-in and resumed production. The resumed production stage is simulated by eluting the calcite column with a synthetic brine at the testing condition of 70 °C and 75 psia back pressure. The synthetic brine is in equilibrium with calcite at the testing condition so that no calcite solid will be precipitated or dissolved during the course of the experiment. The laboratory squeeze simulation test procedure is depicted in Fig. 4. Squeeze simulation test can provide useful information concerning the return behavior of the injected inhibitor chemicals. The key of the squeeze simulation tests in this study is to evaluate the impact of overflush on the return behavior of RM inhibitor nanomaterials. Particularly, the result of overflushing with an organic solvent (isooctane) will be compared with that using a NaCl aqueous overflush brine. Two separate squeeze simulation tests were carried out by overflushing the calcite columns with either isooctane or 1 M NaCl. 1 M NaCl solution is chosen to represent the common aqueous overflush fluid employed in oilfield operations.2 For comparison, a previous squeeze experiment (SE) evaluating a conventional aqueous DTPMP pill overflushed by 1 M NaCl (SE #1 in Table 4)10 was included and compared against the RM returns (SE #2 and 3) in this study. According to conditions of these squeeze studies (Table 4), all three experiments were conducted by eluting the calcite column with a synthetic brine of the same composition at a comparable pore velocity (2.8 cm min−1) at 70 °C. SE #2 was discussed in the previous study.18 A DTPMP return profile for each squeeze simulation study is plotted as the DTPMP concentration in the effluent samples as a function of the PV of the synthetic brine returned. According to the plotted return profiles (Fig. 5), all three studies share a similar pattern of DTPMP return: a peak DTPMP return concentration was achieved just after a few PV and the return concentration entered sub mg L−1 region after ca. 700 PV and drop below the detection limit of 0.12 mg L−1 by another few hundreds of PV.
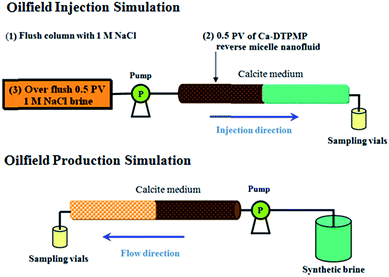 |
| | Fig. 4 Schematic diagram of laboratory squeeze simulation test including injection simulation and production simulation stages. | |
Table 4 Summary of physiochemical conditions of each squeeze simulation test in calcite medium
| Squeeze experiment (SE) |
Form of inhibitor added |
Overflush fluid |
Synthetic brine compositions |
Synthetic brine pH |
Squeeze temperature (°C) |
Flow rate (mL h−1) |
Pore velocity (cm min−1) |
PV (mL) |
| SE – 1 (from ref. 10) |
Acidic pill |
1 M NaCl |
0.025 M CaCl2, 0.015 M NaHCO3, 1 M NaCl and sparged with 100% CO2 |
5.54 for all cases |
70 for all cases |
90 |
2.8 |
8 |
| SE – 2 (from ref. 18) |
RMNF |
1 M NaCl |
60 |
2.8 |
3 |
| SE – 3 (this study) |
RMNF |
Isooctane |
25 |
2.8 |
1 |
 |
| | Fig. 5 Squeeze simulation tests using reverse micelle inhibitor nanomaterials in calcite medium. Three DTPMP return profiles were included: SE#1 using conventional DTPMP pill;10 SE #2 using reverse micelle nanofluid with isooctane overflush;18 SE #3 using reverse micelle nanofluid with 1 M NaCl overflush. (a) is the full return profile and (b) is a part (up to 100 PV) of the main return profile. | |
As summarized in Table 5, the return volumes of these three studies are 870, 1316 and 1015 PV, respectively for conventional pill and two RM tests. Hence, it can be concluded that (1) RM nanomaterials return curves are similar to the shape of the conventional pill with an enhanced squeeze lifetime; (2) by comparing the two RM return profiles, it seems that overflushing with 1 M NaCl aqueous brine outperformed that overflushed by isooctane in terms of squeeze lifetime. The return concentrations within the first 100 PV among the three tests are detailed in Fig. 5b. In general, the DTPMP return concentrations within the first 100 PV are in the order of acidic pill > RM with isooctane overflushing > RM with 1 M NaCl overflushing. This can explain the observed return profiles of interest in that a higher return concentration in the early phase of return test indicates of losing a larger amount of DTPMP early in the test, leading to a reduced squeeze lifetime. As discussed previously,18 inhibitor RM contains