Cinchona alkaloid and di-tert-butyldicarbonate–DMAP promoted efficient synthesis of (E)-nitroolefins†
Nataraj Poomathi and
Paramasivan T. Perumal*
Organic Chemistry Division, CSIR-Central Leather Research Institute, Adyar, Chennai-600020, India. E-mail: ptperumal@gmail.com; Fax: +91-44-24911589
First published on 2nd June 2016
Abstract
The synthesis of nitroolefins from aldehydes and olefins is generally limited by the formation of a mixture of cis and trans compounds. Here we report an alternative, metal-free protocol for the synthesis of β-nitroolefins from arylidinemalononitrile using cinchona alkaloid along with di-tert-butyldicarbonate–DMAP in high yields with total selectivity.
Nitroolefins are a prominent class of synthetic intermediates, which have found wide application in the preparation of a variety of biorelevant compounds and pharmaceuticals.1 These are widely used in different carbon–carbon bond-forming reactions like the Michael reaction,2 cycloaddition,3 Morita–Baylis–Hillman reaction,4 and for the generation of oximes,5 hydroxylamines, nitroalkanes6 and aliphatic amines. They are usually synthesized by Henry reaction, involving base mediated condensation of nitroalkanes with aldehyde or ketones followed by dehydration.7a,b An alternative and relatively simple approach relies upon readily available aldehyde, malononitrile and nitromethane as the starting materials, wherein anion formation and subsequent β-elimination of malononitrile lead to the desired nitroolefins in a stereoselective manner. This convenient and step economical process has drawn significant attention in recent decades.7c,d To date, a number of methods have been developed with different metal based and gaseous nitrating agents.8 These methods, while offering significant improvements in the direct nitration of olefins, often have several drawbacks. Problematic issues include the tendency to form an un-desired mixture of E/Z isomers,8a,b lack of functional group tolerance,8e,g and harsh reaction conditions among others.8d,g
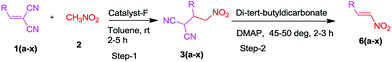
Although a number of methods8h–s for the synthesis of nitroolefins have been developed, more environmentally benign and efficient approaches are still needed to reduce the use of expensive catalysts, solvents and toxic reagents. Further, stereoselective synthesis of nitroolefins with an easy to handle metal free reagent is yet to be developed. Notably, from a practical aspect, metal-free synthesis9 (Fig. 1) are preferred, as the removal of metal contamination can render a process quite expensive.10 In particular, preparation of nitrostyrene under metal-free conditions would be of great significance due to its close association with the pharmaceutical industry. To date, only a few synthetic methods using bifunctional catalysts have been reported, such as those applying transition metal catalyst and bifunctional catalyst.11 To achieve the Michael addition of nitromethane to arylidinemalononitrile, we planned to use thiourea and cinchona alkaloid as a catalyst, where these catalyst systems are able to “intramolecularise” the reactions via simultaneous activation of two reaction partners.12a–d To our knowledge, no reports are available on the β-elimination of malononitrile using cinchona alkaloid and di-tert-butyldicarbonate leading to the desired nitroolefins. This has now been relished in the case of aromatic/heteroaromatic using malononitrile, nitromethane, di-tert-butyldicarbonate, DMAP and catalytic amount of cinchona alkaloid, This newly developed method is operationally simple, functional group tolerant, good yielding and scalable.
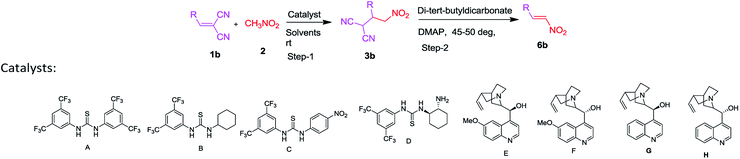
Initially, a feasibility study of our hypothesis revealed that arylidinemalononitrile 1b, nitromethane 2 was investigated using various bases, such as TEA, DABCO, piperidine, DBU and DMAP with di-tert butyldicarbonate using catalyst A–H. Although product 6b can be generated in the presence of all of these bases except entry 1, 2 and 3, only DMAP resulted in a high chemical yield using catalyst F in toluene at 45–50 deg. to furnish the desired product 6b in 87% yield (Table 1, entry 6). Next, several bifunctional catalysts with different solvents such as CH3CN, DCM, toluene and dioxane, were evaluated for their efficacy in this cascade reaction (Table 1, entries 1–18) and cinchona alkaloid F was found to be the most promising catalyst for this cascade reaction (Table 1, entry 6). The results showed that toluene was optimal choice (Table 1, entry 6). However, in the absence of catalyst, the cascade process did not proceed significantly, even in toluene (Table 1, entry 12, 13). Finally, we examined the effect of the catalyst concentration on this cascade reaction. The results revealed that decreasing the amount of catalyst F from 10 to 5 mol% led to an decrease in the yield from 87 to 80% (Table 1, entry 16), whereas by increasing the amount of catalyst F from 10 to 25 mol%, the yield of the product could not be improved significantly (Table 1, entry 17, 18). We found that 2 equiv. of di-tert-butyldicarbonate and 10 mol% of catalyst F in toluene at 50 °C could provide β-nitrostyrene in an excellent 90% isolated yield with complete E-selectivity (Table 2, 6e). With the reaction conditions optimized, the substrate scope was then investigated to show the generality of this cascade reaction (Table 2). In most cases, the reactions afforded the corresponding nitroolefins product with moderate to good yields (65–90%). The structural variation of alkylidenemalononitrile 1 could be well tolerated in this reaction irrespective of the electronic nature or the positions of the substituent on the aromatic ring, compared with the 4-methoxy benzaldehyde (6e, 90%) gave excellent yields of the desired nitro product.
| Entry | Catalyst (mol%) | Time (h) | Temp. (°C) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| DCMa,b | CH3CNa,b | Toluenea,b | Dioxanea,b | ||||
| a The reaction was performed with arylidinemalononitrile 1b (0.1 mmol), nitromethane 2 (0.5 mmol), catalyst, di-tert-butyldicarbonate (0.2 mmol) and DMAP (0.02 mmol) in 2 mL of solvents at 45–50 deg.b Isolated yields.c E/Z ratio is approximately >97/3 for all the synthesized products except entry 6, 8–17 in Table 1 (E/Z ratio: 99 > 1). It was determined by NMR analysis of the crude products. | |||||||
| 1 | A (10) | 4 | 50 | — | — | — | — |
| 2 | B (10) | 4 | 50 | — | — | — | — |
| 3 | C (10) | 4 | 50 | — | — | — | — |
| 4 | D (10) | 4 | 50 | 55 | 45 | 72 | 35 |
| 5 | E (10) | 4 | 50 | 65 | 35 | 62 | 25 |
| 6 | F (10) | 4 | 50 | 78 | 40 | 87 | 35 |
| 7 | G (10) | 4 | 50 | 60 | 35 | 70 | 25 |
| 8 | H (10) | 4 | 50 | 68 | 37 | 75 | 26 |
| 9 | F (10) | 7 | 50 | 77 | 40 | 87 | 30 |
| 10 | F (10) | 10 | 50 | 77 | 40 | 87 | 30 |
| 11 | F (10) | 10 | rt | — | — | — | — |
| 12 | — | 10 | 50 | 30 | 30 | 30 | 25 |
| 13 | — | 15 | 50 | 40 | 32 | 30 | 25 |
| 14 | F (10) | 4 | 60 | 65 | 40 | 83 | 35 |
| 15 | F (10) | 4 | 70 | 60 | 35 | 68 | 20 |
| 16 | F (5) | 4 | 50 | 70 | 35 | 80 | 30 |
| 17 | F (15) | 4 | 50 | 78 | 40 | 87 | 35 |
| 18 | F (25) | 4 | 50 | 77 | 40 | 87 | 33 |
| a Unless otherwise noted, the reactions in step-1 were performed with arylidinemalononitrile/2-formylphenoxy-methyl-3-phenylacrylates/heterocyclic compounds 1 (0.1 mmol), nitromethane 2 (0.5 mmol) and catalyst-F (10 mol%). In step-2, the reactions were performed with di-tert-butyldicarbonate (0.2 mmol) and DMAP (0.02 mmol) in 2 mL of toluene. Yield refers to the column purified products.b E/Z ratio is >99/1 for all the synthesized products. It was determined by NMR analysis of the crude product. |
|---|
 |
It is noteworthy that this cascade process could also be successfully extended to several 2-formylphenoxy-methyl-3-phenylacrylate derivatives (Table 2, 6j–6u with including those containing Cl, Br, Me and OMe) under the optimized conditions in good yields (70–90%). The scope of the reaction was further studied with various heterocyclic compounds under optimized conditions. Oxygen, sulphur and nitrogen based heterocycles such as furan, thiophene and indole gave the nitro products (Table 2, 6(v–x)) in 75, 79 and 82% isolated yield, respectively. Unfortunately alkylidenemalononitrile (e.g. 2-butylidinemalononitrile) did not form the desired nitroolefin product under the reaction conditions.
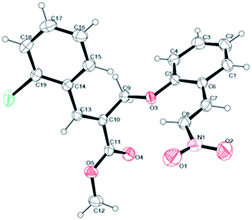
To demonstrate the scalability of the β-elimination of malononitrile method, 4-chlorobenzaldehyde was reacted in a 6 mmol scale. In this experiment, the desired nitrostyrene product was isolated in preoperatively useful yield (87%). The structures of all the nitroolefins 6(a–u) and 6(v–x) are deduced by their satisfactory spectral (1H NMR, 13C NMR and EI-HRMS) studies. The structures of the compounds 6t have been further confirmed by X-ray analysis13 (Fig. 2).
On the basis of the above results a plausible mechanism for the formation of β-nitroolefins is proposed in Scheme 1. The first step is the generation of tert-butoxide from DMAP and di-tert-butyldicarbonate, and which has been proposed previously by our group.12e Cinchona catalyst F containing hydrogen bond donors and a tertiary amine moiety, where the amine could be oriented in such a way as to cooperatively bind a single proton from the nitromethane and the hydroxyl group could activate arylidinemalononitrile 1 electrophilically via hydrogen bonding that would result in efficient assembly of the nitromethane and the arylidinemalononitrile followed by smooth Michael addition and consecutive protonation led to the 2-(2-nitro-1-phenylethyl)malononitrile intermediate 3. We have isolated intermediate 3u in Table 1, which was fully characterized by NMR spectroscopy.14 Next, in the presence of the generated tert-butoxide, intermediate 3 can be deprotonated to furnish cabanion 4, which could be possible two conformations 5 and 5′, stabilized by an hydrogen bonding with catalyst F. The conformations of which are nearly ideal for the subsequent E2 or E1cb reaction process for stereo electronic reasons as depicted in Newman projection 5 and 5′, however intermediate 5′ suffers from severe steric and more dipole repulsion between phenyl, nitro, cyano groups and catalyst F so out of two possible pathways involving 4, preferential formation of energetically more favourable conformation of intermediate 5 over 5′, followed by β-elimination of malononitrile to afford the desired nitro product 6 and recycles malononitrile 8 in Scheme 1. The presence of a single isomer is probably the tertiary amine and hydroxyl group in cinchona alkaloid would activate the nitro group (intermediate-4) through hydrogen bonding, thus the synergistic interactions would ensure high stereoselectivity in this transformation.15
According to the above plausible reaction mechanism and the experimental results, this protocol offers greatly improved the overall yield and E/Z selectivity, is this due to the participation of organocatalyst and Boc-anhydride/DMAP combination provides a better elimination protocol compared to other conventional base-mediated conditions, without cinchona alkaloid, the overall yield of the product was found to be very low and E/Z selectivity also reduced16 which provided further support to the proposed mechanism. Finally, the absolute (E) configuration was obtained in accordance with X-ray crystal structure analysis.
Conclusions
In summary, we have described an easy, efficient and metal-free general route to aromatic β-nitrostyrenes with total E-selectivity has been discovered under mild conditions. The substrate scope of the arylidinemalononitrile bearing aromatic and heterocyclic moieties was explored and E-nitro compounds were obtained selectively. The process is practical, avoiding multistep and expensive purification procedure. Further studies are in progress to broaden the potentiality of the procedure and to better clarify the reaction pathway.Experimental section
General procedure for the synthesis of arylidinemalononitriles and (E)-methyl-2-((4-chloro-2-(2,2-di-cyanovinyl)-phenoxy)-methyl)-3-(4-methoxy-phenyl)-acrylate derivatives
Aldehyde (1 mmol), malononitrile (1 mmol) in ethanol (3 mL) and catalytic amount of water were charged in a 25 mL round bottomed flask and the resulting solution was stirred for 3–5 h at room temperature. The consumption of the starting material was monitored by TLC. The precipitated solid was filtred and washed with ethanol (5–7 mL), dried under vacuum to obtain pure 1a–x in good yields (78–89%). The identities of products were confirmed by NMR giving good agreement with the assigned structures.General procedure for the preparation of E-nitroolefin derivatives
A mixture of arylidine/heteroarylidinemalononitriles/(E)-methyl 2-((4-chloro-2-(2,2-di-cyanovinyl)-phenoxy)-methyl)-3-(4-methoxy-phenyl)-acrylate derivatives 1(a–x) (0.1 mmol), nitromethane 2 (0.5 mmol) in toluene (3 mL) and catalytic amount of cinchona alkaloid F (10 mol%) were charged in a 25 mL round bottomed flask and the resulting solution was stirred for 2–5 h at room temperature. The consumption of the starting material was monitored by TLC. After completion of the reaction, DMAP (0.020 mmol) and di-tert-butyldicarbonate (2.0 equiv.) was then added to a stirred solution of corresponding crude product 3. After stirring the reaction at 45–50 deg. for 2–3 h followed by TLC, the solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97% ethylacetate and petether) to afford pure products 6(a–x) in average to good yields 65–90%. The identities of products 6(a–x) were confirmed by NMR and EI-HRMS, giving good agreement with the assigned structures.
97% ethylacetate and petether) to afford pure products 6(a–x) in average to good yields 65–90%. The identities of products 6(a–x) were confirmed by NMR and EI-HRMS, giving good agreement with the assigned structures.
Acknowledgements
One of the authors N. Poomathi, thanks to the Department of Science and Technology (DST), New Delhi for the award of the Women Scientist Fellowship under women scientist scheme (WOS-A) and SAIF IITM Chennai for EI-HRMS analysis.Notes and references
- (a) A. G. M. Barret and G. G. Graboski, Chem. Rev., 1986, 86, 751 CrossRef; (b) P. Talalay, M. J. Delong and H. Prochaska, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 8261 CrossRef CAS PubMed; (c) T. A. Alston, D. J. T. Porter and H. Bright, Bioorg. Chem., 1985, 13, 375 CrossRef CAS; (d) P. Cheng, Z. Y. Jiang, R. R. Wang, X. M. Zhang, Q. Wang, Y. T. Zheng, J. Zhou and J. J. Chen, Bioorg. Med. Chem. Lett., 2007, 17, 4476 CrossRef CAS PubMed; (e) S. Kaap, I. Quentin, D. Tamiru, M. Shaheen, K. Eger and H. Steinfelder, Biochem. Pharmacol., 2003, 65, 603 CrossRef CAS PubMed; (f) J. H. Kim, G. E. Lee, J. E. Lee and I. K. Chung, Mol. Pharmacol., 2003, 63, 1117 CrossRef CAS PubMed; (g) H. Uehara, R. Imashiro, G. Hernandez-Torres and C. F. Barbas, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20672 CrossRef CAS PubMed; (h) Y. Meah and V. Massey, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 10733 CrossRef CAS PubMed; (i) K. Sakai, A. Nakazawa, K. Kondo and H. Ohta, Agric. Biol. Chem., 1985, 49, 2331 CAS; (j) M. A. Reddy, N. Jain, D. Yada, C. Kishore, V. J. Reddy, P. S. Reddy, A. Addlagatta, S. V. Kalivendi and B. Sreedhar, J. Med. Chem., 2011, 54, 6751 CrossRef CAS PubMed; (k) L. Q. Lu, J. R. Chen and W. Xiao, Acc. Chem. Res., 2012, 45, 1278 CrossRef CAS PubMed.
- (a) T. Ishii, S. Fujioka, Y. Sekiguchi and H. Kotsuki, J. Am. Chem. Soc., 2004, 126, 9558 CrossRef CAS PubMed; (b) H. B. Huang and E. N. Jacobsen, J. Am. Chem. Soc., 2006, 128, 7170 CrossRef CAS PubMed; (c) C. B. Tripathi, S. Kayal and S. Mukherjee, Org. Lett., 2012, 14, 3296 CrossRef CAS PubMed.
- (a) D. A. Evans, S. Mito and D. Seidel, J. Am. Chem. Soc., 2007, 129, 11583 CrossRef CAS PubMed; (b) J. March, Advanced Organic Chemistry, John Wiley & Sons, New York, 3rd edn, 1985 Search PubMed; (c) R. C. Larock, Comprehensive Organic Transformations, VCH, New York, 1989 Search PubMed; (d) L. Albrecht, G. Dickmeiss, F. C. Acosta, C. Rodriguez-Escrich, R. L. Davis and K. A. Jorgensen, J. Am. Chem. Soc., 2012, 134, 2543 CrossRef CAS PubMed; (e) Y. K. Liu, M. Nappi, E. Arceo, S. Vera and P. Melchiorre, J. Am. Chem. Soc., 2011, 133, 15212 CrossRef CAS PubMed; (f) T. Arai, A. Mishiro, N. Yokoyama, K. Suzuki and H. Sato, J. Am. Chem. Soc., 2010, 132, 5338 CrossRef CAS PubMed; (g) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CAS PubMed.
- (a) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS PubMed; (b) D. K. Nair, S. M. Mobin and I. N. N. Namboothiri, Org. Lett., 2012, 14, 4580 CrossRef CAS PubMed.
- A. Corma, P. Serna and H. Garcia, J. Am. Chem. Soc., 2007, 129, 6358 CrossRef CAS PubMed.
- N. J. A. Martin, L. Ozores and B. List, J. Am. Chem. Soc., 2007, 129, 8976 CrossRef CAS PubMed.
- (a) R. Ballini and G. Bosica, J. Org. Chem., 1997, 62, 425 CrossRef CAS PubMed; (b) S. Fioravanti, L. Pellacani, P. A. Tardella and M. C. Vergari, Org. Lett., 2008, 10, 1449 CrossRef CAS PubMed; (c) P. A. Clarke, S. Santos and W. H. C. Martin, Green Chem., 2007, 9, 438 RSC; (d) J. P. Wan, Y. Lin, Q. Huang and Y. Liu, J. Org. Chem., 2014, 79, 7232 CrossRef CAS PubMed.
- (a) P. K. Kancharla, Y. S. Reddy, S. Dharuman and Y. D. Vankar, J. Org. Chem., 2011, 76, 5832 CrossRef CAS PubMed; (b) I. Jovel, S. Prateeptongkum, R. Jackstell, N. Vogl, C. Weckbecker and M. Beller, Adv. Synth. Catal., 2008, 350, 2493 CrossRef CAS; (c) P. J. Campos, B. Garcia and M. A. Rodriguez, Tetrahedron Lett., 2000, 41, 979 CrossRef CAS; (d) H. Suzuki and T. Mori, J. Org. Chem., 1997, 62, 6498 CrossRef CAS; (e) T. Mukaiyama, E. Hata and T. Yamada, Chem. Lett., 1995, 505 CrossRef CAS; (f) W. W. Sy and A. W. By, Tetrahedron Lett., 1985, 26, 1193 CrossRef CAS; (g) R. S. Varma, K. P. Naicker and P. J. Liesen, Tetrahedron Lett., 1998, 39, 3977 CrossRef CAS; (h) E. Hata, T. Yamada and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1995, 68, 3629 CrossRef CAS; (i) S. Ranganathan and S. K. Kar, J. Org. Chem., 1970, 35, 3962 CrossRef CAS; (j) S. W. Tinsley, J. Org. Chem., 1961, 26, 4723 CrossRef; (k) E. J. Corey and H. Estreicher, J. Am. Chem. Soc., 1978, 100, 6294 CrossRef CAS; (l) S. S. Jew, H. D. Kim, Y. S. Cho and C. H. Cook, Chem. Lett., 1986, 1747 CrossRef CAS; (m) A. Zhao, Q. Jiang, J. Jia, B. Xu, Y. Liu, M. Zhang, Q. Liu, W. Luo and C. Guo, Tetrahedron Lett., 2016, 57, 80 CrossRef CAS; (n) Z.-G. Luo, F. Xu, Y.-Y. Fang, P. Liu, X.-M. Xu, C.-T. Feng, Z. Li and J. He, Res. Chem. Intermed., 2016, 42, 6079 CrossRef CAS; (o) V. Sudhakar Chary, K. C. Rajanna, G. Krishnaiah and P. Srinivas, Catal. Sci. Technol., 2016, 6, 1430 RSC; (p) D. Baruah, P. Pahari and D. Konwar, Tetrahedron Lett., 2015, 56, 2418 CrossRef CAS; (q) A. Ying, S. Xu, S. Liu, Y. Ni, J. Yang and C. Wu, Ind. Eng. Chem. Res., 2014, 53, 547 CrossRef CAS; (r) B. V. Rokade and K. R. Prabhu, Org. Biomol. Chem., 2013, 11, 6713 RSC; (s) S. Maity, S. Manna, S. Rana, T. Naveen, A. Mallick and D. Maiti, J. Am. Chem. Soc., 2013, 135, 3355 CrossRef CAS PubMed; (t) T. Naveen, S. Maity, U. Sharma and D. Maiti, J. Org. Chem., 2013, 78, 5949 CrossRef CAS PubMed.
- (a) C. Zhu, G. Q. Li, D. H. Ess, J. R. Falck and L. Kurti, J. Am. Chem. Soc., 2012, 134, 18253 CrossRef CAS PubMed; (b) J. M. O'Brien and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7712 CrossRef PubMed; (c) A. A. Kantak, S. Potavathri, R. A. Barham, K. M. Romano and B. DeBoef, J. Am. Chem. Soc., 2011, 133, 19960 CrossRef CAS PubMed; (d) S. H. Cho, J. Yoon and S. Chang, J. Am. Chem. Soc., 2011, 133, 5996 CrossRef CAS PubMed; (e) G. Pelletier, W. S. Bechara and A. B. Charette, J. Am. Chem. Soc., 2010, 132, 12817 CrossRef CAS PubMed; (f) S. Manna, S. Jana, T. Saboo, A. Maji and D. Maiti, Chem. Commun., 2013, 49, 5286 RSC; (g) S. maity, T. Naveen, U. Sharma and D. Maiti, Synlett, 2014, 25, 603 CrossRef CAS; (h) S. Maity, T. Naveen, U. Sharma and D. Maiti, Org. Lett., 2013, 15, 3384 CrossRef CAS PubMed; (i) U. Dutta, S. Maity, R. Kancherla and D. Maiti, Org. Lett., 2014, 16, 6302 CrossRef CAS PubMed; (j) I. Jovel, S. Prateeptongkum, R. Jackstell, N. Vogl, C. Weckbecker and M. Beller, Adv. Synth. Catal., 2008, 350, 2493 CrossRef CAS; (k) F. A. Ramirez and A. Burger, J. Am. Chem. Soc., 1950, 72, 2781 CrossRef CAS.
- (a) X. D. Bu, K. Koide, E. J. Carder and C. Welch, Org. Process Res. Dev., 2013, 17, 108 CrossRef CAS; (b) J. C. Hermann, Y. S. Chen, C. Wartchow, J. Menke, L. Gao, S. K. Gleason, N. E. Haynes, N. Scott, A. Petersen, S. Gabriel, B. Vu, K. M. George, A. Narayanan, S. H. Li, H. Qian, N. Beatini, L. H. Niu and Q. F. Gan, ACS Med. Chem. Lett., 2013, 4, 197 CrossRef CAS PubMed; (c) F. H. Qiu and D. L. Norwood, J. Liq. Chromatogr. Relat. Technol., 2007, 30, 877 CrossRef CAS; (d) C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889 CrossRef CAS; (e) C. J. Welch, J. Albaneze-Walker, W. R. Leonard, M. Biba, J. DaSilva, D. Henderson, B. Laing, D. J. Mathre, S. Spencer, X. D. Bu and T. B. Wang, Org. Process Res. Dev., 2005, 9, 198 CrossRef CAS.
- (a) L. Ping, Y. C. Chang, C. Zhe, L. Hua, Y. Yu and S. Wei-Guo, Chem. Commun., 2012, 48, 10541 RSC; (b) N. Raveendran Shiju, A. H. Albert, K. Syed, R. B. David and R. Gadi, Angew. Chem., Int. Ed., 2011, 50, 9615 CrossRef PubMed; (c) Y. Yan, L. Xiao, L. Xiaobo, Z. Jiao, B. Shiyang, L. Jian and Y. Qihua, Angew. Chem., Int. Ed., 2012, 51, 9164 CrossRef PubMed; (d) J. P. Das, P. Sinha and H. P. Ray, Org. Lett., 2002, 4, 3055 CrossRef CAS PubMed.
- (a) A. Hamza, G. Schubert, T. Soós and I. Pápai, J. Am. Chem. Soc., 2006, 128, 13151 CrossRef CAS PubMed; (b) J. D. Bass, A. Solovyov, A. J. Pascall and A. Katz, J. Am. Chem. Soc., 2006, 128, 3737 CrossRef CAS PubMed; (c) P. Hammar, T. Marcelli, H. Hiemstra and F. Himo, Adv. Synth. Catal., 2007, 349, 2537 CrossRef CAS; (d) S. J. Zuend and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 15872 CrossRef CAS PubMed; (e) K. Ramachandiran, K. Karthikeyen, D. Muralidharan and P. T. Perumal, Tetrahedron Lett., 2010, 51, 3006 CrossRef CAS.
- Crystal data for product 6t (CCDC: 1051895,† Fig. 2): C19H16ClNO5, crystallised in the monoclinic, space group P2(1)/n with the following unit cell parameters a = 9.0038(2) A alpha = 90 deg., b = 13.6511(3) A beta = 102.1556(12) deg., c = 14.6295(4) A gamma = 90 deg.
- 3u: (E)-methyl-2-((2-(1,1-di-cyano-3-nitro-propan-2-yl)-phenoxy)-methyl)-3-(p-tolyl)-acrylate: isolated as yellow gelly semi solid, 95%, 1H NMR (400 MHz, CDCl3) 8.00 (1H, s), 7.36–7.22 (3H, m), 7.19 (3H, t, J = 8.0), 6.97 (1H, t, J = 7.5), 6.87 (1H, d, J = 8.3), 4.96 (1H, dd, J = 13.7, 9.0), 4.89 (2H, s), 4.78 (1H, dd, J = 13.7, 5.3), 4.61 (3H, s), 4.24 (1H, dd, J = 14.3, 8.7), 3.82 (3H, s), 2.32 (3H, s) ppm. 13C NMR δC (100 MHz, CDCl3) 167.64, 155.71, 146.51, 140.76, 131.46, 131.10, 130.10, 129.91, 129.53, 125.09, 122.16, 120.47, 112.85, 111.18, 111.15, 74.24, 63.70, 52.60, 24.77, 21.44 ppm.
- (a) P. M. Pihko, Hydrogen bonding in organic synthesis, WILEY-VCH Verlag GmbH and Co. KGaA, 2009 Search PubMed; (b) S. J. Connon, Chem. Commun., 2008, 2499 RSC; (c) Y. Iwabuchi, M. Nakatani, M. Yokoyama and S. Hatakeyama, J. Am. Chem. Soc., 1999, 121, 10219 CrossRef CAS; (d) P. Deslongchamps, Stereoelectronic Effects in Organic Chemistry, Pergamon, Oxford, 1983, p. 252 Search PubMed; (e) L. J. Brzezinski, S. Rafel and J. W. Leahy, J. Am. Chem. Soc., 1997, 119, 4317 CrossRef CAS.
- See ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H NMR, and 13C NMR spectra of all compounds and experimental procedure. CCDC 1051895. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra06644e |
| This journal is © The Royal Society of Chemistry 2016 |