Covalent immobilization of KR-12 peptide onto a titanium surface for decreasing infection and promoting osteogenic differentiation
Bin'en Nie†
a,
Haiyong Ao†a,
Chi Chenb,
Kai Xiea,
Jianliang Zhouc,
Teng Longa,
Tingting Tanga and
Bing Yue*a
aShanghai Key Laboratory of Orthopedic Implants, Department of Orthopedic Surgery, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, 639, Zhizaoju Road, Shanghai, 200011, P.R. China. E-mail: advbmp2@163.com; Fax: +86-21-64085875; Tel: +86-21-63139920
bInstitute and Department of Endocrinology and Metabolism, Shanghai Ninth People's Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, China
cDepartment of Cardiothoracic Surgery, The Second Affiliated Hospital of Nanchang University, P.R. China
First published on 6th May 2016
Abstract
Infection and poor bone-implant integration are the two main reasons for titanium (Ti) implant failure. Here, we investigated the feasibility of functionalizing Ti with the antimicrobial peptide, KR-12, derived from the human cationic antimicrobial peptide. The minimal inhibitory concentration and cell viability effects of KR-12 were investigated prior to immobilization on the Ti surface. The results showed that KR-12 possessed a wide anti-bacterial spectrum with no cytotoxicity to human bone marrow mesenchymal stem cells (hBMSCs). Successful covalent immobilization of KR-12 onto an amine-functionalized Ti (Ti-KR-12) surface was characterized by X-ray photoelectron spectroscopy. Gram-positive bacteria, Staphylococcus epidermidis and methicillin resistant Staphylococcus epidermidis were employed for antibacterial characterization. Ti-KR-12 substrates could significantly inhibit adhesion and colonization of common pathogenic bacteria and the drug resistance of pathogenic bacteria. The results of the CCK-8 assay, confocal laser scanning microscopy, and scanning electron microscopy showed that KR-12 covalently immobilized on Ti improved adhesion and proliferation of hBMSCs. The osteogenic differentiation of hBMSCs on samples was investigated by alkaline phosphatase staining, sirius red staining, alizarin red staining, and real-time PCR. The staining and real-time PCR results demonstrated that hBMSCs grown on Ti-KR-12 surfaces for 10 and 14 days under conditions inducing osteogenic differentiation displayed significantly higher alkaline phosphatase activity, larger extracellular matrix calcium deposition area, and higher expression of alkaline phosphatase, osteocalcin, osteopontin, and collagen type-1 mRNA than bare Ti. Our results demonstrated the KR-12 peptide was suitable for improving the biological properties of bioinert titanium. KR-12 showed antibacterial activity and the capability to promote cell proliferation and Ti-KR-12 surfaces significantly decreased bacteria adhesion, whilst promoting the osteogenic differentiation of hBMSCs.
1. Introduction
Titanium (Ti) and its alloys are widely used for orthopedic implants such as hip prosthesis, fracture fixation, and dental implants. Infection and poor bone-implant integration are the two main reasons for Ti implant failure. Infection associated with the clinical use of Ti implants is the major and most severe complication in orthopedic surgery. To overcome this, numerous attempts have been made to enhance the anti-infection efficacy of Ti implants, including modifications in surface topography and immobilization of antimicrobial agents and bioactive molecules on their surfaces.1–4 Antimicrobial agents such as antibiotics, silver, and chitosan and its derivatives have been widely applied to fabricate an anti-infective orthopedic implant.5–7 However, these antimicrobial agents may impair the viability of osteoblast cells and lead to the inhibition of osteogenic differentiation.8 Other approaches have investigated surface modification to promote osteogenic differentiation. Modifications to the Ti surface have included immobilizing collagen, vascular endothelial growth factor (VEGF), Arg-Gly-Asp (RGD) peptide, hydroxyapatite (HAp) nanoparticles, and bone morphogenic protein-2 (BMP-2) on the substrate.9,10 These bioactive agents either enhanced the adhesion of human bone marrow mesenchymal stem cells (hBMSCs), or induced the osteogenesis of the stem cells on the Ti substrate. However, as these studies have not taken infection into consideration it has remained an issue for Ti bioactive modification.The ultimate goal of fabricating an ideal implant should not only mitigate infection but also enhance osseointegration.11 Therefore, when immobilizing antimicrobial agents, or delivering them locally onto orthopedic implants, we need to take the osteoconductivity into consideration.12 Antimicrobial peptide (AMP) is considered to be a new class of antibiotics with the potential to replace the use of traditional antibiotics. AMP has broad-spectrum antimicrobial properties and participates in the innate immune response, protecting a host from pathogen infection.13,14 Moreover, this peptide is unlikely to lead to the development of bacterial resistance compared to the conventional antibiotics. For example, antimicrobial peptide LL-37 can recruit neutrophils, monocytes, and T cells and can enhance bacterial phagocytosis by human macrophages.15 Innate defense regulator (IDR) peptide can kill bacteria through chemokine induction, promote leukocyte recruitment, and regulate neutrophil function.16 Due to its anti-bacterial properties, ability to regulate the immune system, and other complex bioactivities, AMP has been widely applied to functionalize biomaterial surfaces.17
Several approaches had been used to immobilize AMP on Ti implants such as the layer-by-layer technique, processing of nanotubular structures on the Ti surface, and calcium phosphate coatings onto which antimicrobial peptides can be locally delivered. These approaches have demonstrated that AMP can effectively kill bacteria in vitro, and did not impair bone growth.18–20 When using peptide drugs, such as AMP or IDR, as a coating for orthopedic implants, it is important to remember that they are proteins and can easily degrade.21 Covalently immobilized AMPs on Ti implant surfaces have many advantages such as long-term stability and low toxicity. In addition, a covalent bond with the metal provides a strong and stable coating able to withstand surface forces exerted during surgical implantation as well as the flow of fluids in vivo.22,23 Therefore, covalent immobilization is a promising approach to preserve the biological activity of AMP.