pre-formulated Ca–DTPMP complexes dissolved in the aqueous domain inside the micellar system and these Ca–DTPMP complexes will attach to the calcite surface during shut-in period following the overflush treatment. Compared with RM, conventional pill injection will leave a considerable amount of aqueous DTPMP in the calcite medium pore space after shut-in and these aqueous DTPMP compounds will be rapidly flushed out of the column following the onset of synthetic brine return. On the other hand, the difference between two RM returns can be elaborated from the standpoint of adsorption–desorption dynamics and the difference in the role of aqueous and non-aqueous overflush fluids in displacing DTPMP inhibitors in formation medium. Jordan et al.22 compared the effects of using diesel as the overflush fluid against the aqueous overflush fluid via field observation and simulation studies. These authors demonstrated that aqueous overflush fluid can desorb inhibitor which was previously adsorbed by formation during inhibitor injection and subsequently displace the desorbed inhibitor deeper into formation so that the desorbed inhibitor can re-adsorb farther away from the well. Diesel overflush, on the other hand, will not desorb and displace the previously adsorbed inhibitor. Such statement can be verified by modeling the overflush impact on squeeze lifetime using a two-phase simulator.6 As in this study, it is speculated that, during the overflush treatment, aqueous overflush can desorb the previously attached Ca–DTPMP RM from calcite surfaces back into the aqueous phase and subsequently push them farther along the flowing direction towards the other half of the calcite column. The desorbed RM will re-adsorb and attach onto calcite surfaces. This will allow a larger fraction of the injected RM nanomaterials attached onto the calcite surfaces, compared with the scenario of isooctane overflush. This argument correlates well with the observed return data within 100 PV (Fig. 5b) where SE #2 (aqueous overflush) showed a lower initial return concentration within the first 100 PV, suggesting a smaller fraction of the injected inhibitor being eluted out of the column. Eventually, the lower initial inhibitor return in SE #2 resulted in a longer squeeze lifetime compared with SE #3 (isooctane overflush) as shown in Table 5.
Table 5 Summary of the experimental results of each squeeze simulation test in calcite medium
| Squeeze experiment (SE) |
Form of inhibitor added |
Overflush fluid |
DTPMP injecteda (mg) |
Percent of inhibitor returnedb (%) |
Total volume returnedc (PV) |
Initial pIP |
Final pIP |
NSLd (bbl kg−1) |
Protection volumee (bbl) |
| DTPMP injected is the amount of DTPMP in RMNF injected prior to the onset of the squeeze simulation test. Percent of inhibitor returned is the ratio of the mass of inhibitor returned by the end of the squeeze simulation test to the mass of inhibitor injected initially. Total volume returned is the total volume of synthetic brine eluted during the squeeze simulation test. NSL stands for normalized squeeze lifetime and is a reflection of the barrels of brine treated per kilogram of inhibitor added. Protection volume is the estimated volume of production brine protected by injecting 1000 kg of DTPMP equivalent RMNF in a single squeeze treatment. |
| SE – 1 (from ref. 10) |
Acidic pill |
1 M NaCl |
32 |
87 |
870 |
50.4 |
53.9 |
1370 |
1.4 × 106 |
| SE – 2 (from ref. 18) |
RMNF |
1 M NaCl |
8.6 |
91 |
1316 |
50.8 |
53.7 |
2900 |
2.9 × 106 |
| SE – 3 (this study) |
RMNF |
Isooctane |
2.6 |
88 |
1015 |
50.8 |
54.0 |
2500 |
2.5 × 106 |
DTPMP return profiles can be investigated by calculating the negative logarithm of ion activity product (pIP) throughout the duration of squeeze simulation test at a similar experimental condition (1 M NaCl, 5.5 pH and 70 °C) (details in the ESI†).30 Fig. 6 shows that during the course of the simulation test, the calculated pIP values of the three squeeze tests gradually increase from initially ca. 50 to ca. 54 by the end of the test. According to Tomson et al.,30 the increase of the calculated pIP from 50 to ca. 54 is an indication of the morphology change of the Ca–DTPMP complex from initially an amorphous phase into a crystalline phase. The crystalline phase