Synthesis, decoration, and cellular effects of magnetic mesoporous silica nanoparticles†
J. L. Nyalosasoa,
E. Rascola,
C. Pisaniab,
C. Dorandeua,
X. Dumaila,
M. Maynadierc,
M. Gary-Bobod,
J. Lai Kee Hime,
P. Brone,
M. Garciad,
J. M. Devoissellea,
O. Pratb,
Y. Guaria,
C. Charnaya and
J. Chopineau*af
aInstitut Charles Gerhardt de Montpellier (ICGM), CNRS UMR 5253/UM/ENSCM, Université Montpellier, Campus Triolet, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France. E-mail: joel.chopineau@enscm.fr
bCEA – Marcoule, Direction de la Recherche Fondamentale, BIAM, BP 17171, 30207 Bagnols-sur-Cèze, France
cNanoMedSyn, 15 avenue Charles Flahault, 34093 Montpellier, France
dIBMM, CNRS UMR 5247/UM/ENSCM, Faculté de Pharmacie de Montpellier, 15 avenue Charles Flahault, 34093 Montpellier, Cedex 05, France
eCentre de biochimie structurale CNRS UMR 5048/UM/INSERM U 1054, 34090 Montpellier, France
fUniversité de Nîmes Rue Georges Salan, 30000 Nîmes, France
First published on 8th June 2016
Abstract
Mesoporous Silica Nanoparticles (MSN) are now considered as multifunctional platforms for pharmaceutical development. The goal of this study was to optimize a synthesis procedure to obtain reproducible monodisperse magnetic core@shell Fe3O4@MSN with different coatings and study their uptake by cells. 100 nm core@shell nanoparticles with a unique 18 nm magnetic core were synthesized and covered with PEG groups or coated with a lipid bilayer in a controlled manner and their cellular fate was investigated. Both PEG and lipidic coated nanoparticles exhibit a low toxicity when incubated with Hep-G2 cells compared to pristine ones. Furthermore, the different real-time impedance cellular profiles that were observed and the particles uptake by the cells investigated by TEM suggest different internalization mechanisms or uptake kinetics depending on MSN coverage. This study is a first essential step to ensuring the preparation of well-defined nanomaterials for medical applications; it is considered as a crucial step to be able to perform detailed research about cellular trafficking and signaling pathways.
1 Introduction
The emerging field of nanomedicine has been extensively developed over the past two decades taking advantage of the dual benefits of materials at the nanoscale. Firstly, due to the size reduction, new physicochemical properties such as optical, magnetic or thermal properties appear. Second, nanodrugs are able to overcome certain biological barriers and would therefore have a specific biological fate in terms of biodistribution and biokinetics that can be exploited for biomedical purposes. A major field of application of nanomedicines is that of cancer for diagnosis, imaging, drug delivery and cell targeting and many research studies are directed towards this broad area of application.1 As part of this studies, it was shown that the biological behaviour of nanoparticles (NPs), i.e. the biodistribution, cellular uptake, or cytotoxicity is very dependent on their physicochemical properties.2–4 Among the most important physicochemical parameters were identified the particle size, shape and surface chemistry.2 As a general rule, in terms of size, it has been shown that the smaller nanoparticles are more toxic than their larger counterparts, any other parameter (chemical composition, coating, etc.) equivalent otherwise.5,6 Also, more specifically to the core@shell nanoparticles, the fact that in a given sample be present NPs without a core (underestimation) or with multiple cores (overestimation) false the real dose of used NPs. It is therefore necessary to establish standards for the use of nanoparticles and indeed the recent development of nanomedicine is now associated to official recommendations depending on countries. In France, the required characterizations are listed by the norm ISO/TR 13014. This norm argues the careful measured knowledge of NPs' physicochemical properties for comprehension of their biological fate. The European Medicinal Agency published guidelines for the evaluation of the safety of nanomaterials where it is notified that the physicochemical and pharmacokinetic comparison of generic NPs to a reference is not sufficient to approve the safety of those generic NPs (EMA/CHMP/SWP/100094/2011).7The FDA created a section dedicated to the evaluation of nanotechnology since the first Nanotechnology Task Force Report published in 2007.8 This section elaborated guidance for industry considering whether an FDA-regulated product involves the application of nanotechnology in 2014.8 Each of these official recommendations refers to the major influence of the fundamental parameters that are the physicochemical properties, in particular size, chemical composition and surface properties on the NPs biological behaviour. It is therefore essential to have nano-objects that meet these standards in prior to any detailed research about the cellular trafficking and signaling pathways.
