Sorafenib-fortified zein–chondroitin sulphate biopolymer nanoparticles as a novel therapeutic system in gastric cancer treatment
Abstract
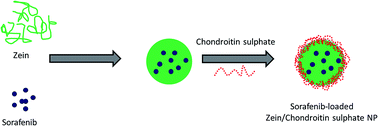
Gastric cancer is the second most common cause of cancer related death worldwide and lacks a highly effective treatment for the advanced disease. In this study, we have successfully demonstrated the preparation of core–shell type biopolymer based zein/chondroitin sulphate nanoparticles for the delivery of sorafenib in gastric cancers. The SRF-loaded zein/chondroitin sulphate nanoparticles (SZCS) were nanosized with a spherical morphology and exhibited a higher encapsulation of more than >90%. The biopolymer nanoparticles showed the ability to release the drug in a controlled manner for 120 h, indicating their potential application in systemic delivery. The nanoparticles showed a remarkable uptake in gastric cancer cells in a time-dependent manner. The SZCS displayed an improved cytotoxic effect compared to that of free SRF in the equivalent concentrations in SGC7901 cancer cells. Also, we demonstrated a higher apoptosis and caspase 3/7 activity for the SZCS nanoparticle system. Based on our results, we can conclude that SZCS might hold great potential in the treatment of gastric cancers.

 Please wait while we load your content...
Please wait while we load your content...