Host defense antimicrobial peptides are key components of human innate immunity that play an indispensable role in human health. LL-37, the only human cationic antimicrobial peptide, is composed of 37 amino acids with a broad spectrum of antibacterial activity and can enter the cell to play a role in immune regulation.24 LL-37 plays a decisive part in eliminating bacteria under physiological conditions and studies have proven it can clear extracellular and intracellular S. aureus within low peptide concentration.25 It is thought that LL-37 kills bacteria directly through regulating the body's defense system. When bacterial infection occurs, LL-37 is produced by epithelial cells and released from the neutrophil granules.26,27 LL-37 has also been proven to enhance migration of mesenchymal cells inside bone defects without affecting the osteogenic differentiation of hBMSCs.28 The KR-12 peptide was designed based on the human cathelicidin LL-37 antimicrobial peptide and possessed both the antimicrobial and anti-inflammatory activities of LL-37 without the associated mammalian cell toxicity.29 The disadvantages of the LL-37 peptide include toxicity and affects on keratinocyte viability at 10 μM.30 Use of the KR-12 peptide eliminates the toxicity problems associated with LL-37 and, as KR-12 is a shorter peptide, the cost to synthetically produce KR-12 is much lower than LL-37.
In this study, the amino acid sequence 18–29 of the human cathelicidin (LL-37) was chosen as an antimicrobial agent to develop a new antimicrobial surface for application in the biofunctionalization of Ti implants and named KR-12. We investigated the feasibility of covalent immobilization of the KR-12 peptide on the Ti surface to inhibit bacterial colonization whilst promoting osteogenic differentiation.
2. Experiment
2.1 Materials
Ti–6Al–4V substrates (ϕ 10 × 2 mm), KR-12: KRIVQRIKDFLR-NH2 and Gly-Gly-Tyr-Arg peptides was synthesized with >95% purity by GL BioChem (Shanghai, China), toluene, 3-aminopropyltriethoxysilane (APTES, Sinopharm Chemical Reagent Co., China), CCK-8 Kit (Donjindo, Japan), LIVE/DEAD® BacLight. Bacterial Viability Kits (Molecular Probes), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, TOKYO, Japan), N-hydroxysulfosuccinimide (sulfo-NHS, Shanghai), alpha minimum essential medium (α-MEM, Hyclone), fetal bovine serum (FBS, Gibco, Australia), trypsin–EDTA (0.5%), Alexa Fluor 488 secondary antibody, 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich), dexamethasone, ascorbic acid, β-glycerol phosphate (Sigma-Aldrich), ALP staining kit, ALP quantitative kit (Beyotime Biotechnology, China), Pierce™ BCA Protein Assay Kit (ThermoFisher), TRIZOL (Invitrogen), cDNA Synthesis Kit, real-time PCR kit (SYBR Premix EX Taq, TaKaRa), Staphylococcus aureus (S. aureus, ATCC 25923) and methicillin-resistant Staphylococcus aureus (MRSA, ATCC, 43300), Staphylococcus epidermidis (S. epidermidis, ATCC 35984) and methicillin resistant Staphylococcus epidermidis (MRSE, ATCC), Escherichia coli (E. coli, ATCC 25922).2.2 Methods
2.2.6.1 Early cell adhesion. The early adhesion of hBMSCs on each Ti substrate was detected by nuclear DAPI fluorescence staining at 1, 2, and 3 h. Briefly, after 1, 2, and 3 h the Ti samples were washed with PBS to detach non-adherent cells, fixed with 4% paraformaldehyde for 1 h, then 0.2% TritonX-100 was added to each Ti sample and incubated for 15 min. The cells were incubated in DAPI (5 μg mL−1) for 15 min at room temperature in the dark. Each Ti sample was washed with PBS and observed with a CLSM (Leica TCS SP2, Germany). Cell numbers were counted by Image-Pro Plus 6.0 software.
2.2.6.2 Cell proliferation. Cell proliferation on each Ti sample was measured at 1, 3, and 5 days post seeding. Cells were cultured as described above. Ti samples were washed twice with PBS and transferred to new 24 well plates. Then 500 μL of fresh culture medium and 50 μL of CCK-8 solution was added to each well and incubated at 37 °C for 2 h. The optical absorbance of the solution was determined using a microplate reader at a wavelength of 450 nm. Blank growth medium was used as the control.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Optical density at 540 nm was measured using a spectrophotometer (Bio-Tek, USA). Matrix mineralization by primary osteoblasts was evaluated by alizarin red staining. After culturing for 10 and 14 days, cells were washed twice with PBS and fixed in 75% ethanol for 1 h. Cell cultures were stained with alizarin red in distilled water for 10 min at room temperature. Samples were carefully washed with distilled water and images were acquired. For quantitative analyses, the stain was dissolved in 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH = 7) and the absorbance measured at 620 nm.