Among nanomedicines, the mesoporous silica nanoparticles (MSN) have been considered as a multifunctional platform for pharmaceutical development9 and found particularly advantageous for theranostic applications10,11 MSN surface exhibits silanols groups, which could be functionalized by covalent ligands or by electrostatic coupling paving the way for an easy modification of their surface properties which could be one way to control the biodistribution of particles.12 Concerning cancer applications, accumulation of the NPs (size range 30–200 nm) in the tumour has been explored due to the enhanced permeation and retention (EPR) effect.12 The rapid neovascularisation (angiogenesis) occurring in the solid tumour presents imperfections, leading to fenestration; and the association of the blood pressure effect with fenestration is favourable for NPs accumulation into the tumour. Nevertheless, NPs could also accumulate into spleen and liver, due to important macrophages uptake of NPs by phagocytosis. To avoid the latter, surface functionalization with polyethylene glycol (PEG) moieties is now a well-known strategy for the production of furtive MSN.13,14 An alternative strategy modifying the fate of NPs consists in wrapping the nano-objects by a lipid bilayer, the latter including PEG grafted molecules and/or targeting probes.14 Among numerous properties, MSN pores could be loaded with cytotoxic molecules.15,16 Otherwise, MSN having a magnetic core allows following nanoparticles (NPs) using magnetic resonance imaging (MRI),17 or for drug release induced by the magnetic field.15 Indeed, drug release could be enhanced by heating through magnetic field application to a temperature of 50 °C. Plus, after MSN cell internalization, heating could enhance the intra-cellular temperature from 37 °C to 50 °C, leading to hyperthermia.18 Cell death generally occurred for a temperature upper to 42 °C, depending of cell type as cancer cells seemed more sensitive to heat stress than normal cells.
Sol–gel chemistry was developed since 2008 as a popular soft chemistry route to core@shell nanoparticles synthesis with well-ordered mesoporosity and tuneable pores.19 Since this date, numerous publications reported this synthesis; however few reached the initial required quality for nanomedecine. Most synthesis resulted in non-homogeneous shaped NPs15,20–23 or provide NPs presenting several magnetic cores per NPs.24–26 The goal of this work was to synthesize Fe3O4@MSN core–shell nanoparticles, monodisperse, homogeneous, and containing one magnetic core per particle. Even if MSN are generally considered as biocompatible, as previously mentioned their surface properties are often modified for biomedical applications27 in order to enhance their colloidal stability in biologically relevant conditions12 and then to improve their bio-stability and biocompatibility (reduced protein adsorption and resistance to nonspecific uptake).13 Plus, comparison of particles with sizes comprised between 150 and 500 nm with zeta potentials between −40 mV and +35 mV showed that NPs with slight negative charge (zeta potential of −15 mV) and 150 nm accumulates more efficiently in tumour than the other ones.28 In the present work, a MSN size of about 100 nm was chosen for optimal penetration in tumors,28 and the surface properties of NPs were modified by grafting PEG or coating with lipid bilayers. The cellular toxicity of the three types of particles, pristine, PEGylated or lipid coated, was measured on Human hepatocyte carcinoma cell line (Hep-G2 cells). Using the same cell line, real-time cellular effect of pristine, PEGylated or lipid coated NPs were compared by cellular impedance measurements. The cellular fate of NPs was analysed in relation with the nature of NPs coverage; the uptake of NPs was monitored using transmission electronic microscopy.
2 Results and discussion
2.1 Synthesis and characterization of pristine Fe3O4@MSN, PEG-Fe3O4@MSN and phospholipid coated Fe3O4@MSN
It is important for MIONs to be well dispersed in chloroform in a way that each single nanocrystal compound ideally occupies one micelle of CTAB so that to obtain single core Fe3O4@MSN. Thus, in order to reduce the formation of multi-core MSN, MIONs were dispersed in chloroform during 3 hours using ultrasound bath and nanocrystals were injected by 10 steps into the CTAB micellar reaction medium, to enhance their distribution in micelles. However, the presence of chloroform in the core–shell reaction mixture interferes with the sol–gel reaction and does not promote the formation of uniform and regular mesoporous silica particles. Therefore, it is essential to eliminate the chloroform from the reaction mixture by applying a brief vacuum at 70 °C after each injection of the MIONs suspension in the reaction mixture. Before silica precursor injection, the reaction medium temperature was stabilized at 80 °C during one hour. After reaction completion, differential centrifugation steps were performed. Firstly, 5200g centrifugation was accomplished to pellet multicore MSNs. After that, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700g centrifugation step was performed. MSN without magnetic core were recovered in the supernatant. Finally, Fe3O4@MSN were extensively washed in an alcoholic solution of ammonium nitrate by reflux during 12 hours. The as-prepared Fe3O4@MSN core–shell sample is a black powder. TEM images showed that the particles are monodisperse and regular silica spheres with radial mesostructures encapsulating 18 nm spherical Fe3O4 nanocrystals are observed (Fig. 1). It should be noted that no core-free silica particles were observed. The core–shell NPs have an overall diameter of 100 ± 5 nm. The size and monodispersity of native Fe3O4@MSN were confirmed by dynamic light scattering (DLS). Hydrodynamic diameter (HD) and zeta potential (ZP) were measured in low ionic strength buffered medium (20 mM Hepes, 5 mM NaCl, pH = 7.4) to prevent NPs aggregation. The HD was measured at 125.47 ± 1.61 nm (see Table 2). The surface properties have been studied using a zeta potential (ZP) analyzer. The measurements revealed that the sample exhibits negative ZP values around −32.09 ± 1.30 mV (Table 2). This relatively high negative value confers to the nanoparticles a colloidal stability in aqueous solution and prevents them from aggregation in agreement with previous studies which argued that above the isoelectric point (pH > 2), the surface of silica is negatively charged due to the presence of deprotonated silanol groups (SiO−).25,31
