Exterior and small carbide particle promoted platinum electrocatalyst for efficient methanol oxidation
Zaoxue Yan*a,
Lina Gaoa,
Xinhong Zhaob,
Wei Weia,
Jimin Xie*a,
Pei Kang Shen*c and
Fen Zhua
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: yanzaoxue@163.com; xiejm391@sohu.com; Fax: +86 11 88791800; Tel: +86 11 88791708
bSchool of Mechanical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China
cCollaborative Innovation Center of Sustainable Energy Materials, Guangxi University, Nanning, Guangxi 530004, P. R. China. E-mail: pkshen@gxu.edu.cn
First published on 8th July 2016
Abstract
Carbides have a synergistic effect on noble metal based electrocatalysts. However, carbides usually have large particles or are wrapped in an inert carbon block, which greatly reduces their interaction with other loaded functional particles. Typically in this paper, we report the synthesis of tungsten carbide particles with a diameter of no more than 10 nm on the exterior surface of carbon spheres (denoted as WC-on-CS) with the help of surfactant as dispersant. Pt particles are then loaded on the material to form a Pt/WC-on-CS electrocatalyst. The above materials are characterized by XRD, SEM, TEM and electrochemical measurements. The results indicate that the exterior and small WC particles have a much higher promotion effect on the Pt electrocatalyst than carbon-wrapped WC particles for methanol oxidation reaction (MOR). The exterior and small WC particles have more exposed surfaces and closer contact with the loaded Pt particles, leading to excellent MOR activity and stability.
1. Introduction
Carbides such as tungsten carbide (WC),1–4 molybdenum carbides (Mo2C and MoC),5–7 vanadium carbide (V8C7),8,9 iron carbide (Fe3C),10 titanium carbide (TiC),11 silicon carbide (SiC),12,13 zirconium carbide (ZrC),14 and bimetallic carbides (Fe2MoC,15 Co3W3C,16 Co3ZnC (ref. 17) and Co6Mo6C2 (ref. 18)) have been widely studied and applied as catalysts, catalyst supports, and electrode, mechanical and coating materials. In the electrochemical field, carbides show a synergistic effect on noble metal electrocatalysts due to electron transfer between carbides and metals, which improves both electrocatalytic activity and the stability of the electrocatalysts.19–31 The compounding of carbides and noble metals can reduce the loading of noble metals while maintaining or even improving the electrocatalytic activity and stability, which in turn reduces the cost of electrocatalysts. Therefore, carbide promoted noble metal catalysts have been widely applied to the electro-oxidation of urea,20 methanol29 and ethanol32 as well as the electro-reduction of oxygen23 and hydrogen evolution22 reactions.To exert the full promotion effect of the carbide on the noble metal, the carbide and the noble metal should have close contact. To obtain this aim, carbide particles should have small sizes and not be embedded in other inert materials. There have been many methods to synthesize carbides with nanometer sizes. Oh et al. synthesized WC layers with a thickness of about 10 nm by heating a mixture of platelet type-carbon fibers and tungsten hexa-carbonyl (W(Co)6) in N2 flow at 200 °C.32 Sullivan et al. synthesized 2–5 nm Mo2C particles by reducing ammonium paramolybdate with CH4 and H2 at 600 °C.33 Alekseev et al. synthesized mesoporous SiC with the diameter of 11 nm by using SiO2 nanoparticles as a template for nanocasting and decomposing polycarbosilane as a replica precursor.34 Uniformly dispersed WC, V8C7, Mo2C and MoC particles with controllable diameters of 0.8–10 nm were synthesized through ion exchange methods.35–38 However, most carbide particles were wrapped by carbon block, reducing their contact with the loaded metals. To load small-sized carbide particles on exterior surface of carbon matrix is of great significance.
In this paper, we report synthesis of WC particles on exterior surface of carbon spheres with the WC diameter of no more than 10 nm. Electrochemistry measurements show that exterior WC particles have higher promotion effect on Pt electrocatalyst than embedded WC particles for methanol oxidation reaction (MOR).