1. Optical density at 540 nm was measured using a spectrophotometer (Bio-Tek, USA). Matrix mineralization by primary osteoblasts was evaluated by alizarin red staining. After culturing for 10 and 14 days, cells were washed twice with PBS and fixed in 75% ethanol for 1 h. Cell cultures were stained with alizarin red in distilled water for 10 min at room temperature. Samples were carefully washed with distilled water and images were acquired. For quantitative analyses, the stain was dissolved in 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH = 7) and the absorbance measured at 620 nm.| Osteogenesis gene | F | R |
|---|---|---|
| COL1 | 5′-CCTGAGCCAGCAGATCGAGAA-3′ | 5′-GGTACACGCAGGTCTCACCAGT-3′ |
| ALP | 5′-TTGACCTCCTCGGAAGACACTC-3′ | 5′-CCATACAGGATGGCAGTGAAGG-3′ |
| OPN | 5′-CTGAACGCGCCTTCTGATTG-3′ | 5′-ACATCGGAATGCTCATTGCTCT-3′ |
| OC | 5′-GGCGCTACCTGTATCAATGGC-3′ | 5′-TGCCTGGAGAGGAGCAGAACT-3′ |
| GAPDH | 5′-CCTGCACCACCAACTGCTTA-3′ | 5′-AGGCCATGCCAGTGAGCTT-3′ |
3. Results
3.1 MIC and hBMSC proliferation effects of KR-12 peptide
The KR-12 peptide possessed broad-spectrum anti-bacterial properties. The MIC of KR-12 was 64 μg mL−1 for S. aureus and MRSA, 32 μg mL−1 for S. epidermidis and MRSE, and 16 μg mL−1 for E. coli. The effect of the KR-12 peptide on cell viability was tested in hBMSCs using a CCK-8 test. As shown in Fig. 1 the KR-12 peptide showed no cytotoxicity to hBMSCs, even at a concentration of 1000 μg mL−1. Furthermore, KR-12 can promote the proliferation of hBMSCs at concentrations of 128–1028 μg mL−1. | ||
| Fig. 1 hBMSCs were treated with different concentrations of KR-12 peptide for 1 day, 3 days and 7 days prior to measuring the cell viability by a CCK-8 test (*P < 0.05). | ||
3.2 Characterization
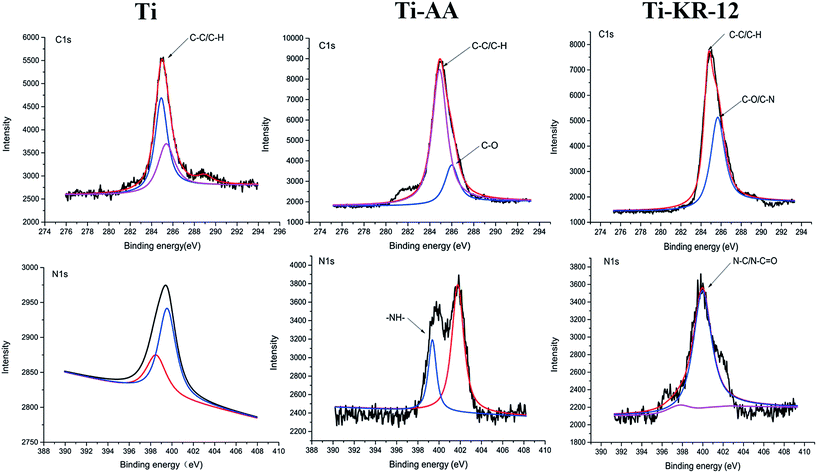
After ultrasonic cleaning to remove the non-grafted KR-12, the atomic composition of all Ti surfaces was investigated by means of XPS. Silanization and KR-12 peptide covalent immobilization resulted in an increase in the percentage of C 1s and N 1s, and a reduction in O 1s and Ti 2p signals compared with control Ti (Table 2). The reduction of Ti and increase of N 1s also indicate that the titanium surface was amine-functionalized with 3-aminopropyltriethoxysilane (APTES). The KR-12 peptide was covalently immobilized on the Ti surface. The amine groups on the Ti surface covalently bonded with the carboxyl group of the KR-12 peptide, resulting in an increase of C 1s and N 1s, and a decrease in O 1s and Ti. Deconvolutions of the C 1s and N 1s peaks from high-resolution XPS spectra were shown in Fig. 2. The C 1s signal of Ti-KR-12 showed two peaks at binding energies of approximately 285 (aliphatic C–C) and 286 eV (C–O/C–N groups). KR-12 peptide immobilization via APTES silanization resulted in a significant increase in the high-energy component of C 1s compared to controls. The XPS narrow scan of the N 1s regions of Ti-KR-12 showed two peaks, at approximately 397 and 400 eV. The 400 eV peaks were from the amine and/or amide bonds of the KR-12 peptides. XPS data confirmed the covalent immobilization of KR-12 on the Ti surface.| C 1s% | O 1s% | Si 2p% | N 1s% | Ti 2p% | |
|---|---|---|---|---|---|
| Ti | 9.19 | 65.28 | 4.77 | 0.97 | 17.92 |
| Ti-AA | 24.25 | 51.14 | 4.51 | 3.58 | 16.04 |
| Ti-KR-12 | 24.13 | 48.16 | 7.46 | 4.34 | 14.81 |
The peptide concentration and the absorbance showed good linear correlation, which was described by the equation: Y = 0.054X + 0.056 (r = 0.997). The yield of this covalently coupling reached to 36.5 ± 4.9%. The quantity of conjugated KR-12 peptide on the Ti surface reached to the amount of 72.9 ± 9.87 μg per Ti disc, which was much higher than the MIC of KR-12 against S. epidermidis.