700g centrifugation step was performed. MSN without magnetic core were recovered in the supernatant. Finally, Fe3O4@MSN were extensively washed in an alcoholic solution of ammonium nitrate by reflux during 12 hours. The as-prepared Fe3O4@MSN core–shell sample is a black powder. TEM images showed that the particles are monodisperse and regular silica spheres with radial mesostructures encapsulating 18 nm spherical Fe3O4 nanocrystals are observed (Fig. 1). It should be noted that no core-free silica particles were observed. The core–shell NPs have an overall diameter of 100 ± 5 nm. The size and monodispersity of native Fe3O4@MSN were confirmed by dynamic light scattering (DLS). Hydrodynamic diameter (HD) and zeta potential (ZP) were measured in low ionic strength buffered medium (20 mM Hepes, 5 mM NaCl, pH = 7.4) to prevent NPs aggregation. The HD was measured at 125.47 ± 1.61 nm (see Table 2). The surface properties have been studied using a zeta potential (ZP) analyzer. The measurements revealed that the sample exhibits negative ZP values around −32.09 ± 1.30 mV (Table 2). This relatively high negative value confers to the nanoparticles a colloidal stability in aqueous solution and prevents them from aggregation in agreement with previous studies which argued that above the isoelectric point (pH > 2), the surface of silica is negatively charged due to the presence of deprotonated silanol groups (SiO−).25,31
 | ||
| Fig. 1 TEM images of (a) magnetic iron oxide nanocrystals and (b) monodisperse magnetic mesoporous silica core–shell particles (Fe3O4@MSN) prepared according to the optimized synthesis protocol. | ||
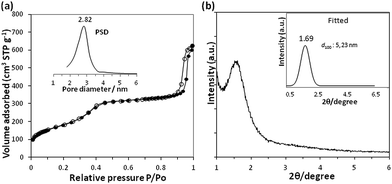
Textural properties of native Fe3O4@MSN have been characterized by N2 sorption and small-angles X-ray diffraction (sa-XRD). The textural parameters are given in Table 1. The material exhibits a type IV isotherm according to the IUPAC classification. The isotherm profile is characteristic of MCM-41 type materials with a surface area of 675 ± 7 m2 g−1 and uniform mesopores of 2.84 ± 0.2 nm of diameter, according to the pore size distribution. The hysteresis observed at P/P0 > 0.85 is likely related to inter-particles porosity and packing defects.32 The small-angle XRD pattern revealed that only the first-order scattering peak (d100) appears well resolved at 2θ = 1.69°. This may be due to the spherical morphology of the core–shell nanoparticles implying a constraint on the pore organisation. The wide-angle XRD pattern (ESI Fig. 3†) evidenced the presence of Fe3O4 nanocrystals with the related indexed diffraction peaks planes (311, 400 and 440) embedded in the characteristic broad signal of amorphous SiO2.29,33 The observed diffraction lines confirmed the magnetite crystals structure of magnetic iron oxide nanocrystals (Fig. 2).
| SBET (m2 g−1) | Sexta (m2 g−1) | Vpb (cm3 g−1) | DBJHc (nm) | |
|---|---|---|---|---|
| a Sext: external surface as inferred from the t-plot analysis applied to N2 adsorption isotherms.b Vp: pore volume calculated at the saturation point of the N2 sorption isotherms at the relative pressure P/P0 = 0.85.c DBJH: mesopore volume, as determined from the BJH method (desorption branch). | ||||
| Fe3O4@MSN | 675 ± 7 | 58 ± 1 | 0.97 ± 0.02 | 2.84 ± 0.20 |
| PEG Fe3O4@MSN | 475.0 ± 3.6 | 55 ± 1 | 0.64 ± 0.02 | 2.75 ± 0.20 |
The PEG Fe3O4@MSN core–shell sample is a black powder. TEM images (ESI Fig. 7†) showed that the particles are monodisperse and regular silica spheres with radial mesostructures were observed. There is no difference on TEM images between pristine Fe3O4@MSN and PEG Fe3O4@MSN. The PEGylation induced an HD increased to 141.76 ± 13.96 nm due to PEG groups and an increase of ZP from −32.03 to −26.30 mV (see Table 2). The presence of the PEG moieties at the particle surface was evidenced using Raman spectroscopy (Fig. 3c). Here, the Raman active vibrational modes of the PEG grafted at the MSN particle surface are highly decreased in intensity, twisting vibrations of methylene group of PEG at 1221–1366 cm−1 and CH2–CH2 bending vibration at 1458–1479 cm−1. This could be due to hydrogen bonding/van der Waals' interaction with the silanol groups of the silica surface.35
| NPs | Hydrodynamic diameter (nm) | Polydispersity index | Zeta potential (mV) |
|---|---|---|---|
| Fe3O4@MSN | 125.47 ± 1.61 | 0.14 ± 0.04 | −32.09 ± 1.30 |
| PEG Fe3O4@MSN | 141.76 ± 13.96 | 0.14 ± 0.05 | −26.30 ± 1.35 |
| DMPC Fe3O4@MSN | 179.40 ± 2.40 | 0.20 ± 0.02 | −10.80 ± 0.25 |
TGA analysis of pristine particles (Fig. 3a) exhibits a gradual change of mass loss of 4.4% in the temperature range 200–800 °C ascribed to a progressive dehydroxylation of the silica shell. It is known that a slow condensation of germinals, vicinals, and isolated silanols occurred upon heating of amorphous silica.36 The grafted amount of PEG onto Fe3O4@MSN was determined based on TGA analysis (Fig. 3b). The thermal decomposition of PEG is observed to start at 251 °C. The measured mass loss of 12% arisen from a grafted PEG amount of 2.36 mmol g−1 of particles taking into account the dehydroxylation of silica.
DMPC coated structures were firstly characterized using HD and ZP measurements. After lipid coating, the HD was increased to 179.40 ± 2.40 nm and ZP was increased to −10.80 ± 0.25 mV as compared to 125.47 ± 1.61 nm and −32.09 ± 1.30 mV for naked nanoparticles. The effective coating of Fe3O4@MSN by the DMPC bilayer was confirmed by cryoTEM observations. CryoTEM observations show that no free SUV was present in the preparation; a unilamellar lipid bilayer is effectively wrapping the surface of the NPs (Fig. 4). Moreover, most of the NPs observed were covered by a complete DMPC lipid bilayer around the NPs in our experimental conditions. After counting 250 particles 84% were found completely wrapped. Scanning transmission electron microscopy (STEM) was used to confirm the presence of phospholipids around Fe3O4@MSN. After TEM observation of Fe3O4@MSN samples, topochemical analysis was performed by scanning with energy dispersive X-ray spectrometer (EDS). The STEM images (Fig. 4c and d) and the corresponding elemental analysis (ESI Fig. 8†) clearly distinguished from the inside to the outside part of the particles, the iron oxide core, the silica shell and in case of lipid covered particles the presence of a sphere mapping the presence of phosphorus due to the presence of DMPC.
2.2 Cell viability and cell impedance measurements
We investigated the biocompatibility of the 3 types of nanoparticles. For this, the cytotoxicity of MSNs (ranging from 1 to 150 μg mL−1) on human hepatocyte carcinoma (Hep-G2) cells was measured with the MTT assay. The percentage of living cells was up to 90% for 100 μg mL−1 of PEG Fe3O4@MSN, and 80% and 90% for 50 μg mL−1 of DMPC Fe3O4@MSN and pristine ones, respectively (Fig. 5). This result demonstrates the absence of a significant toxicity of these batches of nanoparticles for the dose of 50 μg mL−1 and a higher biocompatibility for PEG Fe3O4@MSN.Following this cell viability assay, the two doses 50 and 100 μg mL−1 were chosen for real-time cell impedance analyses. The impact of nanoparticles on cells was performed using the xCELLigence System (ACEA Biosciences), a cell-based microelectronic biosensor which provides real-time and label-free cellular analyses, represented by a Cell Index (CI). This assay allows exceeding the limits of endpoint analysis by capturing data throughout the entire time course of an experiment and obtaining more physiologically relevant data. CI reflects both modifications of cell morphology and cell viability. As control, all these nanoparticles were tested in acellular conditions and no interference on impedance measurements was recorded (data not shown). Impedance was recorded for 60 h of exposure. Pristine Fe3O4@MSN (blue curves in Fig. 6) induced a reduction of the CI in a dose-dependent manner, compared to control cells (black curve in Fig. 6). At both doses, i.e. 100 and 50 μg mL−1, a biphasic CI response was observed with an increase followed by a decrease from 10 h of exposure until the end of the experiment. PEG–Fe3O4@MSN (purple curves in Fig. 6) at 100 μg mL−1 induced a dose-dependent reduction of the CI with a biphasic pattern, similar to that observed with pristine Fe3O4@MSN. Interestingly, PEG Fe3O4@MSN at 50 μg mL−1 had no effect on the CI of Hep-G2 cells. DMPC Fe3O4@MSN (red curves in Fig. 6) induced an early biphasic CI response (after 5 h of exposure) at both doses. After 60 h of exposure, DMPC Fe3O4@MSN at 100 μg mL−1 had the same CI than pristine Fe3O4@MSN at 50 μg mL−1. To summarize, pristine Fe3O4@MSN are the most deleterious for Hep-G2 cells, followed by DMPC Fe3O4@MSN and PEG Fe3O4@MSN.
Finally, the uptake of the Fe3O4@MSN, decorated or not with PEG or DMPC was assessed after 6 h of exposure at 50 μg mL−1 via TEM. A high amount of Fe3O4@MSN was observed inside the Hep-G2 cells, as showed in Fig. 7b. Pristine Fe3O4@MSN were shown as individual dispersed NPs around the nucleus (Fig. 7b). In contrast, in the same experimental conditions, we observed a lower uptake of PEG Fe3O4@MSN and DMPC Fe3O4@MSN by Hep-G2 cells (Fig. 7c and d).
Results obtained in cellular assays shown, firstly, that pristine Fe3O4@MSN, PEG grafted Fe3O4@MSN, and lipid coated Fe3O4@MSN, induced a dose-dependent response on liver Hep-G2 cells. Fe3O4@MSN grafted with PEG did not impact the normal behavior of Hep-G2 cells at a dose of 50 μg mL−1. After the cells were exposed to NPs; changes in the cell impedance profiles were observed earlier for DMPC Fe3O4@MSN than for pristine and PEG Fe3O4@MSN. The use of PEG is well known to produce a steric hindrance around nanoparticles which prevents the adsorption of serum proteins and can counteract the opsonization process.14 Similar observations were also applicable to DMPC Fe3O4@MSN, but to a lesser extent. Indeed, DMPC Fe3O4@MSN (i.e. 50 or 100 μg mL−1), induced a decrease of CI values all along the experiment, reflecting earlier and more deleterious effects on Hep-G2 cells than those observed with PEG Fe3O4@MSN. Nevertheless, these two coverages were found to diminish the toxic potential of the pristine Fe3O4@MSN.
Previous studies described a longer half-life in vivo (longer circulation time)39 and better accumulation in tumors16 for MSN coated with lipid bilayers, associated to PEG groups. However, the individual effect of the lipid bilayer or PEG groups is not clear. Similarly, grafting of PEG onto commercially available liposomal formulations is currently used to reduce the amount of proteins adsorbed on liposome. However, the molecular consistency of the corona is not significantly different in the absence or in the presence of PEG coating.40,41 Plus, corona formation on liposomes inhibits their internalization and their pegylation reduces also the internalization of liposomes.
3 Conclusion
In this work, the synthesis procedure of magnetic NPs was fully optimized to obtain Fe3O4@MSN core@shell nanoparticles, monodisperse, homogeneous, and containing one magnetic core per particle. These criteria were defined to obtain reproducible biological results after interactions of NPs with cells. Then, we performed a preliminary study of the impact on cell fate of the pristine, PEG-grafted and lipid-coated Fe3O4@MSN.The Fe3O4@MSN were synthesized according to an adapted protocol that allows obtaining spherical NPs presenting one magnetic core per MSN and a primary diameter of 100 ± 5 nm. The PEG grafting was achieved in situ, after the last step of the Fe3O4@MSN synthesis; whereas SLB formation was achieved by vesicle fusion and rupture upon contact with the MSN surface. The polydispersity index for the hydrodynamic diameters measured for pristine Fe3O4@MSN, PEG grafted ones and for lipid coated nanoparticles are always inferior to 0.2 indicating a monodispersity, and the absence of aggregates when NPs were dispersed in 20 mM Hepes pH 7.4 buffer containing 5 mM NaCl. It is noteworthy that the lipid coating ascertained using cryoTEM imaging was confirmed by appropriate STEM analyses.
The NPs behaviors with three different surfaces were compared using cellular tests. The results show differences in cell responses upon the exposure with NPs having three different surface properties. PEG Fe3O4@MSN appear less toxic than lipid coated and pristine ones. Only the PEG Fe3O4@MSN exhibits no toxic effect at a dose of 50 μg mL−1 which was not the case for DMPC and pristine Fe3O4@MSN. DMPC Fe3O4@MSN (i.e. 50 or 100 μg mL−1), induced a decrease of CI values all along the experiment, reflecting earlier and more deleterious effects on Hep-G2 cells than those observed with PEG Fe3O4@MSN. Nevertheless, these two coverages were found to diminish the toxic potential of the pristine Fe3O4@MSN.
To the best of our knowledge, this is the first study that compares the cellular effect of PEG grafting or lipid coating as individual strategies for enhancing the stealth effect of MSN. This is particularly interesting because different internalization mechanisms are involved for the MSN presenting different surface coatings. In a previous study, in which MSN coated with lipids were compared to pristine MSN for their interactions towards cells, the data suggested internalization of large aggregates of pristine MSN inside vesicles whereas lipid-coated MSN enter in the cells individually.42 The optimized synthesis protocol of Fe3O4@MSN developed in this work allows the comparison of MSN that were fully characterized in terms of physicochemical properties. This study is an essential step to ensure the quality of NPs material for medical applications. As a lower toxicity was observed for the PEG grafted or lipid coated Fe3O4@MSN compared to the pristine ones, as the real-time impedance profiles suggested different internalization processes; future work should be orientated towards intracellular trafficking of NPs and in depth analysis of cellular responses. Moreover, in vitro studies using model membranes will be needed to clarify the interactions between pristine, PEG and DMPC Fe3O4@MSN with membranes.
4 Experimental
4.1 Synthesis
4.2 Methods of characterization
Transmission electron micrographs (TEM) were obtained with a JEOL 1200 EX II microscope. Nitrogen adsorption and desorption isotherms were measured using a TriStar 3000 (V6.06 A) automated gas adsorption system with nitrogen as adsorbate at −196 °C. Prior to the sorption experiment, the sample air was evacuated under vacuum at 110 °C for 15 h. The specific surface area (SBET) was calculated according to the Brunauer–Emmett–Teller (BET) method, taking the value of 0.162 nm2 as a cross-sectional area per nitrogen molecule in the BET calculation. The total pore volume (Vp) was taken at P/P0 = 0.85. Pore size distribution (PSD), used to measure the pore diameter (Dp), was obtained from the desorption branch of the nitrogen isotherm using the Barrett–Joyner–Halenda (BJH) equation. X-ray diffraction patterns (XRD) were collected on a Bruker AXS D8 Advance with Solid 1D Lynx Eyes Detector, using Ni-filtered Cu Kα radiation. XRD techniques were applied to determine the pore ordering and the presence of crystalline phases of Fe3O4. BJH parameters were employed to calculate the effective pore diameter.20 Thermogravimetric analysis (TGA) was performed with DSC 7 (Perkin Elmer), from 30 to 780 °C.Hydrodynamic diameter and zeta potential were determined using a Nano ZS apparatus (Malvern). Each measurement was performed at 20 μg mL−1 dilution NPs from a stock suspension at 10 mg ml−1 in EtOH 95%. The dilution required a 30 s bath sonication step of the stock suspension and 1 min bath sonication step of the dilution. The suspensions were prepared by dilution in Hepes 20 mM, NaCl 5 mM buffered at pH 7.4 and 25 °C. Data were collected from the He–Ne laser light source (633 nm) at 173° from the transmitted beam. Results are presented as Z-average obtained in intensity mode, associated to the polydispersity index.
CryoTEM experiments have been performed using a JEOL 220FS transmission electron microscope (Japan) with a 4k × 4k slow-scan CCD camera (Gatan, USA). Samples were prepared on copper quantifoil grids R 2/2 (Quantifoil, Germany). DMPC Fe3O4 MSN suspensions were prepared at a concentration of 3 mg mL−1 of particles. A 3 μL drop of suspension was filed on the copper grid, dried 2 seconds and frozen in ethane for approximately 6 seconds. Grids were kept in liquid nitrogen until microscope observation. Effective NPs coverage was characterized with cell counter (Image J). Scanning transmission electron microscopy (STEM) was performed using a JEOL 2100F transmission electron microscope (Japan) with 200 kV field emission and energy dispersive X-ray spectrometer. Pristine and DMPC Fe3O4@MSN samples were analyzed on copper grids from suspensions containing a concentration of 3 mg mL−1 of particles. After TEM observation of Fe3O4@MSN samples, element analysis have been performed by scanning with a counting rate of 398 and 422 counts per sec for pristine Fe3O4@MSN and DMPC Fe3O4@MSN, respectively. The scanning was performed using a 0.7 nm EDX probe with a probe current of 1 nA.
4.3 Biological materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solution absorbance was read at 540 nm.
1). The solution absorbance was read at 540 nm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells in 200 μL total volume. E-plates were placed into the Real-Time Cell Analyzer (RTCA) station (ACEA) and incubated at 37 °C and 5% CO2. Cells were grown for 48 hours corresponding to the adhesion and growth phases. After 48 hours, cells were exposed (n = 3) to three types of nanoparticles: pristine Fe3O4@MSN, PEG grafted and lipid-bilayer coated Fe3O4@MSN, exposed at 50 and 100 μg mL−1. Real-time impedance measurements of control cells and nanoparticles alone in culture cell media were also recorded as controls. Cell index (CI) raw data values were calculated by the RTCA software 2.0. Normalized cell indexes were also calculated at a selected normalization time point, which was chosen at the time of addition of nanoparticles.
000 cells in 200 μL total volume. E-plates were placed into the Real-Time Cell Analyzer (RTCA) station (ACEA) and incubated at 37 °C and 5% CO2. Cells were grown for 48 hours corresponding to the adhesion and growth phases. After 48 hours, cells were exposed (n = 3) to three types of nanoparticles: pristine Fe3O4@MSN, PEG grafted and lipid-bilayer coated Fe3O4@MSN, exposed at 50 and 100 μg mL−1. Real-time impedance measurements of control cells and nanoparticles alone in culture cell media were also recorded as controls. Cell index (CI) raw data values were calculated by the RTCA software 2.0. Normalized cell indexes were also calculated at a selected normalization time point, which was chosen at the time of addition of nanoparticles.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for 1 h, and twice in EPON for 2 h. The polymerization is performed by embedding cells in EPON resin for 12 h at 60 °C, plunged in liquid nitrogen at −195 °C to detach the coverslip, and placed for two days at 60 °C for polymerization. The ultrathin sections (70 nm) were obtained using an ultramicrotome and disposed on the copper grids. The grids were incubated in uranyl acetate for 2 min, rinsed in water, and then incubated in lead citrate for 2 min, and finally rinsed in water.
1, v/v) for 1 h, and twice in EPON for 2 h. The polymerization is performed by embedding cells in EPON resin for 12 h at 60 °C, plunged in liquid nitrogen at −195 °C to detach the coverslip, and placed for two days at 60 °C for polymerization. The ultrathin sections (70 nm) were obtained using an ultramicrotome and disposed on the copper grids. The grids were incubated in uranyl acetate for 2 min, rinsed in water, and then incubated in lead citrate for 2 min, and finally rinsed in water.Abbreviations
| BET | Brunauer Emmett Teller |
| CI | Cell index |
| CTAB | Cetyltrimethylammonium bromide |
| CryoTEM | Cryogenic transmission electron microscopy |
| DMPC | 1,2-Dimyristoyl-sn-glycero-3-phosphocholine |
| EDS | Energy dispersive X-ray spectrometer |
| EDX | Energy dispersive X-ray |
| EPR | Enhanced permeation and retention |
| EtOH | Ethanol |
| FCS | Fetal calf serum |
| HD | Hydrodynamic diameter |
| Hepes | (4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) |
| Hep-G2 cells | Human hepatocyte carcinoma cells |
| MIONs | Magnetic iron oxide nanocrystals |
| MRI | Magnetic resonance imaging |
| MSN | Mesoporous silica nanoparticles |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NPs | Nanoparticles |
| PEG | Polyethylene glycol |
| RPMI | Roswell Park Memorial Institute medium |
| STEM | Scanning transmission electron microscopy |
| SUV | Small unilamellar vesicles |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| TEOS | Tetraethoxysilane |
| XRD | X-ray diffraction |
| ZP | Zeta potential |
Acknowledgements
This work was supported by the French national research agency (ANR-13-NANO-0007, BioSiPharm project). We thank technological platform of microscopy of Montpellier University for the use of their electron microscope and their technical support, in particular Franck Godiard and Véronique Richard. We thank Gilles Renou from SIMaP of Grenoble for STEM analysis.References
- S. Nie, Y. Xing, G. J. Kim and J. W. Simons, Annu. Rev. Biomed. Eng., 2007, 9, 257–288 CrossRef CAS PubMed.
- A. E. Nel, L. Madler, D. Velegol, T. Xia, E. M. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- H. Yang, C. Liu, D. Yang, H. Zhang and Z. Xi, J. Appl. Toxicol., 2009, 29, 69–78 CrossRef CAS PubMed.
- N. Ma, C. Ma, C. Li, T. Wang, Y. Tang, H. Wang, X. Moul, Z. Chen and N. Hel, J. Nanosci. Nanotechnol., 2013, 13, 6485–6498 CrossRef CAS PubMed.
- B. Fadeel and A. E. Garcia-Bennett, Adv. Drug Delivery Rev., 2010, 62, 362–374 CrossRef CAS PubMed.
- T. Yu, K. Greish, L. D. McGill, A. Ray and H. Ghandehari, ACS Nano, 2012, 6, 2289–2301 CrossRef CAS PubMed.
- EMA, Reflection paper on non-clinical studies for generic nanoparticle iron medicinal product applications, 2011 Search PubMed.
- FDA, Nanotechnology task force report, 2007 Search PubMed.
- D. Tarn, C. E. Ashley, M. Xue, E. C. Carnes, J. I. Zink and C. J. Brinker, Acc. Chem. Res., 2013, 46, 792–801 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef CAS PubMed.
- Z. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart and J. I. Zink, Chem. Soc. Rev., 2012, 41, 2590–2605 RSC.
- K. Pombo Garcia, K. Zarschler, L. Barbaro, J. A. Barreto, W. O'Malley, L. Spiccia, H. Stephan and B. Graham, Small, 2014, 10, 2516–2529 CrossRef PubMed.
- V. Cauda, C. Argyo and T. Bein, J. Mater. Chem., 2010, 20, 8693–8699 RSC.
- C. Graf, Q. Gao, I. Schutz, C. N. Noufele, W. Ruan, U. Posselt, E. Korotianskiy, D. Nordmeyer, F. Rancan, S. Hadam, A. Vogt, J. Lademann, V. Haucke and E. Ruhl, Langmuir, 2012, 28, 7598–7613 CrossRef CAS PubMed.
- J. Liu, C. Detrembleur, D. Pauw-Gillet, S. Mornet, L. Vander Elst, S. Laurent, C. Jérôme and E. Duguet, J. Mater. Chem. B, 2014, 2, 59–70 RSC.
- H. Meng, M. Wang, H. Liu, X. Liu, A. Situ, B. Wu, Z. Ji, C. H. Chang and A. E. Nel, ACS Nano, 2015, 9, 3540–3557 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425–443 CrossRef CAS PubMed.
- F. M. Martin-Saavedra, E. Ruiz-Hernandez, A. Bore, D. Arcos, M. Vallet-Regi and N. Vilaboa, Acta Biomater., 2010, 6, 4522–4531 CrossRef CAS PubMed.
- J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed. Engl., 2008, 47, 8438–8441 CrossRef CAS PubMed.
- M. Kruk, M. Jaroniec and A. Sayari, J. Phys. Chem. B, 1997, 101, 583–589 CrossRef CAS.
- Y.-S. Lin and C. Haynes, Chem. Mater., 2009, 21, 3979–3986 CrossRef CAS.
- K. R. Hurley, Y.-S. Lin, J. Zhang, S. M. Egger and C. L. Haynes, Chem. Mater., 2013, 25, 1968–1978 CrossRef CAS PubMed.
- R. G. Digigow, J.-F. Dechézelles, H. Dietsches, I. Geissbühler, D. Vanhecke, C. Geers, A. M. Hirt, B. Rothen-Rutishauer and A. Petri-Fink, J. Magn. Magn. Mater., 2014, 362, 72–79 CrossRef CAS.
- V. Maurice, T. Georgelin, J.-M. Siaugue and V. Cabuil, J. Magn. Magn. Mater., 2009, 321, 1408–1413 CrossRef CAS.
- E. Bringas, O. Koysuren, D. V. Quach, M. Mahmoudi, E. Aznar, J. D. Roehling, M. D. Marcos, R. Martinez-Manez and P. Stroeve, Chem. Commun., 2012, 48, 5647–5649 RSC.
- M. Perrier, M. Gary-Bobo, L. Lartigue, D. Brevet, A. Morère, M. Garcia, P. Maillard, L. Raehm, Y. Guari, J. Larionova, J.-O. Durand, O. Mongin and M. Blanchard-Desce, J. Nanopart. Res., 2013, 15, 1–17 CrossRef.
- F. Tang, L. Li and D. Chen, Adv, Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- C. He, Y. Hu, L. Yin, C. Tang and C. Yin, Biomaterials, 2010, 31, 3657–3666 CrossRef CAS PubMed.
- W. W. Yu, J. C. Falkner, C. T. Yavuz and V. L. Colvin, Chem. Commun., 2004, 2306–2307 RSC.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS PubMed.
- J.-F. Dechezelles, V. Malik, J. J. Crassous and P. Schurtenberger, Soft Matter, 2013, 9, 2798–2802 RSC.
- J. L. Nyalosaso, G. Derrien, C. Charnay, L.-C. de Menorval and J. Zajac, J. Mater. Chem., 2012, 22, 1459–1468 RSC.
- W.-Y. Rho, H.-M. Kim, S. Kyeong, Y.-L. Kang, D.-H. Kim, H. Kang, C. Jeong, D.-E. Kim, Y.-S. Lee and B.-H. Jun, J. Ind. Eng. Chem., 2014, 20, 2646–2649 CrossRef CAS.
- M. Catauro, F. Bollino, F. Papale, M. Gallicchio and S. Pacifico, Mater. Sci. Eng., C, 2015, 48, 548–555 CrossRef CAS PubMed.
- H. Y. Jung, R. K. Gupta, E. O. Oh, Y. H. W. Kim and C. M. Whang, J. Non-Cryst. Solids, 2005, 351, 372–379 CrossRef CAS.
- S. Ek, A. Root, M. Peussa and L. Niinista, Thermochim. Acta, 2001, 379, 201–212 CrossRef CAS.
- R. Veneziano, G. Derrien, S. Tan, A. Brisson, J. M. Devoisselle, J. Chopineau and C. Charnay, Small, 2012, 8, 3674–3682 CrossRef CAS PubMed.
- H. Wang, J. Drazenovic, Z. Luo, J. Zhang, H. Zhou and S. L. Wunder, RSC Adv., 2012, 2, 11336–11348 RSC.
- M. M. van Schooneveld, E. Vucic, R. Koole, Y. Zhou, J. Stocks, D. P. Cormode, C. Y. Tang, R. E. Gordon, K. Nicolay, A. Meijerink, Z. A. Fayad and W. J. Mulder, Nano Lett., 2008, 8, 2517–2525 CrossRef CAS PubMed.
- M. A. Dobrovolskaia, B. W. Neun, S. Man, X. Ye, M. Hansen, A. K. Patri, R. M. Crist and S. E. McNeil, Nanomedicine, 2014, 10, 1453–1463 CAS.
- M. Hadjidemetriou, Z. Al-Ahmady, M. Mazza, R. F. Collins, K. Dawson and K. Kostarelos, ACS Nano, 2015, 9, 8142–8156 CrossRef CAS PubMed.
- D. B. Tada, E. Suraniti, L. M. Rossi, C. A. Leite, C. S. Oliveira, T. C. Tumolo, R. Calemczuk, T. Livache and M. S. Baptista, J. Biomed. Nanotechnol., 2014, 10, 519–528 CrossRef CAS PubMed.
- S. Dib, M. Boufatit, S. Chelouaou, F. Sadi-Hassaine, J. Croissant, J. Long, L. Raehm, C. Charnay and J. O. Durand, RSC Adv., 2014, 4, 24838–24841 RSC.
- A. D. Bangham, M. M. Standish and J. C. Watkins, J. Mol. Biol., 1965, 13, 238–252 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra09017f |
| This journal is © The Royal Society of Chemistry 2016 |