has a much lower Ca–DTPMP solid solubility, which is responsible for the low DTPMP return concentration post 700 PV in the RM return tests. Finally, the significance of the RM nanomaterials and their potential field application can be evaluated by calculating the normalized squeeze lifetime (NSL, i.e., barrels of brine treated per kilogram of inhibitor added) and also inhibitor protection volume.31 NSL evaluates the squeeze lifetime on the same inhibitor mass basis by calculating the ratio of experimentally obtained return volumes and the mass of inhibitor injected. Protection volume is the estimated volume of production brine protected by injecting RMNF containing 1000 kg of DTPMP in a single squeeze treatment (details of NSL and protection volume calculations are in the ESI†). Based on the calculations for SE #1 to #3, it is evident that RM outperformed conventional pill in terms of the calculated NSL and protection volume; and aqueous overflush treatment can enhance the RM squeeze lifetime compared with the RM squeeze with isooctane overflush. The protection time of each squeeze treatment will also depend on the daily water production rates of the production well of interest. As for low water cut wells, the water production rates account for a small fraction of the total fluid production rates. Therefore, the total volume of protected production fluids (hydrocarbons and water) can be considerably larger than the volume of produced water protected as shown in Table 5. It should be noted that for certain low water cut and water sensitive wells, execution of an aqueous overflush might induce formation impairment or even formation damage due to formation incompatibility with aqueous brines. Thus, precautions should be taken in injecting aqueous fluids into water sensitive formation during a squeeze job.
 |
| | Fig. 6 The calculated negative logarithm of ion activity product of three return profiles. The small inserted figure at the lower right corner is a part (up to 100 PV) of the main figure. | |
Conclusions
In the present study, Ca–DTPMP inhibitor reverse micelle nanomaterials were evaluated in laboratory column transport and squeeze simulation studies. Preflush and overflush effects were investigated for their roles in nanomaterial transport and return performance. It is evident that flow rate can impact the inhibitor nanomaterial transport in calcite medium and an increase in flow rate can lead to an enhanced breakthrough level and also a reduction in the tendency of RM particles to be collected by calcite medium. The retardation factor is calculated to be less sensitive to flow rate variation; while the removal coefficient is highly depended on flow rates. Isooctane preflush can enhance the nanomaterial transport compared with aqueous brine preflush at different flow rates (pore velocities) evaluated in this study. Squeeze simulation studies suggest that inhibitor reverse micelle nanomaterials show an improved inhibitor return performance compared with the conventional pill. It has been observed that aqueous brine overflush can enhance reverse micelle return performance compared with isooctane overflush. This is believed to be a result of desorption of previously attached inhibitor nanomaterial from calcite surface and subsequent re-adsorption of these materials deeper in calcite medium. During the course of squeeze simulation test, the Ca–DTPMP complex inside the inhibitor reverse micelle developed from an amorphous phase into a crystalline phase with a much lower solubility, accounting for an extended squeeze lifetime. Furthermore, the potential field application of the inhibitor reverse micelle nanomaterial can be assessed by calculating the normalized squeeze lifetime and the protection volume of the produced brine.
Acknowledgements
The authors would like to acknowledge the financial support by Brine Chemistry Consortium including Baker Hughes, BWA, CARBO, Cenovus, Chevron, ConocoPhillips, Dow, EOG Resources, GE, Hess, Halliburton, Italmatch, Kemira, Kinder Morgan, Lubrizol, Marathon Oil, NALCO Champion, Occidental, Petrobras, RSI, Saudi Aramco, Schlumberger, Shell, SNF, Statoil and Total. This work was supported by the NSF Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (ERC-1449500).