3.3 Water contact angle
The Ti and Ti-AA substrates displayed contact angles of 71.9 ± 1.67° and 38.08 ± 3.75°, respectively. Following silanization and the covalent immobilization of the KR-12 peptide on the Ti surface, the contact angles for the Ti surfaces decreased to 19.01 ± 1.25° (Fig. 3). The silanized and KR-12 peptide functionalized Ti surfaces exhibited increased hydrophilicity compared to Ti.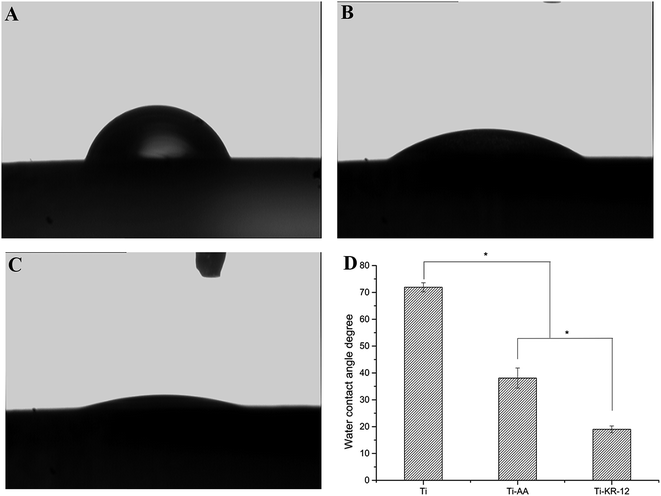 | ||
| Fig. 3 Water contact angle measurement. (A) Ti substrate, (B) Ti-AA substrate, (C) Ti-KR-12 substrate. (D) The quantitative measurement of the water contact angle of each Ti substrate (*P < 0.05). | ||
3.4 Confocal laser-scanning microscopy (CLSM), spread plate method and scanning electron microscopy (SEM) for the characterization of bacteria viability on each Ti surface
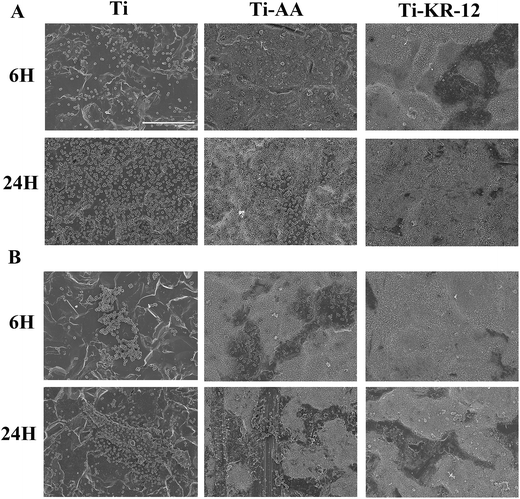
After 6 h and 24 h each Ti substrate was incubated with S. epidermidis and MRSE. A large number of bacterial colonies were apparent on the Ti control and the Ti-AA groups at both time points. Significantly fewer viable bacterial colonies were seen to adhere to the Ti-KR-12 surface at 6 h or 24 h (P < 0.05, Fig. 4B and D). Viable bacteria (stained green) can be seen on the Ti and Ti-AA substrates at 6 and 24 h (Fig. 4A and C). Significantly less green staining was observed in the Ti-KR-12 group at both time points. The antibacterial activity of Ti-KR-12 was around 90% for S. epidermidis and MRSE at each time point and dead bacteria (stained red) are evident on the Ti-KR-12 substrate. The Ti-AA surface did not inhibit bacterial colonization and may have promoted the growth of MRSA on the Ti surface.The amount of bacteria on each Ti sample was confirmed by SEM. Fig. 5 showed that the Ti and Ti-AA substrates had many adherent S. epidermidis and MRSE colonies after 6 and 24 h, while the Ti-KR-12 surface had very few adherent bacterial colonies at the either time point.
3.5 hBMSC adhesion, proliferation, and morphology
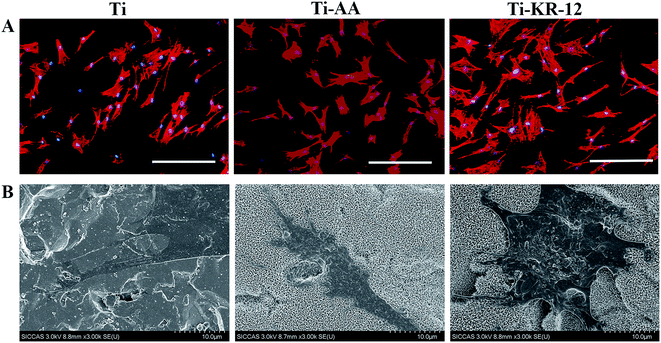
The early adhesion of hBMSCs was investigated at 1 h, 2 h, and 3 h after culture on Ti discs and the cell proliferation was investigated at days 1, 3, and 7 after culture on Ti discs. Early cell adhesion indicated that Ti-KR-12 could recruit more cells to the surface than the Ti and Ti-AA substrates (Fig. 6A). Cell numbers were significant higher in the Ti-AA and Ti-KR-12 groups than the Ti group at the same time point, (P < 0.05, Fig. 6B). More cells on the Ti surface means more rapid proliferation and is of great importance to osteoblast differentiation. One and three days after seeding, cell counts were significantly higher in the Ti-AA and Ti-KR-12 groups (P < 0.05) than the Ti group. Five days after seeding, the cell counts were significantly higher in the Ti-KR-12 group (P < 0.05) than those of the Ti and Ti-AA groups (Fig. 6C).Cell morphology on each Ti surface was characterized by fluorescence microscopy and SEM. As shown in Fig. 7A, after hBMSCs were cultured with each Ti sample, cells can colonize close to the Ti surface and spread well. Cells colonized on Ti-KR-12 have a larger spreading area (Fig. 7B), indicating that hBMSCs grow well on the Ti-KR-12 surface.
3.6 Osteogenic differentiation of hBMSCs
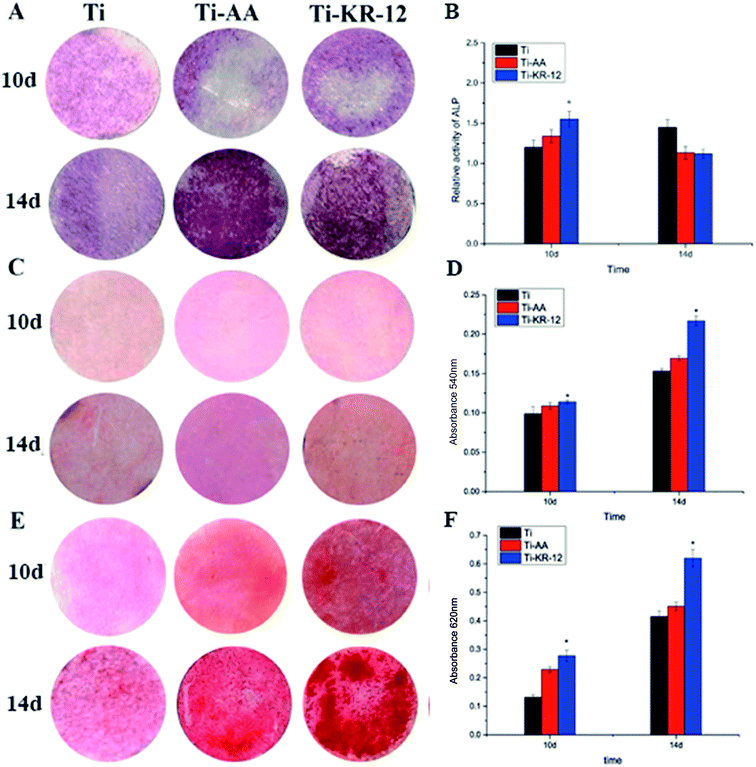
We measured the ALP activity on various Ti surfaces co-cultured with hBMSCs after 10 and 14 days. Whilst ALP staining was not obvious on each Ti sample quantitation, shown in Fig. 8B, demonstrated that Ti-KR-12 displayed higher ALP activity than in the Ti and Ti-AA groups at day 10 (P < 0.05). ALP activity was higher in the Ti group than in the Ti-AA and Ti-KR-12 groups after 14 days. We measured the collagen secretion of hBMSCs on various Ti surfaces. The quantitative assay indicated that the Ti-KR-12 group secreted more collagen than the Ti and Ti-AA groups (P < 0.05) (Fig. 8D). Furthermore, the ECM calcium deposition area was larger on Ti-KR-12 surfaces than on Ti and Ti-AA surfaces, and quantitative results were consistent with staining results (Fig. 8E and F).3.7 Osteogenesis-related gene expression
We investigated osteogenesis-related gene expression in various Ti surfaces co-cultured with hBMSCs at days 10 and 14. We examined the expression of ALP and COL as gene markers of early osteogenic differentiation, and OPN and OC as markers of late osteogenic differentiation (Fig. 9). At day 10, Ti-KR-12 samples showed significantly upregulated early osteogenesis-related gene expression (ALP and COL) compared with the Ti and Ti-AA samples (P < 0.05, Fig. 9). At day 14 the Ti-KR-12 samples showed upregulated OPN expression, and the Ti-AA and Ti-KR-12 samples showed upregulated expression of OC compared with the Ti (P < 0.05, Fig. 9).4. Discussion
Orthopedic implants face two devastating complications in vivo: poor integration of fixtures with bone tissue, and orthopedic implant infections (OII). These complications are primarily due to the bio-inert nature of the orthopedic implant, which lacks both the ability to reduce bacterial growth, and the bioactivity to promote implant-bone integration. Here we presented, for the first time, that the Ti functionalized with a novel KR-12 peptide derived from human LL-37, could effectively prevent infection and promote osteogenic differentiation.Traditional antibiotics inhibit the proliferation and differentiation of mesenchymal stem cells to some extent.31–34 Unlike antibiotics, AMP exhibited very low cell toxicity. In recent years, AMP has been a popular bioactive component for the surface modification of inert Ti.22 Different AMPs may possess different bioactivities. For example, human lactoferrin 1-11 (hLF1-11) has been widely applied in the modification of Ti,35–37 and has been shown to exhibit toxicity on bone cells or erythrocytes with concentrations up to 400 μg mL−1.38 Our study, however, indicated that that the KR-12 peptide possessed a wide antimicrobial spectrum without any cell toxicity to hBMSCs, even at a concentration of 1024 μg mL−1, which far exceeded its MIC (Fig. 1). In addition, our results indicated that the KR-12 peptide could promote hBMSCs proliferation at concentrations of 128–1024 μg mL−1. This unique bioactivity made KR-12 an attractive candidate for the application of implant biofunctionalization.
