Multiple overlap extension PCR (MOE-PCR): an effective technical shortcut to high throughput synthetic biology†
Saeid Kadkhodaei*a,
Hamid Rajabi Memaribc,
Sahar Abbasiliasid,
Morvarid Akhavan Rezaeie,
Ali Movahedif,
Tan Joo Shung and
Arbakariya Bin Ariff*h
aInstitute of Tropical Agriculture, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. E-mail: admin@bioinfobase.info
bBiotechnology and Life Science Center, Shahid Chamran University, Ahvaz 6135783151, Iran
cSynHiTech, 609-7191, Thornhill, Ontario L3T 0C7, Canada
dLaboratory of Halal Science Research, Halal Products Research Institute, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
eFaculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia
fKey Laboratory of Forest Genetics and Biotechnology, Nanjing Forestry University, Nanjing 210037, China
gBioprocess Technology, School of Industrial Technology, Universiti Sains Malaysia, 11800 Penang, Malaysia
hDepartment of Bioprocess Technology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
First published on 8th July 2016
Abstract
The current study describes multiple-overlap-extension PCR (MOE-PCR) as a simple and effective approach to assembling multiple DNA fragments with various sizes and features in a single in vitro reaction. In this research, 50 bp of homology in overlapping DNA fragments and a specific touchdown PCR program resulted in successful assembly of eight different DNA fragments using a single PCR protocol. The simplicity, speed, reliability and cost-effectiveness of MOE-PCR offers it as an attractive method for assembling and/or cloning single and multiple DNA fragments. Indeed, the method is a one-step approach that eliminates some of the routine steps such as ligation and complex enzymatic reactions as well as sequence limitations of the other methods. The applications of this relatively high fidelity method could be extended to the construction of chimeric recombinant sequences that can be widely used in metabolic engineering, functional analysis of genes and genetic elements, expression studies of multi-domain proteins, protein engineering and the most recent genome editing strategies which together with synthetic biology are revolutionizing the life sciences. We expect the technique to be used as a robust, reliable and fast method in synthetic biology.
Introduction
In modern synthetic biology, scientists have attempted to benefit mankind and other organisms by developing new biological systems in which the natural system is redesigned and/or rebuilt. Using BioBricks, scientists attempt to introduce abstraction and standardization principles1 in biosynthetic engineering.2 To this end, various useful enzymes and recombinant proteins have been produced for harnessing the synthetic biology techniques, which may lead to a kind of artificial evolution in the near future.Currently, synthetic biology faces critical limitations, including time and cost effectiveness of the related procedures, as well as the efforts required to construct genetically engineered sequences. Therefore, a more reliable and rapid DNA synthesis and fragment assembly method is required to expedite the synthetic biology processes. In order to overcome the limitations of the use of traditional restriction enzymes-based cloning methods such as Golden gate,3 BglBricks,4 the SfiI method,5 ePathBrick6 and OGAB,7 several alternatives have been developed in synthetic biology. These include in vitro8 and in vivo homologous recombination,9,10 ligation-independent cloning (LIC),11 sequence and ligation independent cloning (SLIC);9 and next generation PCR-based methods12–14 as well as hybridization-based methods such as successive hybridization assembly (SHA).15 Various methods used in multiple DNA fragment assembly have been previously reviewed.16,17
However, these techniques are not without their limitations. In Gateway Multisite technology, two to four DNA fragments can be inserted into a destination vector; among the drawbacks include the site-specific recombination which require additional specific sequences, leaving extra sequences (after recombination) and consequently amino acids (for gene fragments to be transformed and expressed), the high cost of the kit and the insert size limitation.18,19 Alternatively, Jiang et al. (2012) developed a hybridization-based method known as SHA (successive hybridization assembling). In this technique, two adjacent DNA fragments could be joined to each other first through OE-PCR or SLIC and then these two-half structures are used as substrate fragments (SF) for assembly in a hybridization procedure after denaturation–renaturation treatment. In the latest ligation independent cloning method known as SLIC,9 an enzyme having 3′–5′ exonuclease activity (T4 DNA polymerase) is used to generate single stranded-end fragments and then by annealing of the adjacent overlapped sequences, the final recombinant DNA will be assembled. In spite of its similarity to LIC, this method does not require specific sequences and that is why it is called as sequence independent. In the SLIC technique, extra enzymatic procedures are required, which may hinder its widespread adoption. However, In-Fusion (Clontech)20 and Gibson (NEB) kits were developed and commercialized based on the LIC method.
Gibson DNA assembly is analogous to SLIC except that it uses the dedicated T5 exonuclease, a ligase to seal the single stranded nicks and no dNTP addition step. The advantage of Gibson over SLIC is its simultaneous one pot reaction. However, the enzymatic cocktail is more expensive than that required for SLIC. Fragment size is another practical limitation of the Gibson method where the fragments smaller than 250 bp are more likely to be entirely chewed by T5 exonuclease prior to the annealing and extension steps. Another sequence-independent and overlap cloning method is USER (uracil-specific excision reagent). This method initially requires specific primers to incorporate one or more uracil to the amplified fragments. The uracil(s) are excised by uracil DNA glycosylase and an AP-lyase is used to cleave the subsequent abasic sites. The resultant overhangs can then be assembled in a single ligase-independent method. Since AP-lyase enzymes leave a 5′ PO4 ligation is technically feasible with this method.21 Through USER cloning method parallel assembly is possible and no assembly scars are left. This method is not truly sequence-independent because at least one thymidine is needed adjacent to the end of the sequence to be replaced with a uracil. Furthermore, multi-step process and the requirements for USER reagents and compatible vectors are among the disadvantages of this method.
In PCR-based techniques, the polymerase extension principle of PCR is used to synthesize genes by assembling overlapping oligonucleotides.22 Circular polymerase extension cloning (CPEC)13 and mutagenic OE-PCR14 are the most recent methods in this regard. In CPEC, overlapping regions between the adjacent fragments are extended to form a circular plasmid and is thus named circular polymerase extension cloning. The linearized vector and insert prime each other in this method, as in Gibson/SLIC assembly. Like Gibson assembly and SLIC, CPEC is standardized, largely sequence-independent and scar-less. But, unlike SLIC and Gibson there is only a single polymerase enzyme needed without any extra reagents such as exonuclease and dNTP. The common disadvantages of PCR-based techniques are mis-priming events and polymerase-derived mutations which may occur in the PCR products (sequences of the fragments to be assembled). The main weaknesses of the previous PCR-based methods, CPEC13 and mutagenic OE-PCR,14 is that in CPEC technique the maximum number of DNA fragments which could be assembled was four, which is not enough for large scale and high-throughput synthetic biology processes; on the other hand, in mutagenic OE-PCR at least three rounds of OE-PCR are used to assemble the final sequence. In this method, adjacent fragments are mixed together two and two in individual OE-PCRs. After the first round of OE-PCR, all reactions with the joined fragments are mixed with the reactions containing the joined adjacent fragments. The completely assembled sequences of interest are built exponentially with each round of OE-PCR during the third round of OE-PCR until all fragments had been joined together. Besides increasing the error rate of polymerase exponentially, time and cost effectiveness of the method will not be reasonable.
Here, we describe an OE-PCR-based technique that can easily assemble up to eight DNA fragments in an efficient, economical and highly simplified approach. We utilized 50 bp homologous ends for multiple DNA fragments assembly to construct around 20 kb of both linear and circular recombinant DNAs. The efficiency, speed and cost-effectiveness of the technique referred to as MOE-PCR as the more productive synthetic biology approach were discussed.
Results
In vitro homologous recombination of multiple fragments
Schematic diagram of the MOE-PCR for production of multi-way recombinant DNA in a single in vitro reaction is illustrated in Fig. 1. A comparison of the results from each MDFA method revealed that the SLIC and MOE-PCR methods were more efficient than SHA; however, none were able to assemble all nine fragments together (Fig. 2). In the first attempts, we tried to assemble two, three, four and five DNA fragments with fragment sizes ranging from ∼300 to ∼2200 bp (Table 1). The efficiency of the two to four-way recombinations was robust, and good enough for the five-way reaction in both SLIC and MOE-PCR. In the two-way reactions, almost 100% of the fragments were assembled and increasing the number of fragments up to five affected the percentage of recombinant fragments (Table 2). | ||
| Fig. 2 Virtual construct predesigned by CLCbio Main Workbench, and all DNA fragments for multiple assembling. The blue color part at the end of each fragment represents the 50 bp homologous sequence with the adjacent fragment. The DNA fragments as described in Table 3 are coded as A to I in alphabetical order. The coverage score of 2 demonstrates the overlapping regions in the sequence map. | ||
| No. | Fragment name | Size (bp) | No. | Fragment name | Size (bp) |
|---|---|---|---|---|---|
| 1 | A – ML_MARs | 3578 | 7 | B – GW-RFA | 3387 |
| B – GW-RFA | C – GFP_pUC18-a28-tGBC | ||||
| C – GFP_pUC18-a28-tGBC | D – 3′UTR_pHsp70A_RbcS2-cgLuc | ||||
| D – 3′UTR_pHsp70A_RbcS2-cgLuc | E – 3′UTR2-eNOS_pBI121 | ||||
| 2 | E – 3′UTR2-eNOS_pBI121 | 2072 | 8 | F – BAR_pUC18-a28-tGBC | 1985 |
| F – BAR_pUC18-a28-tGBC | G – CamV_pBI121 | ||||
| G – CamV_pBI121 | H – MR_MARs | ||||
| 3 | H – MR_MARs-DSM2 | 3002 | 9 | I – pUC_pUC18-a28-tGBC | 2994 |
| I – pUC_pUC18-a28-tGBC | A – ML_MARs | ||||
| 4 | D – 3′UTR_pHsp70A_RbcS2-cgLuc | 916 | 10 | B – GW-RFA | 3946 |
| E – 3′UTR2-eNOS_pBI121 | C – GFP_pUC18-a28-tGBC | ||||
| D – 3′UTR_pHsp70A_RbcS2-cgLuc | |||||
| E – 3′UTR2-eNOS_pBI121 | |||||
| F – BAR_pUC18-a28-tGBC | |||||
| 5 | G – CamV_pBI121 | 1712 | 11 | G – CamV_pBI121 | 4656 |
| H – MR_MARs | H – MR_MARs | ||||
| I – pUC_pUC18-a28-tGBC | |||||
| A – ML_MARs | |||||
| 6 | A – ML_MARs | 8502 | 12 | C – GFP_pUC18-a28-tGBC | 6809 |
| B – GW-RFA | D – 3′UTR_pHsp70A_RbcS2-cgLuc | ||||
| C – GFP_pUC18-a28-tGBC | E – 3′UTR2-eNOS_pBI121 | ||||
| D – 3′UTR_pHsp70A_RbcS2-cgLuc | F – BAR_pUC18-a28-tGBC | ||||
| E – 3′UTR2-eNOS_pBI121 | G – CamV_pBI121 | ||||
| F – BAR_pUC18-a28-tGBC | H – MR_MARs | ||||
| G – CamV_pBI121 | I – pUC_pUC18-a28-tGBC | ||||
| H – MR_MARs | A – ML_MARs | ||||
| I – pUC_pUC18-a28-tGBC |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (vector
2 (vector![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) insert) and 15 cycles were used in all experiments. The experiments were performed using three (7 fragment assembly) to five (3 fragment assembly) replicates
insert) and 15 cycles were used in all experiments. The experiments were performed using three (7 fragment assembly) to five (3 fragment assembly) replicates
Amplification of two- to four-way SLIC fragments using a forward primer for the left-most fragment and a reverse primer for the right-most fragment was successful. In this way, the low amount of initial SLICs could be increased. Obviously, a high fidelity polymerase and the minimum possible number of cycles are required to minimize the mutation rate in PCR. On the other hand, PCR amplification efficiency for the SLIC assembled fragments declined for large fragments and assemblies. The assembly of larger fragments (F1R4 = 3578 and F8R9 = 3002 bp) was poor, which may be attributed to the type of polymerase used, and may need further optimization.
The high efficiency of SLIC suggested that it might be feasible to create recombinant DNAs with more fragments. To attempt a nine-way recombination, we amplified nine fragments of various sizes (Table 3) by PCR with 50 bp overlapping homology for each. In this experiment, the assembly of all nine fragments and circularization to generate the designed recombinant plasmid was not successful. The same was true for the nine-way reaction using MOE-PCR. Therefore, in the next round of experiments, we eliminated the Gateway fragment from the destination vector because of the potential problems caused by its repeated recombination sequences. This resulted in successful assembly and circularization of all eight fragments.
| Fragment name | Accession no. | Source | Primers | Size (bp) | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| A – ML_MARs | In progress | Synthetic (artificially designed) | F1_pUC-ML | R1_GW-ML | 567 |
| B – GW-RFA | — | GWrFA | F2_ML-GW | R2_GFP-GW | 1793 |
| C – GFP_pUC18-a28-tGBC | [GenBank: KF780167] | Synthetic | F3_eGW-GFP | R3_3′UTR-GFP | 778 |
| D – 3′UTR_pHsp70A_RbcS2-cgLuc | pHsp70A_RbcS2-cgLuc | F4_His6-3′UTR | R4_eNOS-3′UTR | 304 | |
| E – 3′UTR2-eNOS_pBI121 | pBI121 | R5_BAR-3′UTR2 | F5_3′UTR-eNOS | 662 | |
| F – BAR_pUC18-a28-tGBC | [GenBank: KF780168] | Synthetic | R6_CamV-BAR | F6_3′UTR2-BAR | 609 |
| G – CamV_pBI121 | pBI121 | R7_MR-CamV | F7_BAR-CamV | 901 | |
| H – MR_MARs | In progress | Synthetic (artificially designed) | F8_CamV-MR | R8_pUC-MR | 575 |
| I – pUC_pUC18-a28-tGBC | pUC18 | F9_eMR-pUC | R9_ML-pUC | 2191 | |
According to the number of colonies on the amp plates obtained from each method, SLIC and MOE-PCR were comparable, while there were no colonies obtained for the SHA technique. In addition, in terms of integrity of the assemblies, both SLIC and MOE-PCR demonstrated reasonable results, especially for the assembly of five or fewer fragments.
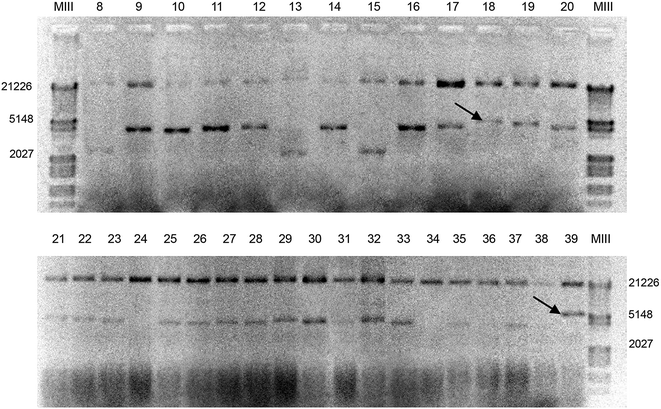
For eight-way recombination we picked 150 colonies. Only MOE-PCR colonies passed all verification tests from colony cracking (Fig. 3) to restriction mapping (Fig. 4a), PCR analysis (Fig. 4b) and primer walking sequencing. Restriction analysis of the positive colonies revealed correct digestion patterns and successful assembly of all fragments in four colonies, which was a lower frequency compared with the four-way reactions. We selected the final positive colonies for complete sequence analysis, among which one showed only a PCR-induced silent mutation in the vector backbone.
In SLIC, after T4 DNA polymerase and dCTP treatments, we performed T4 DNA ligase reactions by incubating overnight at 4 °C and then kept at −20 °C until further use. Similar negative controls (same reactions but without T4 ligase treatment) were kept at −20 °C after T4 polymerase and dCTP treatments. As can be seen from ESI,† some ligations seemed to have occurred but the desired one (around 9 kb) is very faint and is more visible in SLIC-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (1 molar ratio for vector backbone and 2 molars for each inserts) without T4 ligase. Thus, no clear benefit of T4 DNA ligase in the facilitation of DNA fragment assembly could be identified in this analysis.
2 (1 molar ratio for vector backbone and 2 molars for each inserts) without T4 ligase. Thus, no clear benefit of T4 DNA ligase in the facilitation of DNA fragment assembly could be identified in this analysis.
The MOE-PCR method showed to be more efficient in assembling the fragments (Fig. 5). Increasing the cycles from 15 to 25 improved the product yield and also increased the number of colonies when more fragments were used. The higher molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 increased the number of colonies as well.
2 increased the number of colonies as well.
 | ||
| Fig. 5 Multi-way assemblies through MOE-PCR. The arrows represent the positive bands among the faulty recombinations. MIII and 100p are the molecular size markers (Roche, Germany) and the numbers represent different multiway fragment assemblies (Table 1); 1-ABCD (3578 bp), 2-EFG (2072 bp), 3-HI (3002 bp), 4-DE (916 bp), 5-GH (1712 bp), 6-ABCDEFGHI (8502 bp), 7-BCDE (3387 bp), 8-FGH (1985 bp), 9-IA (2994 bp), 10-BCDEF (3946 bp), 11-GHIA (4656 bp) and 12-CDEFGHIA (6809 bp). The arrows indicate the correct assembly bands in each lane among faulty recombinations. | ||
After the SHA procedure, the number of fragments seemed to have decreased, but without any assembly of a new, larger fragment. Also, after transformation of E. coli, no colonies grew on Amp + Cam plates. In the original paper,15 the authors suggested putting the mixture of SFs in boiling water and leaving them to cool down to room temperature (around 2 h). Nevertheless, in this study the procedure was simulated using a thermal cycler to gradually decrease the temperature from boiling point to room temperature, which took around 30 min. There was no recombination obtained through SHA; therefore, this method was not used for further DNA assemblies.
Fidelity of the methods
We sequenced a total of around 30 kb comprising all constructs. When KAPA HiFi HotStart was used to amplify the fragments, we found only one mutation (a silent mutation in the vector backbone) introduced into the final construct by DNA polymerase. The polymerase error rate was higher for Qiagen and Phusion (4.4 × 10−7) in the preliminary experiments and therefore we employed KAPA HiFi HotStart as the preferred DNA polymerase for all cloning studies (ESI†). On the other hand, gel purification of the fragments was carried out in a minimum exposure time under UV to avoid DNA damage by UV.Discussion
In the present study, we developed a one-step one-pot approach which was efficient and accurate enough to join multiple DNA fragments in a defined order. The novelty of our study is modification of the OE-PCR in a way to be simply utilized in synthetic biology and assembly of multiple (small and large) DNA fragments in a single PCR reaction. In the previous study (CPEC) based on OE-PCR, published in Nature Protocol,13 the authors were able to assemble four DNA fragments in smaller sizes. But, our modified method could be easily used for assembly of at least eight fragments ranging from 300 to 2200 bp. Another attempt on using the same PCR principle in multiple DNA fragment assembly is mutagenic OE-PCR method,14 in which the authors assembled multiple fragments in at least three rounds of OE-PCR.For comparison, we constructed a set of multiple-fragment assemblies using different PCR-based (MOE-PCR), hybridization-based (SHA) and exonuclease-based (SLIC) methods. In the eight-way assemblies constructed with SLIC and MOE-PCR, we observed clones with varied inserts because of the faulty recombinations. Almost 20% of the eight-way assemblies showed correct combination, which would be sufficient for single in vitro complex component assembly. Li and Elledge (2007) reported 20% recombination in a ten-fragment assembly using SLIC, although the fragments they assembled were smaller, nearly half the size of the fragments used in this assembly. However, SLIC requires more steps, including T4 DNA polymerase and dCTP treatments.
Although the idea seems theoretically sound, in the present study, the results of SHA were not satisfactory compared to the other evaluated methods. However, the authors mentioned that the method was not affected by repeated sequences, our experiences do not justify this claim. This is possibly because of the presence of repeated sequences, the high similarity of some fragments and AT/GC richness, all of which increase the probability of secondary structures formation leading to lower efficiency. Furthermore, in practice, the preparation of two-half SFs and the subsequent procedures for purification of the fragments, as extra steps, makes it costlier and time consuming. In addition, the concentration of the prepared SFs is too low, requiring an extra round of PCR.
Another alternative method is ligation independent cloning (LIC). The basis of this technique in earlier versions was the application of certain enzymes with 3′ exonuclease activity, such as T4 DNA polymerase, to generate single strand overhanging ends in both vector and insert. A specific sequence lacking dCMP was required in this context to create 12 nucleotide overhangs.11 The modified version of LIC knows as SLIC method was reported to have successfully constructed a ten-way recombination of a 7.5 kb plasmid using 40 bp homologous ends. However, compared with PCR-based techniques, it requires more enzymatic reactions, fragment purification and more concentrated DNA to be used in a single low volume reaction. In addition, T4 DNA ligase treatments in the SLIC method did not show advantageous results and, surprisingly, lack of this enzyme was more beneficial in DNA fragment assemblies in the present study. The robustness and varied applications of PCR, such as amplification of a desired sequence from a template with small quantities, addition or deletion of specific sequences in the fragment of interest (general or site-directed mutagenesis to add of REs or other expression elements) and screening of the positive transformants, make it efficient for use in cloning procedures. Exploiting the basic polymerase extension feature of PCR, assembly of DNA fragments having overlapping sequences can be facilitated. While no primer is used in this regard, these overlapping sequences act as the priming sites and enable annealing of adjacent fragments to construct recombinant DNAs. Using PCR-based techniques, gene fragments up to 5 kb have been assembled in vitro.16 In a four-fragment assembly through CPEC, Quan and Tian (2009) demonstrated high accuracy of the assembled vectors. Quan and Tian (2011) extended the use of this approach for single-gene library and multi-fragment cloning. They reported the successful cloning of four PCR fragments having 15–25 bp overlapping regions through PCA (polymerase cycling assembly). Here, we modified a version of the overlap extension PCR and successfully assembled up to eight DNA fragments with various sizes in a single in vitro reaction. We designed 50 bp overlapping regions between all fragments (the vector and eight inserts) such that they had similar melting temperatures (Tm), typically between 69 and 74 °C. This range of Tm should both maximize the specificity of the correct hybridization and reduce mis-hybridizations. This is critical to achieve accurate and successful multi-assembly. To this end, the overlapping region for those junctions which were not flexible to the sequence changes (such as promoter-gene or gene-terminator) was designed based on the sequences of each fragment. This was considered as the basis and threshold for the other flexible junctions where the extra sequences (spacers) were allowed to be inserted. This strategy was used to adjust the GC content and subsequent Tm of the flexible overlapping regions to be similar to those of non-flexible regions as high as possible. The overlapping sequences are typically introduced to the fragments through PCR; however, for fragments that were prepared through gene synthesis, the homologous areas can be directly added to the desired sequences. In this study, the MARs sequences were synthesized by outside suppliers because of their complex structure (repeated sequences and AT richness), which made them difficult to prepare in-house.
In MOE-PCR, accurate design of overlapping ends that share similar GC contents, and have as high a Tm as possible, is critical. The higher the Tm for the homologous ends, the better the hybridization specificity and efficiency. However, for MOE-PCR, the overlapping sequences should be long enough such that the Tm is increased, but it should still be close to the extension temperature, which allows running an easy two-step PCR. In our study, the Tms of the fragments were around 72 °C, with differences ranging ±2.5 °C. This strategy, which can be manipulated through ΔG, as one of the criteria to validate a primer pair for PCR, will minimize mismatches to increase the assembly accuracy and efficiency. In this regard, low values of ΔG (>−9 kcal mol−1) can be ignored when the Tm is high enough to avoid unwanted mis-hybridizations. The length of the homologous ends previously considered for 2–4 way assemblies in OE-PCRs were 15–35 nucleotides.13 The higher the number of fragments, the longer the length of overlapping sequence between neighbouring fragments is required. In our experience, 50 bp homologous ends resulted in a reasonable percentage of correct constructs for a single eight-way assembly. Increasing this length may lead to higher cloning efficiency; however, longer primers would be needed, requiring more careful manipulation in primer design and synthesis. Gibson et al. (2008)24 using yeast recombination succeeded in assembling 25 DNA fragments with 80–360 bp overlapping sequences. Such long overlapping ends may not be easily introduced using PCR primers and highly similar fragments may compromise the final product.
A critical issue for the successful assembly of multiple DNA fragments is the accuracy of the amplicons, which should be as high as possible.23 To this end, an oligonucleotide synthesis procedure and DNA polymerase were chosen such they minimized the number of mutations that could potentially be introduced by the primer and polymerase enzyme. Primer synthesis was performed using PAGE purification to maximize elimination of possible errors in priming sites. This would increase the cost, but would be justified by the decrease in the number of errors in subsequent amplicons. On the other hand, the scale of synthesis can be reduced to minimize costs because only a very small amount of primer is required for PCR optimization and amplification. For example, the OD scale of two (2 OD) in primer synthesis provides enough primers for at least 150 (70 bp oligonucleotide) to more than 300 (50 bp) of 20 μL PCR reactions, depending on the size and GC content of the primer. The properties of each primer including the length, GC content and sequence are shown in Table 4. We used KAPA HiFi HotStart DNA polymerase (KAPA Biosystems, Capetown, South Africa), which is the highest fidelity DNA polymerase available. The error rate of this enzyme is 2.8 × 10−7, which is 100 times lower than the wild type Taq (2.28 × 10−5) and 10 times lower than other B-family DNA polymerases. The use of this enzyme would minimize the number of mutations introduced. There have been reports of using alternative high fidelity DNA polymerases25,26 but a comprehensive analysis is lacking. However, our findings are supported by Quail et al. (2012) study which revealed that the best enzyme overall for library preparation was KAPA HiFi (Kapa Biosystems). They demonstrated that KAPA HiFi performed well using either a standard amplification or quantitative PCR premix formulation.25 Proofreading high fidelity enzymes are extremely accurate, but do not perform well over longer target distances or with low template concentration, because the 3′–5′ exonuclease (proofreading) activity destroys primers and affects sensitivity. To amplify large fragments, other specific polymerases such as KAPA™ LongRange HotStart (KAPA Biosystems) are recommended. However, the fidelity rates of these polymerases are somewhat lower than that of HiFi polymerase. In addition, the 3′-exonuclease activity of the enzyme removes the extra nucleotide at the ends of the amplification products. Proofreading PCR enzymes might potentially damage the ends of amplicons particularly in the exceeded cycles. This is attributed to the 3′ exonuclease activity of such enzymes. The ends of PCR products can be protected from this 3′ exonuclease activity in the presence of dNTPs. The reduction of dNTPs at a higher number of PCR cycles would weaken the protection of the 3′ ends against 3′-exonuclease activity of the DNA polymerase, resulting in damage to the PCR products and making cloning extremely difficult.19 However, in MOE-PCR, increasing the number of cycles from 18 to 25 significantly improved the number of positive transformants. Multi-way assembly using MOE-PCR follows the principles of standard PCR. However, the cycle number and conditions need to be optimized according to the number of fragments. In our experience, 18 cycles resulted in 315 c.f.u. (colony forming units), among which 20% showed positive and correct assembly. This relatively low number of cycles guaranteed very low or no errors being introduced by the polymerase during PCR. The number of PCR cycles used in this study was comparable or lower than the four-fragment assembly (20 cycles) carried out by Quan and Tian (2011). We believe that more cycles would improve the efficiency of the assembly, providing a robust high fidelity polymerase is used. The total time required for this method is determined by the fragments sizes and processivity of the polymerase enzyme. The eight-way reaction with fragments ranging from 900 to 2200 bp took around 1 h to be accomplished, demonstrating the speed of the technique.
| Name | Sequence (5′–3′) | Size (bp) | GC% |
|---|---|---|---|
| F1_pUC-ML | TGTAAACAGGTACCGCCGCCCGCCTTTCGATTAAAAATCCCAATTATCGG | 50 | 48 |
| R1_GW-ML | TTTGTACAAACTTGTGATAGATCTGGTACCCGGCCCGCCGCGATTTAGATTAAAAATCCCAATTAACCGA | 70 | 43 |
| F2_ML-GW | ATCTAAATCGCGGCGGGCCGGGTACCAGATCTATCACAAGTTTGTACAAA | 50 | 48 |
| R2_GFP-GW | GAACAGCTCCTCGCCCTTGCTGGCCATCTTGGCTTGCTCGAGTAAATATTATCACCACTTTGTACAAG | 68 | 48.5 |
| F3_eGW-GFP | AATATTTACTCGAGCAAGCCAAGATGGCCAGCAAGGGCGAGGAGCTGTTC | 50 | 52 |
| R3_3′UTR-GFP | CCTCCATTTACACGGAGCGGTCTAGATTATTAGTGGTGGTGGTGGTGGTGGTCCATGCCGTGGGTGATGCCG | 72 | 57 |
| F4_His6-3′UTR | CACCACCACCACCACCACTAATAATCTAGACCGCTCCGTGTAAATGGAGG | 50 | 52 |
| R4_eNOS-3′UTR | CGATTTAGTGCTTTACGGCACCTCGACTTCGAACCGTACCCGCTTCAAATACGCCCAG | 58 | 53.4 |
| F5_3′UTR-eNOS | ATTTGAAGCGGGTACGGTTCGAAGTCGAGGTGCCGTAAAGCACTAAATCG | 50 | 50 |
| R5_BAR-3′UTR2 | CGCCCCGTGCTGCCCGTGACCGAGATTTAATAAGTTAACATCAACAACTCTCCTGGCGCA | 60 | 53.3 |
| F6_3′UTR2-BAR | GAGTTGTTGATGTTAACTTATTAAATCTCGGTCACGGGCAGCACGGGGCG | 50 | 50 |
| R6_CamV-BAR | GAAGTTCATTTCATTTGGAGAGAACACGCCCGGGAAGATGAGCCCCGAGCGCCGCCCCG | 59 | 61 |
| F7_BAR-CamV | GCTCGGGGCTCATCTTCCCGGGCGTGTTCTCTCCAAATGAAATGAACTTC | 50 | 54 |
| R7_MR-CamV | CCGATAATTGGGATTTTTAATCGAAACGCGCCCTGCAGGTCCCCAGATTAG | 51 | 49 |
| F8_CamV-MR | TAATCTGGGGACCTGCAGGGCGCGTTTCGATTAAAAATCCCAATTATCGG | 50 | 48 |
| R8_pUC-MR | CGCTCTTTTCTCTTAGGTTTACTTAATTAACCGCCGCCGCCGCCTGCAGGATTTAGATTAAAAATCCCAATTAACCGA | 78 | 43.6 |
| F9_eMR-pUC | CCTGCAGGCGGCGGCGGCGGTTAATTAAGTAAACCTAAGAGAAAAGAGCG | 50 | 54 |
| R9_ML-pUC | CCGATAATTGGGATTTTTAATCGAAAGGCGGGCGGCGGTACCTGTTTACACCACAATATA | 60 | 45 |
The design of the assembly was particularly complex for the fragments that were supposed to be assembled in reverse order (fragments 4, 5, 7 and 8, Fig. 2 and Table 3). To this end, the forward and reverse primers were designed based on the antisense and sense strands, respectively. The virtual design of the whole recombinant sequence using the appropriate software is particularly useful.
Agarose gel electrophoresis of a small aliquot of the MOE-PCR reaction may help to evaluate the efficiency of the experiment. If the recombinations are successful, the band of interest representing the full-length assembly of all fragments will be visible with a size corresponding to the sum of all the fragments sizes. Some faulty recombinations or intermediate assemblies may be observed, and the circular ones may increase the rate of false positive colonies (Fig. 5). Analysis of the sequencing results demonstrated almost 100% fidelity of the amplicons assembled in the new recombinant DNA. This highlights the key importance of using high fidelity enzymes, rigorous primer design and synthesis, and purification strategies.
To achieve the highest number of transformants in MDFA, the competency of the bacterial cells is crucial. Therefore, employing competent cells with high transformation efficiencies (>108 c.f.u. per μg) is highly recommended.13 Running all experiments simultaneously, including MOE-PCR, SLIC and SHA, resulted in many colonies, including those containing faulty recombinations. Therefore, the large number of colonies obtained after transformation required a fast and efficient procedure to screen the positive colonies. Colony cracking was useful in this respect because it just needs very simple materials and takes around half an hour to complete. Normally there are 1–3 bands in colony cracking gels: the upper one corresponds to bacterial genomic DNA, the middle one is the recombinant plasmid, and the lower one is RNA.
In one of the assembled vectors, we finally cloned the Gateway reading frame (A) upstream of the main CDS, to facilitate promoter analysis studies. The Gateway technique has a disadvantage in that it leaves extra sequences after recombination. We resolved this problem using the two similar REs sequences; one was embedded at the end of Gateway fragment before the att site and another one at the beginning of CDS before the translation initiation site. The vector was then digested and self-ligated to form the final construct. However, this requires an extra cloning and self-ligation step.
The inability of the MDFA methods studied to assemble all nine fragments can be attributed to the complexity of some of the fragments (e.g. MARs and Gateway recombination sequences) particularly at the 5′- and 3′-ends, which contained repeated sequences, AT or GC rich regions and high similarity between some of the sequences. However, removing the Gateway fragment resulted in construction of the vectors and constructs of interest in an eight-way in vitro recombination reaction. Sequences that are present in various building blocks more than once may result in faulty recombination and an undesirable final product.27 In our experience, the developed PCR based method seems to be more tolerant in this regard. The relatively high thermal cycling procedure during the annealing step of PCR inhibits secondary structures formation due to the complexity of the sequences, making the assembly more accurate. The lower rate of faulty recombinations in our modified OE-PCR method compared with SLIC can be attributed to the fact that the fragments were subjected to a higher touch down temperature, at which mismatches and the formation of stable secondary structures would be significantly inhibited. In SLIC, a lower temperature (37 °C) is used for the final assembly reaction. Li and Elledge (2007), using SLIC, reported 0 and 83% faulty recombination for 5- and 10-fragment assembly, respectively. In a four-way assembly using SLIC, disappointing results were obtained when short repeats were present in the fragments: only 20% of the randomly picked transformants were correctly assembled.15
Comparing MOE-PCR with SLIC, the latter was more sensitive for large fragments, because the efficiency dropped compared with the assembly of smaller ones (SLIC1 + SLIC3). Li and Elledge (2007) reported successful assembly of nine fragments of sizes ranging from 275 to 980 bp and having 40 bp overlap ends into a 3.1 kb vector. However, in the present study the larger and more complex DNA fragments with sizes ranging from ∼300 to ∼2200 bp were used (Table 3). As an alternative for large constructs, a combination of the two approaches might be beneficial: SLIC could be used to ligate the adjacent 2 to 5 fragments and then, in the second round, these new larger fragments could be assembled using MOE-PCR, or vice versa. To amplify large fragments, highly efficient specific DNA polymerases are recommended. Some of the fragments included repeated and GC/AT rich sequences, which make the contig assembly and aligning difficult. Among the software used for contig assembly, Sequencher® v5.1 (sequence analysis software, Gene Codes Corporation, Ann Arbor, MI USA http://www.genecodes.com) and CodonCode Aligner v4.1 (CodonCode corporation, Centerville, MA, USA http://www.codoncode.com) were less sensitive to these types of sequences and gave better results.
As a comparison for the use of efficient molar ratio, doubling the concentration of inserts notably improved the number of transformants in both the sequence and ligation independent method, and in our PCR-based method. However, the number of faulty recombinations also increased. In most E. coli strains, the biosynthesized template plasmid is methylated. DpnI can digest the methylated parental DNA; therefore, DpnI treatment after PCR amplification of the required fragments is recommended to reduce background transformants. However, Sleight et al. (2010) reported that the dilution of PCR template reduces background sufficiently such that DpnI digestion is unnecessary. In addition, DpnI treatment is not required if the fragments are gel purified during the fragment preparation procedure. In vitro PCR reactions produce unmethylated DNA amplicons. Therefore, this can be considered as another advantage of MOE-PCR method; i.e., eliminates the extra step of DpnI treatment in the assembly process.
Faulty recombinations caused by mismatching at overhangs were observed for both SLIC and MOE-PCR methods. The number of unassembled fragments and faulty or incomplete recombinations were much higher in SLIC rather than in MOE-PCR, while there was no recombination detectable for SHA on gel electrophoresis. Colony counting using ImageJ 1.48c (National Institutes of Health, Bethesda, MD, USA) and the ITN plugin demonstrated that the number of colonies in SLIC was considerably more than in the MOE-PCR method indicating more faulty recombinations (ESI†).
Owing to the presence of Gateway reading frame A as one of the DNA fragments for vector construction, which comprises a ccdB toxic gene, specific E. coli strain One Shot® ccdB Survival™ T1R was used as the host competent cell for transformation of all assembled fragments containing this recombination sequence. In a comparison between competent cell preparation protocols, the number of colonies obtained using the Top10 protocol was almost twice that gained using the Inoue method. However, in terms of the long-term stability of the competent cells, the Inoue method lasted at least 6 months, whereas Top10 method competent cells lost their competency in less than 6 months.
Taking the results together, we deduced that using simply PCR with some modifications we could assemble up to eight DNA fragments, a number that could be increased. The key point is that the homologous end should be long enough to ensure specific hybridization in the first step. Care should also be taken to minimize the presence of sequences capable of forming secondary structures in the homologous ends. However, this may not be problematic in the proposed thermal cycling program because the annealing temperature is high enough to prevent formation of these structures. The 50 bp overlapping region for adjacent fragments in this study was sufficient for eight-way assembly. Controlled increases in the size of overlapping region may further improve the efficiency of the method.
Conclusions
In summary, we have developed a technique (MOE-PCR) that enables complex assembling or cloning of multiple DNA sequences of various sizes and features (BioBricks) in a single PCR reaction. Both SLIC and MOE-PCR assembly techniques can be used to assemble complex recombinant DNA. But, in terms of simplicity and efficiency, the PCR-based MOE-PCR could be the method of choice for single in vitro recombination assemblies. The applications of this relatively high fidelity method could be extended to the construction of chimeric recombinant sequences, including operons, metabolic pathways, the construction of genomic or cDNA libraries, fusion protein strategies, mutation studies, functional analysis, expression of multi-domain proteins, protein engineering and last but certainly not the least genome editing which together with synthetic biology are revolutionizing life sciences. We expect the novel modified technique to be a robust, reliable and fast method in synthetic biology.Experimental details
Preparation of the fragments
Multiple DNA fragment assembly (MDFA)
We tried to utilize different techniques such as hybridization-based SHA,15 ligation independent-based SLIC9 and PCR-based CEPC,13 as well as some modifications to assemble the DNA fragments.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vector to insert molar ratio in a 15 or 30 μL (depending on the molar ratio) annealing reaction containing 1× ligation buffer with or without T4 DNA ligase (NEB). The mixture was incubated at 37 °C for 30 min and 5 μL of the reaction was transformed into 100 μL of chemically competent Escherichia coli cells. Finally, we plated them on agar plates containing ampicillin.9 Further verification steps were performed as described later.
1 vector to insert molar ratio in a 15 or 30 μL (depending on the molar ratio) annealing reaction containing 1× ligation buffer with or without T4 DNA ligase (NEB). The mixture was incubated at 37 °C for 30 min and 5 μL of the reaction was transformed into 100 μL of chemically competent Escherichia coli cells. Finally, we plated them on agar plates containing ampicillin.9 Further verification steps were performed as described later.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were used. Thermal cycling conditions were: 1 cycle at 95 °C for 5 min; 15 or 25 cycles at 98 °C for 20 s, 80–70 °C (gradient as 0.1 °C s−1), 72 °C for 2 min; and a final extension of 10 min at 72 °C. After running the MOE-PCR reactions, the products were directly transformed into high efficiency competent cells (108 to 109 cfu μg−1) without further purification.13 To verify the positive colonies containing all fragments of interest, 200 colonies were screened through a number of verification tests (see Verification section). An excel sheet was programmed to perform all the calculations related to multiple DNA fragments assemblies.
2 were used. Thermal cycling conditions were: 1 cycle at 95 °C for 5 min; 15 or 25 cycles at 98 °C for 20 s, 80–70 °C (gradient as 0.1 °C s−1), 72 °C for 2 min; and a final extension of 10 min at 72 °C. After running the MOE-PCR reactions, the products were directly transformed into high efficiency competent cells (108 to 109 cfu μg−1) without further purification.13 To verify the positive colonies containing all fragments of interest, 200 colonies were screened through a number of verification tests (see Verification section). An excel sheet was programmed to perform all the calculations related to multiple DNA fragments assemblies.Competent cell preparation
One of the fragments contained a Gateway recombination sequence; therefore, we used ccdB Survival™ 2 T1R competent cells as a specific bacterial strain in this regard. The methods of Inoue29 and Top10 (ref. 30) were employed to prepare the competent cells.Verification steps
We screened positive colonies containing all fragments of interest from a large number of colonies after plating on media containing suitable antibiotics through four sequential steps:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min, 10 μL of the supernatant was electrophoresed on a 0.8% agarose gel. Twenty positive colonies were identified based on the differences in band sizes (Fig. 3).
000g for 10 min, 10 μL of the supernatant was electrophoresed on a 0.8% agarose gel. Twenty positive colonies were identified based on the differences in band sizes (Fig. 3).The softwares CLC Main Workbench 6.9 (CLC bio, Denmark) and Geneious R7 (Biomatters, New Zealand) were used to manage the sequences.
Competing interests
The authors declare no competing interests.Author's contribution
SK conceived and designed the study with the input of HRM and ABA. SK, SA, MAR, and TJS carried out the experiments. SK and AM drafted the manuscript.List of abbreviations
| CPEC | Circular polymerase extension cloning |
| DA-PCR | Dual asymmetrical PCR |
| MAR | Matrix attachment region |
| MDFA | Multiple DNA fragment assembly |
| MOE-PCR | Multiple overlap extension PCR |
| LIC | Ligation independent cloning |
| OGAB | Ordered gene assembly in B. subtilis |
| SF | Substrate fragments |
| SLIC | Sequence and ligation independent cloning |
| SHA | Successive hybridization assembly |
Acknowledgements
We appreciate Dr Alireza Valdiani for his insightful comments on the manuscript. We thank Prof. Dr Rasedee Abdullah, Prof. Dr Abdul Rahman Bin Omar, Abby Salleh and other members of LIVEs (Laboratory of Immunotherapeutic and Vaccines). This work was supported by the following generous contributions: Research grants from the UPM Institute of Biosciences to ABA, and UPM Graduate Research Fellowship to SK.References
- B. Canton, A. Labno and D. Endy, Nat. Biotechnol., 2008, 26, 787–793 CrossRef CAS PubMed.
- N. Nandagopal and M. B. Elowitz, Science, 2011, 333, 1244–1248 CrossRef CAS PubMed.
- C. Engler, R. Kandzia and S. Marillonnet, PLoS One, 2008, 3, e3647 Search PubMed.
- J. C. Anderson, J. E. Dueber, M. Leguia, G. C. Wu, J. a. Goler, A. P. Arkin and J. D. Keasling, J. Biol. Eng., 2010, 4, 1 CrossRef PubMed.
- C. Lu, K. Mansoorabadi and T. Jeffries, Appl. Biochem. Biotechnol., 2007, 136, 136–140 Search PubMed.
- P. Xu, A. Vansiri, N. Bhan and M. A. G. Koffas, ACS Synth. Biol., 2012, 1, 256–266 CrossRef CAS PubMed.
- K. Tsuge, Nucleic Acids Res., 2003, 31, 133e CrossRef.
- D. L. Cheo, S. a. Titus, D. R. N. Byrd, J. L. Hartley, G. F. Temple and M. a. Brasch, Genome Res., 2004, 14, 2111–2120 CrossRef CAS PubMed.
- M. Z. Li and S. J. Elledge, Nat. Methods, 2007, 4, 251–256 CrossRef CAS PubMed.
- L. Zhang, G. Zhao and X. Ding, Sci. Rep., 2011, 1, 141 Search PubMed.
- C. Aslanidis and P. J. de Jong, Nucleic Acids Res., 1990, 18, 6069–6074 CrossRef CAS PubMed.
- A. V. Bryksin and I. Matsumura, BioTechniques, 2010, 48, 463–465 CrossRef CAS PubMed.
- J. Quan and J. Tian, Nat. Protoc., 2011, 6, 242–251 CrossRef CAS PubMed.
- M. Wäneskog and P. Bjerling, Anal. Biochem., 2014, 444, 32–37 CrossRef PubMed.
- X. Jiang, J. Yang, H. Zhang, H. Zou, C. Wang and M. Xian, PLoS One, 2012, 7, e30267 CAS.
- T. Ellis, T. Adie and G. S. Baldwin, Integr. Biol., 2011, 3, 109–118 RSC.
- P. Xu, N. Bhan and M. A. G. Koffas, Curr. Opin. Biotechnol., 2013, 24, 291–299 CrossRef CAS PubMed.
- L. K. Petersen and R. S. Stowers, PLoS One, 2011, 6, e24531 CAS.
- C. Li, A. Wen, B. Shen, J. Lu, Y. Huang and Y. Chang, BMC Biotechnol., 2011, 11, 92 CrossRef CAS PubMed.
- S. C. Sleight, B. a. Bartley, J. a. Lieviant and H. M. Sauro, Nucleic Acids Res., 2010, 38, 2624–2636 CrossRef CAS PubMed.
- H. H. Nour-eldin, F. Geu-flores and B. A. Halkier, in Methods in Molecular Biology, ed. A. G. Fett-Neto, Humana Press, Totowa, NJ, 2010, vol. 643, pp. 185–200 Search PubMed.
- L. Young and Q. Dong, Nucleic Acids Res., 2004, 32, e59 CrossRef PubMed.
- J. Quan and J. Tian, PLoS One, 2009, 4, e6441 Search PubMed.
- D. G. Gibson, G. a. Benders, K. C. Axelrod, J. Zaveri, M. a. Algire, M. Moodie, M. G. Montague, J. C. Venter, H. O. Smith and C. a. Hutchison, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 20404–20409 CrossRef CAS PubMed.
- M. A. Quail, T. D. Otto, Y. Gu, S. R. Harris, T. F. Skelly, J. A. McQuillan, H. P. Swerdlow and S. O. Oyola, Nat. Methods, 2012, 9, 10–11 CrossRef CAS PubMed.
- P. McInerney, P. Adams and M. Z. Hadi, Mol. Biol. Int., 2014, 2014, 12 Search PubMed.
- J. L. Schmid-Burgk, Z. Xie, S. Frank, S. Virreira Winter, S. Mitschka, W. Kolanus, A. Murray and Y. Benenson, Nucleic Acids Res., 2012, 40, e92 CrossRef CAS PubMed.
- N. a. Shevchuk, A. V. Bryksin, Y. a. Nusinovich, F. C. Cabello, M. Sutherland and S. Ladisch, Nucleic Acids Res., 2004, 32, e19 CrossRef PubMed.
- H. Inoue, H. Nojima and H. Okayama, Gene, 1990, 96, 23–28 CrossRef CAS PubMed.
- J. Sambrook, E. F. Fritsch and T. Maniatis, Molecular Cloning: A Laboratory Manual, 2001, vol. 3 Search PubMed.
Footnote |
† Electronic supplementary information (ESI) available: Additional data file 1 is a comparison of SLIC recombinations with (+T4) or without T4 DNA ligase (−T4) in different molarity ratios of vector and inserts (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Additional data file 2 is a comparison of the mutation rate for different DNA polymerases used in the study. Additional data file 3 demonstrates the efficiency of an eight-way DNA fragment assembly through SLIC and MOE-PCR. Additional data file 4 illustrates the virtual map of a circular recombinant construct containing all required elements; this vector represents a series of expression vectors constructed though MOE-PCR. See DOI: 10.1039/c6ra13172g 2). Additional data file 2 is a comparison of the mutation rate for different DNA polymerases used in the study. Additional data file 3 demonstrates the efficiency of an eight-way DNA fragment assembly through SLIC and MOE-PCR. Additional data file 4 illustrates the virtual map of a circular recombinant construct containing all required elements; this vector represents a series of expression vectors constructed though MOE-PCR. See DOI: 10.1039/c6ra13172g |
| This journal is © The Royal Society of Chemistry 2016 |