Notes and references
- J. W. Mullin, Crystallization, Butterworth Heinemann, Oxford, 4th edn, 2001 Search PubMed.
- J. Fink, Petroleum Engineer's Guide to Oil Field Chemicals and Fluids, Waltham, MA, 2012 Search PubMed.
- P. Zhang, K. Allan and H. Bourne, Selection of Calcium Carbonate Scale Critical Values for Deepwater Production, SPE 173747, in SPE International Symposium on Oilfield Chemistry, The Woodlands, Texas, USA, 2015 Search PubMed.
- M. A. Kelland, Production Chemicals for the Oil and Gas Industry, CRC Press, Boca Raton, FL, 2nd edn, 2014 Search PubMed.
- W. W. Frenier and M. Ziauddin, Formation, Removal, and Inhibition of Inorganic Scale in the Oilfield Environment, Richardson, TX, USA, 2008 Search PubMed.
- O. Vazquez, E. J. Mackay and M. M. Jordan, Modeling the Impact of Diesel vs. Water Overflush Fluids on Scale-Squeeze-Treatment Lives Using a Two-Phase Near-Wellbore Simulator, SPE Prod. Oper., 2009, 24, 473–480 CrossRef CAS.
- M. B. Tomson, A. T. Kan and G. Fu, Control of Inhibitor Squeeze via Mechanistic Understanding of Inhibitor Chemistry, SPE J., 2006, 11, 283–293 CrossRef CAS.
- K. S. Sorbie and E. J. Mackay, Scale Inhibitor Placement: Back to Basics – Theory and Examples, SPE 95090, in SPE International Symposium on Oilfield Scale, Aberdeen, U.K., 2005 Search PubMed.
- M. B. Tomson, A. T. Kan, G. Fu, D. Shen, H. A. Nasr-El-Din, H. Al-Saiari and M. Al-Thubaiti, Mechanistic Understanding of Rock/Phosphonate Interactions and the Effect of Metal Ions on Inhibitor Retention, SPE J., 2008, 13, 325 CrossRef CAS.
- D. Shen, P. Zhang, A. T. Kan, G. Fu, J. Farrell, and M. B. Tomson, Control Placement of Scale Inhibitors in the Formation With Stable Ca–DTPMP Nanoparticle Suspension and its Transport in Porous Media, SPE 114063, in SPE International Oilfield Scale Conference, Aberdeen, UK, 2008 Search PubMed.
- P. Zhang, D. Shen, C. Fan, A. T. Kan and M. B. Tomson, Surfactant-Assisted Synthesis of Metal Phosphonate Inhibitor Nanoparticles and Their Transport in Porous Media, SPE J., 2010, 15, 610–617 CrossRef CAS.
- P. Zhang, A. T. Kan, C. Fan, S. Work, J. Yu, H. Lu, H. A. Al-Saiari and M. B. Tomson, Silica-Templated Synthesis of Zinc–DTPMP Nanoparticles, their transport in carbonate and sandstone porous media and scale inhibition, SPE J., 2011, 16, 662–671 CrossRef CAS.
- P. Zhang, C. Fan, H. Lu, A. T. Kan and M. B. Tomson, Synthesis of the crystalline phase silica-based Ca–phosphonate nanomaterials and their transport in carbonate and sandstone porous media, Ind. Eng. Chem. Res., 2011, 50, 1819–1830 CrossRef CAS.
- P. Zhang, A. T. Kan and M. B. Tomson, Enhanced transport of novel crystalline calcium phosphonate scale inhibitor nanomaterials and their long term flow back performance in laboratory squeeze simulation tests, RSC Adv., 2016, 6, 5259–5269 RSC.
- C. Yan, A. T. Kan, W. Wang, F. Yan, L. Wang and M. B. Tomson, Sorption Study of γ-AlO(OH) Nanoparticle-Crosslinked Polymeric Scale Inhibitors and Their Improved Squeeze Performance in Porous Media, SPE J., 2014, 19, 687–694 CrossRef.
- D. Kumar, S. S. Chishti, A. Rai and S. D. Patwardhan, Scale Inhibition Using nano-silica Particles, SPE 149321, in SPE Middle East Health, Safety, Security, and Environment Conference and Exhibition, Abu Dhabi, UAE, 2012 Search PubMed.
- Z. Kiaei and A. Haghtalab, Experimental study of using Ca–DTPMP nanoparticles in inhibition of CaCO3 scaling in a bulk water process, Desalination, 2014, 338, 84–92 CrossRef CAS.
- P. Zhang, D. Shen, A. T. Kan and M. B. Tomson, Synthesis and laboratory testing of a novel calcium–phosphonate reverse micelle nanofluid for oilfield mineral scale control, RSC Adv., 2016, 6, 39883–39895 RSC.
- J. J. Wylde, The advent of a truly oil soluble scale inhibitor squeeze chemical, NACE 04384, in NACE Annual Corrosion Conference & Exposition, New Orleans, LA, 2004 Search PubMed.
- M. M. Jordan, T. E. Kramer, D. Barbin, S. Linares-Samaniego and W. Arnette, Scale Inhibitor Squeeze Treatments Selection, Deployed and Monitoring in a Deepwater Gulf of Mexico Oilfield, SPE 146400, in SPE Annual Technical Conference and Exhibition, Denver, Colorado, 2011 Search PubMed.
- O. Vazquez, E. Mackay and K. Sorbie, A two-phase near-wellbore simulator to model non-aqueous scale inhibitor squeeze treatments, J. Pet. Sci. Eng., 2012, 82, 90–99 CrossRef.
- M. M. Jordan, E. J. Mackay and O. Vazquez, The Influence Of Overflush Fluid Type On Scale Squeeze Life Time – Field Examples And Placement Simulation Evaluation, NACE International, New Orleans, Louisiana, 2008 Search PubMed.
- A. T. Kan, J. E. Oddo and M. B. Tomson, Formation of Two Calcium Diethylenetriaminepentakis(methylene phosphonic acid) Precipitates and Their Physical Chemical Properties, Langmuir, 1994, 10(5), 1450 CrossRef CAS.
- R. J. Charbeneau, Groundwater Hydraulics And Pollutant Transport, Waveland Press, Long Grove, IL, 1st edn, 2006 Search PubMed.
- M. M. Clark, Transport Modeling for Environmental Engineers and Scientists, John Wiley & Sons, Hoboken, NJ, 2nd edn, 2009 Search PubMed.
- J. N. Ryan and M. Elimelech, Colloid mobilization and transport in groundwater, Colloids Surf., A, 1996, 107, 1–56 CrossRef CAS.
- P. B. Bedient, H. S. Rifai and C. J. Newell, Ground Water Contamination: Transport and Remediation, Prentice Hall, 2nd edn, 1999 Search PubMed.
- L. W. de Jonge, C. Kjaergaard and P. Moldrup, Colloids and colloid-facilitated transport of contaminants in soils: An introduction, Vadose Zone J., 2004, 3(2), 321–3258 CAS.
- D. F. Evans and H. Wennerström, The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet, Wiley-VCH, 2nd edn, 1999 Search PubMed; M. M. Clark, Transport Modeling for Environmental Engineers and Scientists, John Wiley & Sons, Hoboken, NJ, 2nd edn, 2009 Search PubMed.
- M. B. Tomson, A. T. Kan and J. E. Oddo, Acid/Base and Metal Complex Solution Chemistry of the Polyphosphonate DTPMP versus Temperature and Ionic Strength, Langmuir, 1994, 10, 1442–1449 CrossRef CAS.
- A. T. Kan, G. Fu, D. Shen, H. Al-Saiari and M. B. Tomson, Enhanced Scale-Inhibitor Treatments With the Addition of Zinc, SPE J., 2009, 14, 617–626 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra07445f |
| ‡ Presently with Baker Hughes, Inc. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.