We successfully covalently immobilized KR-12 onto the Ti surface. The antibacterial capability of Ti-KR-12 group measured against adherent bacteria was higher than that of the Ti and Ti-AA groups. This indicated that the KR-12 peptide could maintain its antibacterial capabilities when covalent immobilized on the Ti surface. The amino acid sequence of KR-12 is KRIVQRIKDFLR. This sequence included five positively charged amine groups (3 Rs, and 2 Ks), that could recruit negatively charged bacteria to the KR-12 modified surface by the charge effect.39 In addition, the KR-12 peptide could disrupt the bacterial cell wall, leading to bacterial death.40 We therefore proposed that the mechanism of action of the KR-12 functionalized Ti was to recruit bacteria to the Ti surface through the charge effect and then kill them.
Bone mesenchymal stem cells (BMSCs) are the key repair cell in bone healing and implant osseointegration. Cell adhesion, spreading state, proliferation, and cell differentiation on the implant surface are critical for bone regeneration.41 Increasing hBMSCs adhesion, proliferation and osteogenic differentiation on Ti surface can promote the bone-implant integration.42 DAPI staining showed that Ti-KR-12 could significantly improve early cell adhesion on the Ti-KR-12 surface and the CCK-8 assay indicated that Ti-KR-12 could significantly promote cell proliferation on the Ti-KR-12 surface.
Early adhesion of hBMSCs on the modified Ti surface plays a decisive role in its biofunction. On one hand, cell adhesion on the Ti surface is the first step of cell proliferation and differentiation, whilst on the other hand, rapid cell adhesion on Ti surface can win the race towards bacteria adhesion on the Ti surface, and inhibit the bacteria adhesion and biofilm formation at early time, which is of great importance to prevent orthopedic implant infection.43,44 It had been reported that LL-37 could promote the migration of mesenchymal stromal cells.45 Our results indicated that the KR-12 peptide retained bioactivity when covalent immobilized on Ti surface and promoted the early adhesion and proliferation of hBMSCs.
Apart from the cell adhesion and proliferation, cell differentiation of hBMSCs on Ti also plays an important role in the process of bone-implant integration. This study showed that Ti-KR-12 substrate could promote both early and late osteogenic differentiation, since the ALP activity and ECM mineralization was increased relative to the Ti and Ti-AA substrates (Fig. 8). To confirm the osteogenic differentiation of KR-12 functionalized Ti, real-time PCR was used to monitor the changes in the expression levels of osteogenic differentiation-related genes (ALP, COL1, OPN, and OC) in hBMSCs cultured on the Ti, Ti-AA and Ti-KR-12 substrates. The Ti-KR-12 group exhibited significantly higher expression of early and late osteogenic differentiation gene markers than the Ti and Ti-AA groups (Fig. 9). These results indicated that the KR-12 peptide could promote osteogenic differentiation of hBMSCs in vitro.
KR-12 modified Ti had several major advantages compared with previously reported Ti biofunctionalization. Firstly, the KR-12 peptide exhibited no cytotoxicity to hBMSCs and might even promote hBMSC proliferation, which played a crucial role in bone-implant integration and bone regeneration. Secondly, traditional biofunctionalization of Ti for anti-bacterial properties often involved immobilization of exogenous antibacterial agents onto the Ti surface. This impaired the hBMSC proliferation and differentiation and might give rise to bacterial resistance. In contrast, the KR-12 modified Ti could promote osteoblast differentiation and matrix mineralization. Thirdly, since the AMP comprised an important part of the body's defense system against bacteria,46 the AMP modified Ti surface could simulate the bodies defense system to eliminate bacteria, without impairing hBMSC proliferation and differentiation. Such unique properties made KR-12 suitable for the biofunctionalization of Ti for anti-bacterial properties and for promoting osteogenic differentiation.
5. Conclusion
We presented a novel antimicrobial peptide, KR-12, with a broad antimicrobial spectrum and the ability to promote hBMSC proliferation. KR-12 was successfully covalently immobilized onto Ti surface through its C-terminal cysteine whilst maintaining its bioactivity. The functionalization of Ti with KR-12 significantly decreased bacterial adhesion as compared to Ti alone. The antibacterial mechanism of KR-12 functionalized Ti included recruiting planktonic bacteria to the Ti surface through the charge effect and then killing them. Furthermore, KR-12 functionalized Ti could promote the osteogenic differentiation of hBMSCs. These covalent modifications were attractive for biomedical implants as they exhibited the ability to prevent implant-associated infection and promote bone-implant osseointegration.Acknowledgements
This work was supported by the National Natural Science Foundation of China (81472119, 81501856), and the National High-tech Research and Development Program (863 Program) of China (No. 2014AA020539).References
- L. Zhao, P. K. Chu, Y. Zhang and Z. Wu, J. Biomed. Mater. Res., Part B, 2009, 91, 470–480 CrossRef PubMed.
- C. Ungureanu, C. Dumitriu, S. Popescu, M. Enculescu, V. Tofan, M. Popescu and C. Pirvu, Bioelectrochemistry, 2016, 107, 14–24 CrossRef CAS PubMed.
- W. T. Lin, H. L. Tan, Z. L. Duan, B. Yue, R. Ma, G. He and T. T. Tang, Int. J. Nanomed., 2014, 9, 1215–1230 Search PubMed.
- L. Zhao, Y. Hu, D. W. Xu and K. Y. Cai, Colloids Surf., B, 2014, 119, 115–125 CrossRef CAS PubMed.
- N. J. Hickok and I. M. Shapiro, Adv. Drug Delivery Rev., 2012, 64, 1165–1176 CrossRef CAS PubMed.
- L. Zhao, P. K. Chu, Y. Zhang and Z. Wu, J. Biomed. Mater. Res., Part B, 2009, 91, 470–480 CrossRef PubMed.
- Y. Zhao, H. Cao, H. Qin, T. Cheng, S. Qian, M. Cheng, X. Peng, J. Wang, Y. Zhang, G. Jin, X. Zhang, X. Liu and P. K. Chu, ACS Appl. Mater. Interfaces, 2015, 7, 17826–17836 CAS.
- P. Dubey, I. Matai, S. U. Kumar, A. Sachdev, B. Bhushan and P. Gopinath, Adv. Colloid Interface Sci., 2015, 221, 4–21 CrossRef CAS PubMed.
- C. Y. Chien, T. Y. Liu, W. H. Kuo, M. J. Wang and W. B. Tsai, J. Biomed. Mater. Res., Part A, 2013, 101, 740–747 CrossRef PubMed.
- C. Y. Chien and W. B. Tsai, ACS Appl. Mater. Interfaces, 2013, 5, 6975–6983 CAS.
- S. B. Goodman, Z. Yao, M. Keeney and F. Yang, Biomaterials, 2013, 34, 3174–3183 CrossRef CAS PubMed.
- M. Kazemzadeh-Narbat, S. Noordin, B. A. Masri, D. S. Garbuz, C. P. Duncan, R. E. W. Hancock and R. Wang, J. Biomed. Mater. Res., Part B, 2012, 100, 1344–1352 CrossRef PubMed.
- N. K. Brogden and K. A. Brogden, Int. J. Antimicrob. Agents, 2011, 38, 217–225 CAS.
- M. Zasloff, Nature, 2002, 415, 389–395 CrossRef CAS PubMed.
- M. Wan, A. M. van der Does, X. Tang, L. Lindbom, B. Agerberth and J. Z. Haeggstrom, J. Leukocyte Biol., 2014, 95, 971–981 CrossRef PubMed.
- S. C. Mansour, C. de la Fuente-Nunez and R. E. Hancock, J. Pept. Sci., 2015, 21, 323–329 CrossRef CAS PubMed.
- M. Bagheri, M. Beyermann and M. Dathe, Bioconjugate Chem., 2012, 23, 66–74 CrossRef CAS PubMed.
- M. Kazemzadeh-Narbat, J. Kindrachuk, K. Duan, H. Jenssen, R. E. Hancock and R. Wang, Biomaterials, 2010, 31, 9519–9526 CrossRef CAS PubMed.
- M. Kazemzadeh-Narbat, B. F. L. Lai, C. Ding, J. N. Kizhakkedathu, R. E. W. Hancock and R. Wang, Biomaterials, 2013, 34, 5969–5977 CrossRef CAS PubMed.
- M. Ma, M. Kazemzadeh-Narbat, Y. Hui, S. Lu, C. Ding, D. D. Chen, R. E. Hancock and R. Wang, J. Biomed. Mater. Res., Part A, 2012, 100, 278–285 CrossRef PubMed.
- M. Salwiczek, Y. Qu, J. Gardiner, R. A. Strugnell, T. Lithgow, K. M. McLean and H. Thissen, Trends Biotechnol., 2014, 32, 82–90 CrossRef CAS PubMed.
- F. Costa, I. F. Carvalho, R. C. Montelaro, P. Gomes and M. C. L. Martins, Acta Biomater., 2011, 7, 1431–1440 CrossRef CAS PubMed.
- S. A. Onaizi and S. S. Leong, Biotechnol. Adv., 2011, 29, 67–74 CrossRef CAS PubMed.
- U. H. N. Dürr, U. S. Sudheendra and A. Ramamoorthy, Biochim. Biophys. Acta, Biomembr., 2006, 1758, 1408–1425 CrossRef PubMed.
- J. Noore, A. Noore and B. Li, Antimicrob. Agents Chemother., 2013, 57, 1283–1290 CrossRef CAS PubMed.
- G. Wang, B. Mishra, R. F. Epand and R. M. Epand, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 2160–2172 CrossRef CAS PubMed.
- D. Vandamme, B. Landuyt, W. Luyten and L. Schoofs, Cell. Immunol., 2012, 280, 22–35 CrossRef CAS PubMed.
- M. Kittaka, H. Shiba, M. Kajiya, T. Fujita, T. Iwata, K. Rathvisal, K. Ouhara, K. Takeda, T. Fujita, H. Komatsuzawa and H. Kurihara, Peptides, 2013, 46, 136–142 CrossRef CAS PubMed.
- B. Jacob, I. S. Park, J. K. Bang and S. Y. Shin, J. Pept. Sci., 2013, 19, 700–707 CrossRef CAS PubMed.
- M. H. Braff, M. Zaiou, J. Fierer, V. Nizet and R. L. Gallo, Infect. Immun., 2005, 73, 6771–6781 CrossRef CAS PubMed.
- I. Pountos, T. Georgouli, K. Henshaw, B. Howard and P. V. Giannoudis, Cell. Mol. Biol., 2014, 60, 1–7 CAS.
- A. Ince, N. Schutze, C. Hendrich, F. Jakob, J. Eulert and J. F. Lohr, Swiss Med. Wkly., 2007, 137, 139–145 CAS.
- A. Ince, N. Schutze, N. Karl, J. F. Lohr and J. Eulert, Int. Orthop., 2007, 31, 223–228 CrossRef PubMed.
- C. R. Rathbone, J. D. Cross, K. V. Brown, C. K. Murray and J. C. Wenke, J. Orthop. Res., 2011, 29, 1070–1074 CrossRef CAS PubMed.
- F. Costa, S. Maia, J. Gomes, P. Gomes and M. C. L. Martins, Acta Biomater., 2014, 10, 3513–3521 CrossRef CAS PubMed.
- M. Godoy-Gallardo, C. Mas-Moruno, M. C. Fernandez-Calderon, C. Perez-Giraldo, J. M. Manero, F. Albericio, F. J. Gil and D. Rodriguez, Acta Biomater., 2014, 10, 3522–3534 CrossRef CAS PubMed.
- M. Godoy-Gallardo, C. Mas-Moruno, K. Yu, J. M. Manero, F. J. Gil, J. N. Kizhakkedathu and D. Rodriguez, Biomacromolecules, 2015, 16, 483–496 CrossRef CAS PubMed.
- H. P. Stallmann, C. Faber, A. Bronckers, J. M. A. de Blieck-Hogervorst, C. Brouwer, A. V. N. Amerongen and P. Wuisman, Peptides, 2005, 26, 2355–2359 CrossRef CAS PubMed.
- E. Glukhov, M. Stark, L. L. Burrows and C. M. Deber, J. Biol. Chem., 2005, 280, 33960–33967 CrossRef CAS PubMed.
- G. S. Wang, J. Biol. Chem., 2008, 283, 32637–32643 CrossRef CAS PubMed.
- P. N. De Aza, D. García-Bernal, F. Cragnolini, P. Velasquez and L. Meseguer-Olmo, Mater. Sci. Eng., C, 2013, 33, 4009–4020 CrossRef CAS PubMed.
- H.-Y. Ao, Y.-T. Xie, S.-B. Yang, X.-D. Wu, K. Li, X.-B. Zheng and T.-T. Tang, Journal of Orthopaedic Translation, 2016, 5, 16–25 CrossRef.
- A. G. Gristina, Science, 1987, 237, 1588–1595 CAS.
- B. L. Foss, N. Ghimire, R. Tang, Y. Sun and Y. Deng, Colloids Surf., B, 2015, 134, 370–376 CrossRef CAS PubMed.
- S. B. Coffelt, F. C. Marini, K. Watson, K. J. Zwezdaryk, J. L. Dembinski, H. L. LaMarca, S. L. Tomchuck, K. Honer zu Bentrup, E. S. Danka, S. L. Henkle and A. B. Scandurro, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 3806–3811 CrossRef CAS PubMed.
- E. H. Mattar, H. A. Almehdar, H. A. Yacoub, V. N. Uversky and E. M. Redwan, Cytokine Growth Factor Rev., 2016, 28, 99–111 Search PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |