High performance asymmetric supercapacitor based on CoAl-LDH/GF and activated carbon from expanded graphite
Abstract
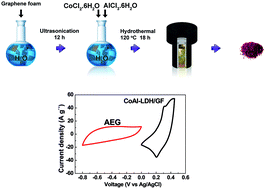
An asymmetric supercapacitor fabricated with a CoAl-layered double hydroxide/graphene foam (LDH/GF) composite as the positive electrode and activated carbon derived from expanded graphite (AEG) as the negative electrode in aqueous 6 M KOH electrolyte is reported. This CoAl-LDH/GF//AEG cell achieved a specific capacitance of 101.4 F g−1 at a current density of 0.5 A g−1 with a maximum energy density as high as 28 W h kg−1 and a power density of 1420 W kg−1. Furthermore, the supercapacitor also exhibited an excellent cycling stability with ∼100% capacitance retention after 5000 charging–discharging cycles at a current density of 2 A g−1. The results obtained show the potential use of the CoAl-LDH/GF//AEG material as a suitable electrode for enhanced energy storage in supercapacitors.

 Please wait while we load your content...
Please wait while we load your content...