2. Experimental
2.1 Synthesis of WC-on-CS, WC-in-CS, CS and WC-on-CS-without-PVA
Surfactants are usually adopted to control particle sizes, shapes and pore sizes of nanomaterials through changing the kind and concentration of surfactant, leading to improved physical, chemical or electrochemical properties.39 Herein, PVA surfactant is used to control carbide particle sizes and distributions.2.2 Preparation of Pt electrocatalysts (Pt/WC-on-CS, Pt/WC-in-CS and Pt/CS)
Before preparation, WC-on-CS and WC-in-CS were firstly treated in 5 mol L−1 HCl solution and washed with deionized water to remove the W metal and W2C impurities. Then, 50 mg of the dried WC-in-CS (or WC-on-CS or CS) was added into a mixture of H2PtCl6 (4.44 mL, containing 33.3 mg Pt) and 20 mL glycol (AR, Tianjin Fuyu Fine Chemicals Co., Ltd, China) with sonication for 30 min to form a uniform ink. The pH of the mixture was adjusted to 10 using 1 mol L−1 NaOH/glycol solution (AR, Sinopharm Chemical Reagent Co., Ltd, China). The sample was then placed into a microwave oven (1000 W, 2450 MHz) for heating treatment with a 10 s on and 5 s off procedure for 20 times. The product was finally washed for 5 times and dried in vacuum at 80 °C for 2 h. The Pt particles loading on the WC-on-CS, WC-in-CS and CS were denoted as Pt/WC-on-CS, Pt/WC-in-CS and Pt/CS respectively. The Pt contents in the electrocatalysts were targeted at 40 wt% stoichiometricly. The actual Pt contents were determined by inductively coupled plasma-atomic emission spectrometry (ICP, IRIS(HR), USA).2.3 Electrochemical characterization
Pt/WC-on-CS (or Pt/WC-in-CS or Pt/CS, 5 mg) or commercial Pt/C (4 mg, 47.6 wt% Pt, TKK, Japan) was dispersed in 1.95 mL ethanol and 0.05 mL 0.5 wt% Nafion suspension (DuPont, USA) under ultrasonic agitation to form electrocatalyst ink. The ink (5 μL) was deposited on surface (0.25 cm2) of a glassy carbon rod and dried at room temperature. The Pt loading was 0.02 mg cm−2. The electrochemical measurements were performed in a three-electrode cell on a potentiostat (IM6e, Zahner-Electrik, Germany) at 30 °C controlled by a water-bath thermostat. A platinum foil (1.0 cm2) and a saturated calomel electrode (SCE) were used as counter and reference electrodes, respectively.All chemicals were of analytical grade and used as received.
2.4 Physical characterization
The morphologies and particle sizes of the materials were characterized by transmission electron microscopy (TEM, JEM-2010HR, JEOL Ltd., Japan) operating at 200 kV. The structures of the samples were determined on an X-ray diffractometer (XRD, D/Max-IIIA, Rigaku Co. Ltd, Japan, CuKα, λ = 1.54056 Å radiation) operating at 40 kV and 30 mA at a scan rate of 10° min−1.3. Results and discussion
Fig. 1 shows XRD patterns of the as-synthesized WC-on-CS, WC-in-CS, CS and WC-on-CS-without-PVA. The diffraction peaks at 2θ of 31.5°, 35.6° and 48.3° correspond to (001), (100) and (101) facets of WC. The peaks at 2θ of 34.5°, 38.0° and 39.6° were assigned to (100), (002) and (101) facets of W2C. The peaks at 2θ of 40.3°, 58.3° and 73.2° correspond to (001), (100) and (101) facets of W metal. There are other minor but obvious peaks that also belong to W, WC or W2C. The WC particle sizes can be calculated according to the well-known Scherrer's eqn (1):
D = Kλ/(B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
Fig. 2 shows SEM and TEM images of the as-synthesized materials. The WC-on-CS is composed of uniform spheres (Fig. 2a). Each sphere has uniformly dispersed WC particles that are loaded on exterior surface of carbon (Fig. 2b and its upper inset), which would contact the loaded Pt particles closely. The WC particle size distribution (lower inset of Fig. 2b) based on 100 WC particles randomly selected shows that the average WC particle diameter in the WC-on-CS is 9.0 nm. The WC in the WC-in-CS also has uniform distribution (Fig. 2c), yet the WC particles are obviously embedded in carbon block (upper inset of Fig. 2c), which would have difficulty in interacting with the loaded Pt particles. The average WC particle diameter in the WC-in-CS is calculated as 18.0 nm (lower inset of Fig. 2c). However, the WC-on-CS-without-PVA has seriously agglomerated particles (Fig. 2d), owing to the lack of PVA dispersant. The WC particle sizes obtained from these TEM results are all consistent with the XRD results (Fig. 1).
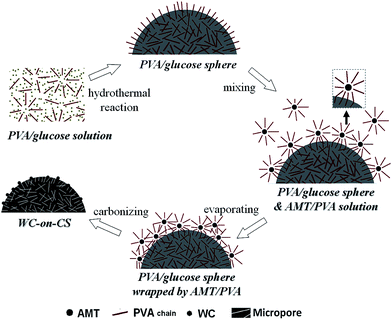
The synthesis procedure of the WC-on-CS is schematically illustrated in Fig. 3. The first step is to mix glucose, water and PVA for hydrothermal reaction. The PVA chains are partially embedded into the synthesized glucose sphere, and form PVA/glucose spheres. The second step is to mix the synthesized PVA/glucose spheres, AMT and another PVA solution. AMT particles are separated by PVA chains that are both in solution and in surface of PVA/glucose spheres. The partially embedded PVA chains in glucose spheres are favorable for the uniform dispersion of AMT particles on surface of glucose spheres. The third step is to dry the mixed solution with violent stirring and sonication, while the PVA/glucose spheres are uniformly wrapped by AMT particles and PVA chains. The last step is to carbonize the dried mixture and form the WC-on-CS sample. At the same time, PVA is partially decomposed to form micropores.
Pt particles were loaded on the WC-on-CS, WC-in-CS and CS to form electrocatalysts. Fig. 4 shows XRD patterns of the electrocatalysts. The diffraction peaks at 2θ of 39.8°, 46.2° and 67.5° correspond to (111), (200) and (220) facets of Pt. It is difficult to calculate the exact particle size since the peaks of WC and Pt overlap. If roughly substrates the background of WC, the average particle sizes of Pt in both Pt/WC-on-CS and Pt/WC-in-CS are calculated as 2.5–3.5 nm.
The TEM and HRTEM images of the typical Pt/WC-on-CS are displayed in Fig. 5. Fig. 5a shows uniform dispersion of the Pt particles. Due to the different contrasts of WC and Pt, the particles with the diameters of around 3 nm can be discerned and should be Pt. The histogram (inset of Fig. 5a) indicates a Gaussian distribution of the Pt particle sizes with the average diameter of 3.0 nm based on 100 Pt particles randomly selected. The Pt size is consistent with the XRD result (Fig. 4). The HRTEM image (Fig. 5b) clearly displays the Pt (111) and WC (100) lattices, proving the coexistence of Pt and WC.
 | ||
| Fig. 5 (a) TEM image of the Pt/WC-on-CS, inset is the Pt particle size distribution, (b) HRTEM image of the Pt/WC-on-CS. | ||
Fig. 6a shows cyclic voltammogram (CV) curves of MOR on the Pt/WC-on-CS, Pt/WC-in-CS, Pt/CS and commercial Pt/C electrodes in 0.5 mol L−1 H2SO4/1.0 mol L−1 methanol solution. The peak current densities and onset potentials obtained from Fig. 6a are listed in Table 1. The results show that the peak current densities are in the following order: Pt/WC-on-CS (1927 mA mgPt−1) > Pt/WC-in-CS (1020 mA mgPt−1) > Pt/C (761 mA mgPt−1) > Pt/CS (695 mA mgPt−1). The onset potentials are in the following order: Pt/WC-on-CS (+0.25 V) < Pt/WC-in-CS (+0.33 V) < Pt/C (+0.40 V) = Pt/CS (+0.40 V). Both the Pt/WC-on-CS and Pt/WC-in-CS have higher peak current densities and lower onset potentials than the Pt/CS and Pt/C, indicating promotion effect of WC on Pt. Moreover, the Pt/WC-on-CS is 1.89 times the peak current density of the Pt/WC-in-CS, indicating that the exterior WC particles have higher promotion effect than the embedded WC particles. Moreover, the peak current density on the Pt/WC-on-CS is 2.53 times that on the Pt/C, and the onset potential of the former is negatively shifted 150 mV than the latter. This is a significant improvement since a direct methanol fuel cell gives only less than 0.5 V output at reasonable current density, leading to an expected 30% improvement in electric efficiency. The results mean promising economic value of the Pt/WC-on-CS.
| Electrocatalyst | Pt mass content | Peak current density (mA mgPt−1) | Onset potential (V) | EASA (m2 gPt−1) |
|---|---|---|---|---|
| Pt/WC-on-CS | 35.6% | 1927 | 0.25 | 61.1 |
| Pt/WC-in-CS | 35.8% | 1020 | 0.33 | 60.0 |
| Pt/CS | 36.2% | 695 | 0.40 | 59.5 |
| Pt/C | 47.6% | 761 | 0.40 | 56.2 |
Fig. 6b compares the ratios of current densities on the Pt/WC-on-CS and Pt/WC-in-CS to those on the Pt/C. The Pt/WC-on-CS shows much advantage at lower potentials. Typically, the ratio of the current density on the Pt/WC-on-CS to that on the Pt/C is 7.5 at 0.43 V, while the ratio of the current density on the Pt/WC-in-CS to that on the Pt/C is only 2.5 at the same potential.
Fig. 6c compares the CV curves on different electrodes in 0.5 mol L−1 H2SO4 solution without methanol. The electrochemical active surface areas (EASAs) can be calculated by assuming that the hydrogen adsorption quantity of electric charge for smooth polycrystalline Pt is 2.10 C m−2. The EASA results are summarized in Table 1. They are in the following order: Pt/WC-on-CS (61.1 m2 gPt−1) > Pt/WC-in-CS (60.0 m2 gPt−1) > Pt/C (59.5 m2 gPt−1) > Pt/CS (56.2 m2 gPt−1). It can be seen that all the EASAs are similar and their ratios are 1.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.01
1.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.94 correspondingly, which are lower than the current density ratios (2.53
0.94 correspondingly, which are lower than the current density ratios (2.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.34
1.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.91). These results prove that the EASA is not the key factor to determine these electrocatalytic activities; therefore, the promotion effect of WC on Pt and their interaction degree play the most important roles on the activities of the electrocatalysts.
0.91). These results prove that the EASA is not the key factor to determine these electrocatalytic activities; therefore, the promotion effect of WC on Pt and their interaction degree play the most important roles on the activities of the electrocatalysts.
The cyclic stabilities of the Pt/WC-on-CS, Pt/WC-in-CS and Pt/C for MOR are tested and shown in Fig. 6d. The shadows are the cyclic differences between the 1st cycle and the 1000th cycle. It is clear that the activity of the Pt/WC-on-CS reduced 11.0% from 1927 mA mgPt−1 to 1715 mA mgPt−1 by comparing the peak current densities. The Pt/WC-in-CS reduced 18.4% from 1020 mA mgPt−1 to 836 mA mgPt−1. The activity of the commercial Pt/C reduced 25.1% from 761 mA mgPt−1 to 570 mA mgPt−1. The results indicate that the WC is more stable than carbon as Pt electrocatalyst support. Furthermore, the exterior WC is more stable than the carbon wrapped WC as support.
Fig. 7 shows the TEM images of the Pt/WC-on-CS and Pt/WC-in-CS before and after the above 1000 CV cycles. The Pt/WC-on-CS does not show obvious change in dispersion of the particles after the 1000 CV cycles; however, the Pt/WC-in-CS shows obvious agglomeration of particle after the 1000 CV cycles. The results confirm the better stability of the Pt/WC-on-CS.
 | ||
| Fig. 7 TEM images of (a) the fresh Pt/WC-on-CS and (c) the fresh Pt/WC-in-CS; TEM images of (b) the Pt/WC-on-CS and (d) the Pt/WC-in-CS after their respective 1000 CV cycles (Fig. 6). | ||
The promotion effect of WC on Pt is believed to be the origin of the high activity and stability of the Pt/WC-on-CS catalyst, which can be explained by strong negative electronic property of WC. The surface electronic structure of Pt/WC is very different from that of the Pt/C as a result of the electron transfer generated by the WC support, leading to an obvious anti-CO-poisoning effect in the electro-oxidation of methanol. The electron transfer also leads to strong interaction force between carbides and noble metals, restricting the loss and agglomeration of Pt particles.40,41
4. Conclusions
Exterior tungsten carbide particles are successfully synthesized (WC-on-CS), which have exposed WC surface and can contact the loaded Pt particles closely. Therefore, the WC-on-CS shows higher promotion effect than the carbon-wrapped WC particles (WC-in-CS) on Pt electrocatalyst for MOR. The electron transfer or strong interaction force between carbides and noble metals is believed to result in the promoted activity and stability of the Pt/WC electrocatalysts. In addition, the Pt/WC-on-CS is 2.53 times the MOR activity of commercial Pt/C and 150 mV more negative onset potential than the latter, indicating promising economic value. The present method is imagined to be adopted to synthesize other exterior carbide or nitride and other nanoparticles.Acknowledgements
This work was funded by National Natural Science Foundations of China (21306067), Natural Science Foundation of Jiangsu (BK20130490), Sponsored project of Jiangsu provincial Six Talent Peaks (No. 2015-XNY-010), China Scholarship Council (CSC No. 201508320098) and Jiangsu University Scientific Research Funding (No. 14JDG164).References
- K. Huang, K. Bi, J. C. Xu, C. Liang, S. Lin, W. J. Wang, T. Z. Yang, Y. X. Du, R. Zhang, H. J. Yang, D. Y. Fan, Y. G. Wang and M. Lei, Electrochim. Acta, 2015, 174, 172–177 CrossRef CAS.
- J. S. Moon, Y. W. Lee, S. B. Han, D. H. Kwak, K. H. Lee, A. R. Park, J. I. Sohn, S. N. Cha and K. W. Park, Phys. Chem. Chem. Phys., 2014, 16, 14644–14650 RSC.
- Y. Shen, L. Li, J. Xi and X. Qiu, J. Mater. Chem. A, 2016, 4, 5817–5822 CAS.
- X. Zhang, Z. Lu and Z. Yang, J. Power Sources, 2016, 321, 163–173 CrossRef CAS.
- X. Liu and D. R. Salahub, J. Am. Chem. Soc., 2015, 137, 4249–4259 CrossRef CAS PubMed.
- M. M. Sullivan and A. Bhan, ACS Catal., 2016, 6, 1145–1152 CrossRef CAS.
- L. Liao, X. Bian, J. Xiao, B. Liu, M. D. Scanlon and H. H. Girault, Phys. Chem. Chem. Phys., 2014, 16, 10088–10094 RSC.
- Z. Yan, M. Zhang, J. Xie and P. K. Shen, J. Power Sources, 2013, 243, 336–342 CrossRef CAS.
- X. Ji, K. Xu, C. Chen, B. Zhang, H. Wan, Y. Ruan, L. Miao and J. Jiang, J. Mater. Chem. A, 2015, 3, 9909–9914 CAS.
- Y. G. Huang, X. L. Lin, X. H. Zhang, Q. C. Pan, Z. X. Yan, H. Q. Wang, J. J. Chen and Q. Y. Li, Electrochim. Acta, 2015, 178, 468–475 CrossRef CAS.
- Y. Ren, J. Dai, B. Pang, X. Liu and J. Yu, Electrochim. Acta, 2015, 176, 402–409 CrossRef CAS.
- M. Giorcelli, M. Pavese, M. I. Shahzad and A. Tagliaferro, Mater. Lett., 2015, 151, 12–15 CrossRef CAS.
- L. Wang, L. Zhao, P. Yu, C. Tian, F. Sun, H. Feng, W. Zhou, J. Wang and H. Fu, J. Mater. Chem. A, 2015, 3, 24139–24147 CAS.
- P. Justin, P. H. K. Charan and G. R. Rao, Appl. Catal., B, 2014, 144, 767–774 CrossRef CAS.
- Z. Yan, M. Zhang, J. Xie, J. Zhu and P. K. Shen, Appl. Catal., B, 2015, 165, 636–641 CrossRef CAS.
- Z. Li, S. Ji, B. G. Pollet and P. K. Shen, Chem. Commun., 2014, 50, 566–568 RSC.
- Y. Xiao, P. Sun and M. Cao, ACS Nano, 2014, 8, 7846–7857 CrossRef CAS PubMed.
- X. Ma, H. Meng, M. Cai and P. K. Shen, J. Am. Chem. Soc., 2012, 134, 1954–1957 CrossRef CAS PubMed.
- M. Rahsepar, M. Pakshir, P. Nikolaev, Y. Piao and H. Kim, Int. J. Hydrogen Energy, 2014, 39, 15706–15717 CrossRef CAS.
- L. Wang, T. Du, J. Cheng, X. Xie, B. Yang and M. Li, J. Power Sources, 2015, 280, 550–554 CrossRef CAS.
- C. He and J. Tao, J. Power Sources, 2016, 324, 317–324 CrossRef CAS.
- C. Tang, D. Wang, Z. Wu and B. Duan, Int. J. Hydrogen Energy, 2015, 40, 3229–3237 CrossRef CAS.
- E. Lust, K. Vaarmets, J. Nerut, I. Tallo, P. Valk, S. Sepp and E. Härk, Electrochim. Acta, 2014, 140, 294–303 CrossRef CAS.
- L. Elbaz, C. R. Kreller, N. J. Henson and E. L. Brosha, J. Electroanal. Chem., 2014, 720–721, 34–40 CrossRef CAS.
- T. Huang, J. Yu, J. Han, Z. Zhang, Y. Xing, C. Wen, X. Wu and Y. Zhang, J. Power Sources, 2015, 300, 483–490 CrossRef CAS.
- A. Hassan, V. A. Paganin, A. Carreras and E. A. Ticianelli, Electrochim. Acta, 2014, 142, 307–316 CrossRef CAS.
- L. Yang, Y. C. Kimmel, Q. Lu and J. G. Chen, J. Power Sources, 2015, 287, 196–202 CrossRef CAS.
- Y. L. Liu, X. Y. Xu, P. C. Sun and T. H. Chen, Int. J. Hydrogen Energy, 2015, 40, 4531–4539 CrossRef CAS.
- M. Shi, W. Zhang, D. Zhao, Y. Chu and C. Ma, Electrochim. Acta, 2014, 143, 222–231 CrossRef CAS.
- J. Yu, X. Gao, G. Chen and X. Yuan, Int. J. Hydrogen Energy, 2016, 41, 4150–4158 CrossRef CAS.
- Y. N. Regmi, G. R. Waetzig, K. D. Duffee, S. M. Schmuecker, J. M. Thode and B. M. Leonard, J. Mater. Chem. A, 2015, 3, 10085–10091 CAS.
- Y. Oh, S. K. Kim, D. H. Peck, J. Jang, J. Kim and D. H. Jung, Int. J. Hydrogen Energy, 2014, 39, 15907–15912 CrossRef CAS.
- M. M. Sullivan, J. T. Held and A. Bhan, J. Catal., 2015, 326, 82–91 CrossRef CAS.
- S. A. Alekseev, D. M. Korytko, S. V. Gryn, V. Iablokov, O. A. Khainakova, S. Garcia-Granda and N. Kruse, J. Phys. Chem. C, 2014, 118, 23745–23750 CAS.
- Z. Yan, L. Gao, M. Zhang, J. Xie and M. Chen, RSC Adv., 2015, 5, 9561–9564 RSC.
- Z. Yan, H. Wang, M. Zhang, Z. Jiang, T. Jiang and J. Xie, Electrochim. Acta, 2013, 95, 218–224 CrossRef CAS.
- Z. Yan, J. Xie, P. K. Shen, M. Zhang, Y. Zhang and M. Chen, Electrochim. Acta, 2013, 108, 644–650 CrossRef CAS.
- G. He, Z. Yan, X. Ma, H. Meng, P. K. Shen and C. Wang, Nanoscale, 2011, 3, 3578–3582 RSC.
- L. Zeng, T. S. Zhao, L. An, G. Zhao, X. H. Yan and C. Y. Jung, J. Power Sources, 2015, 275, 506–515 CrossRef CAS.
- Z. Yan, G. He, P. K. Shen, Z. Luo, J. Xie and M. Chen, J. Mater. Chem. A, 2014, 2, 4014–4022 CAS.
- G. Cui, P. K. Shen, H. Meng, J. Zhao and G. Wu, J. Power Sources, 2011, 196, 6125–6130 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |