Spectral normalisation by error minimisation for prediction of conversion in solvent-free catalytic chain transfer polymerisations†
Samuel J. Richardsona,
Idriss Blakeyab,
Kristofer J. Thurechtabc,
Derek J. Irvined and
Andrew K. Whittaker*abc
aAustralian Institute for Bioengineering and Nanotechnology, University of Queensland, St Lucia, Queensland 4072, Australia. E-mail: a.whittaker@uq.edu.au
bCentre for Advanced Imaging, University of Queensland, St Lucia, Queensland 4072, Australia
cARC Centre of Excellence in Convergent Bio-Nano Science and Technology, Australia
dNational Centre for Industrial Microwave Processing, Department of Chemical and Environmental Engineering, University of Nottingham, Nottingham, NG7 2RD, UK
First published on 11th July 2016
Abstract
Oligomers are useful chemicals for a number of synthetic and industrial applications. Catalytic chain transfer (CCT) polymerisation has been shown to be an extremely effective methodology for the synthesis of oligomers. Monitoring the conversion of monomer during the production of oligomers can present challenges using conventional analytical techniques such as IR or Raman spectroscopy, due to overlap from spectral features from the retained alkene groups at the chain terminus of the oligomers. This can cause ambiguity when assigning monomeric and oligomeric peaks in the vibrational spectra. In addition to this, such reactions are often carried out in solvent-free systems making the normalisation of spectra difficult. Multivariate analysis offers a useful methodology to quantify monomer conversion using Raman spectroscopy, despite a high double bond content within the polymerisation mixture. Chemometric models were also used to determine suitable points at which to normalise the spectra by a process of error minimisation, since conventional normalisation methods are not effective when Raman bands of constant intensity are not present. A number of partial least squares regression (PLSR) models were used to predict conversion for a range of commercially important monomers, such as methyl methacrylate (MMA), tert-butyl methacrylate (t-BMA) and hydroxyethyl methacrylate (HEMA), with goodness-of-fit R2 values typically above 0.99, and root-mean-square error of cross-validation (RMSECV) between 1–3% or within 5% of the maximum conversion. Additionally, the ability to detect the concentrations of dimer and trimer formed in the CCT polymerisation of MMA has been demonstrated.
Introduction
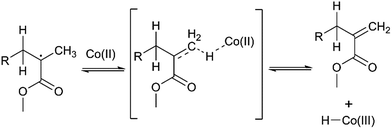
An attractive method for producing oligomers is catalytic chain transfer polymerisation (CCTP).1–4 CCTP not only provides a higher degree of control over free-radical polymerisation using conventional chain transfer agents (CTAs), but by using low-spin CoII complexes as catalysts, CCT yields oligomers or polymer chains with terminal vinyl groups (Scheme 1). These terminal groups are available for subsequent modification.The method of CCTP is principally used to synthesise oligomers and polymers of methacrylate monomers, since the CCT mechanism is most efficient when the monomer possesses an α-methyl group. This is due to the high kinetic efficiency of hydrogen abstraction from the α-methyl group when compared to abstraction of secondary hydrogen atoms, such as those found on the polymer backbone. Following the homolytic abstraction of a nearby hydrogen atom from the α-methyl or methylene group by a CCT catalyst, the propagating tertiary carbon radical forms a double bond with the α-methyl group. The CCT catalyst is regenerated by loss of a hydride radical from the cobalt hydride, which subsequently initiates a new polymer chain.
A number of cobalt catalysts have been developed, with the most successful being bis((difluoroboryl)dimethylglyoximato)cobalt(II) (MeCoBF) and bis((difluoroboryl)diphenylglyoximato)cobalt(II) (PhCoBF) due to their superior air stability.5,6 More recently, air-sensitive cobaloxime catalysts have also been developed, which can be formed in situ, avoiding complicated synthetic steps with low atom efficiency required to isolate the difluoroboryl derivatives.2,4
The degree of oligomerisation has a large effect upon the chemical and material properties, including the volatile organic content, inherent chain transfer activity and copolymerisation rate of the materials, and thus influences their performance in different applications. For example, it has been shown that longer oligomers of methyl methacrylate can exhibit greater chain transfer activity.7 Hence, the ability to monitor the conversion and concentration of specific oligomeric species is important to maximise the efficiency of the synthetic process and quality of the products.8
Ideally, these oligomers should be manufactured in the bulk to maximise atom efficiency, as well as minimising process steps and meeting environmental legislation by eliminating the need for solvents.3,9 While bulk reactions offer numerous advantages, they also introduce problems due to the large increases of viscosity observed during polymerisation. High viscosities resulting from the Trommsdorff–Norrish effect can lead to broadening of the molar mass dispersity, as well as thermal runaway leading to dangerous reaction conditions.10 Bulk reactions also introduce challenges for monitoring of the reaction.11 Typically, when a polymerisation reaction of vinyl monomer is monitored spectroscopically, intensity changes related to the consumption of vinyl groups can be observed and then normalised to a peak due to the solvent or other band of constant intensity. Techniques such as nuclear magnetic resonance (NMR) are sensitive to chemical changes and can provide extensive structural information chemical structure, which allows easy quantification of different oligomeric species.7 However, NMR is often not suitable for industrial implementation due to the high costs associated with the field strengths required to resolve the different molecular weight oligomers.
Raman spectroscopy is an alternative technique that can be used for online reaction monitoring, which provides structural information by examining light that has been inelastically scattered by the sample. The technique is most sensitive to polarisable groups, and thus is an excellent tool for monitoring polymerisations of vinyl monomers due to a strong response from the carbon–carbon double bond stretching mode.12 Raman spectroscopy is an optical technique so spectra can be collected in situ without significant perturbation of the sample. In addition, spectra can be collected through glass reactors, or using fibre optic probes, allowing for versatile use within industrial reactors. Furthermore, in recent times, relatively inexpensive and portable spectrometers have been made available commercially. These factors make Raman spectroscopy attractive for in situ, or online monitoring of addition polymerisations. Raman spectroscopy has previously been used to monitor conventional free radical polymerisations of vinyl monomers by monitoring the decrease in intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration peak as a function of time.13,14 The products of CCT polymerisations, however, possess double bonds that are structurally similar to the monomer, which significantly complicates reaction monitoring using Raman spectroscopy.
C stretching vibration peak as a function of time.13,14 The products of CCT polymerisations, however, possess double bonds that are structurally similar to the monomer, which significantly complicates reaction monitoring using Raman spectroscopy.
For systems consisting of multiple components, such as monomers and a range of oligomeric species, the Raman spectra can become complicated by the numerous overlapping peaks. Therefore, it can be difficult to develop robust methodologies to deconvolute the spectra. Multivariate analysis is a potential method to overcome these problems, where spectra for samples with known chemical and physical properties can be used to build a model for predicting properties of a particular system. The two most common statistical methods used for chemometrics are partial-least-squares regression (PLSR) and principal components regression (PCR).15,16 These multivariate techniques, and others, have been put to use industrially for purposes ranging from predicting research octane number of petrol and amino acid content soybeans, to estimating reaction conversion in complex reactions.17–25
Normalisation of spectral data is an essential process to ensure peak intensities and areas and comparable through multiple spectra. Instrument gain and sensitivity can vary during the reaction, which results in changes in spectral intensity independent of reaction progress. A typical approach for normalisation is to multiply the spectral intensities of each spectrum such that the intensity of an internal standard, which is assumed to be invariant in time, is equal across all spectra. However, normalisation to an internal standard can be difficult in systems where there are no reference peaks of constant intensity. The use of peaks arising from the unreacted moieties in reagents is not generally reliable, since the changes in structure of neighbouring groups can affect the shape and intensity of the peaks being considered. Alternatively peaks due to solvent may be used for normalisation since the concentration of solvent is typically invariant throughout a reaction, however many industrial processes do not use solvents for environmental and waste reduction considerations. Alternative methods, such as normalisation by closure, have been used, however they have not proven to be generally applicable for the calibration of models.26
In this study, a partial least squares regression model was constructed using Raman spectra taken from CCT polymerisation mixtures and used to predict monomer conversion, as well as the concentration of dimers and trimers within a polymerisation reaction mixture. To achieve this, a number of models were trained using extents of conversion measured independently using NMR spectroscopy. The models were then tested to affirm accurate prediction of the composition of the reaction. To demonstrate the scope of the methodology, robust models were generated for the CCT polymerisation of a variety of methacrylate monomers and using a number of different CCT catalysts. In addition a new method of model training in which a point of normalisation is selected based on error minimisation is presented. Hence, this study has significant scope for improving the process monitoring of CCT polymerisations and the methodologies developed here can also be adapted to monitor other complex reactions.
Experimental
Materials
Anti-diphenylglyoxime (dpg, 97%, Sigma-Aldrich), methylene chloride (DCM, analytical reagent, Chem-Supply) and ethyl acetate (EtOAc, analytical reagent, Chem-Supply) were used without further purification. CoBr2 (97%, anhydrous, Alfa Aesar) was used after dehydration at 130 °C, confirmed by a change to a bright green colour. Methyl methacrylate (MMA, 99%, Sigma-Aldrich), hydroxyethyl methacrylate (HEMA, 99%, Sigma-Aldrich) and tert-butyl methacrylate (t-BMA, 99%, Sigma-Aldrich) were purified by elution through a column of basic alumina to remove the inhibitor. 2,2-Azobis(isobutyronitrile) solution (AIBN, 12 wt% in acetone, Sigma-Aldrich) was concentrated in vacuo before recrystallisation in methanol.Bis[(difluoroboryl)diphenylglyoximato]cobalt(II) was synthesised according to the procedure described by Tovrog.5
Synthetic procedures
The cobalt catalyst used for CCT polymerisation was either PhCoBF, or bis(diphenylglyoximato) cobalt dibromide (CoBr2(dpg)2) formed in situ prior to polymerisation by the addition of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of CoBr2 and dpg.2,4
2 ratio of CoBr2 and dpg.2,4
The cobalt catalyst (50 mg) and AIBN (0.250 g, 14 mmol) were added to 50 mL of neat monomer and the flask sealed and purged with argon gas for 15 minutes. The reaction was heated to 80 °C for 2 hours before quenching the reaction. 500 μL samples were collected throughout the reaction at intervals of 10 minutes using a gas-tight syringe, then immediately quenched in liquid nitrogen to ensure an accurate representation of the composition of the reaction mixture at the time of sampling. The resulting samples were then characterised by 1H NMR and Raman spectroscopy. Conversion was measured using 1H NMR by comparing the integrated intensities of peaks arising from hydrogen atoms located on the alkoxy side chain (O–CH2R) or the terminal vinyl trans hydrogen atom.7 The 1H NMR peaks due to oligomers species display an up-field shift. Samples of the dimer were separated from the crude mixture using fractional distillation (50 °C, 0.1 mbar) while column chromatography was used to separate the trimer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96 EtOAc
96 EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM, on silica). The trimer was further purified by eluting through a basic alumina plug, followed by addition of activated charcoal (10 wt%) and finally by filtration. Both catalysts have different chain transfer constants, so the resulting ratio of oligomers differs.4
DCM, on silica). The trimer was further purified by eluting through a basic alumina plug, followed by addition of activated charcoal (10 wt%) and finally by filtration. Both catalysts have different chain transfer constants, so the resulting ratio of oligomers differs.4
Characterisation
1H NMR was performed on a Bruker AVANCE 400 MHz NMR spectrometer with a Bruker 5 mm broadband inverse resonance probe, taking 32 scans per spectrum. All samples were prepared as CDCl3 solutions, and referenced to the residual protonated solvent peak (7.26 ppm).Raman spectra were collected using a Thermo-Fischer Almega dispersive Raman spectrometer, using the 180° backscatter accessory. High resolution spectra spanning approximately 720–1850 cm−1 were collected using a 785 nm laser. The acquisition time was 1 second and 128 spectra were averaged unless otherwise noted. Spectra were resampled to a range of 750–1800 cm−1 and a resolution of 1 cm−1. Baseline correction was accomplished by subtracting a fifth order polynomial from each of the spectra.
Partial least squares analysis and regression
PLSR was performed using a MATLAB script utilising the SIMPLS algorithm. The program code was developed by the authors to create and utilise models to predict variables, specifically conversion, using PLSR. High resolution Raman spectra (1800–750 cm−1), resampled at a resolution of 1 cm−1, of known conversion (measured by 1H NMR) were used to calibrate the loadings vectors. The optimum number of latent variables were determined by a process of minimising RMSECV using a modified Wold's R Criteria, where latent variables were allowed to increase in the case of 0.95 < RMSECVn+1:RMSECVn to prevent over-fitting.27 The RMSECV was calculated using a Monte Carlo method of validation.28 One third of spectra randomly selected from each set were left out in calibration and then used for validation of the model. This process was repeated for three times the total number of spectra in the set. A summary of polymerisation mixtures used in this study can be found in Table 1. A model for predicting individual MMA dimer and trimer concentrations was calibrated using a combined MMA data set.| Sample code | Monomer | Samples | Conversion range (%) | [Initiator] (mg mL−1) | Catalyst | [Catalyst] (mg mL−1) |
|---|---|---|---|---|---|---|
| a Raman spectra for these samples were collected using only 32 scans. | ||||||
| MMA-A | MMA | 13 | 0–50 | 5 | PhCoBF | 1 |
| MMA-B | MMA | 13 | 0–30 | 5 | CoBr2(dpg)2 | 1 |
| MMA-Ca | MMA | 13 | 0–56 | 2.5 | PhCoBF | 0.25 |
| HEMA | HEMA | 11 | 0–66 | 5 | CoBr2(dpg)2 | 1 |
| t-BMAa | t-BMA | 13 | 0–48 | 5 | CoBr2(dpg)2 | 1 |
Spectral normalisation
A new method of selecting a wavenumber for normalisation was employed to obtain the optimum point of normalisation with the minimum error. The normalisation point that minimises the error of prediction was identified by comparison of the results of normalising the spectra on all the possible wavenumbers.Results and discussion
Raman spectra of CCT polymerisation of MMA
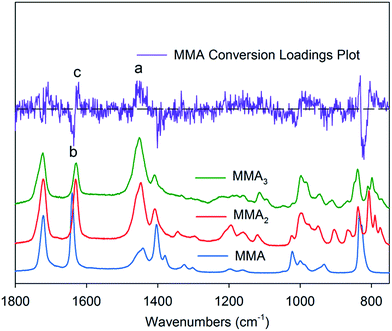
In order to evaluate whether conventional methods of monitoring changes in intensity of Raman bands could be used to determine conversions during the CCT polymerisation of MMA, the Raman spectrum of the MMA polymerised to 30% monomer conversion (determined by 1H NMR) was compared to the spectrum of the MMA monomer (Fig. 1). We observe that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration located at 1641 cm−1 did not shift discernibly in the partially-polymerised sample. While there was a decrease in the intensity of this band, the change was not correlated with conversion, since the total double bond concentration depends on both the conversion and the degree of polymerisation (DP). There appeared to be a general decrease in intensity of all peaks with the exception of the methyl hydrogen bending modes between 1415–1550 cm−1 and methylene hydrogen wagging mode, which both appeared to change in shape slightly. Beyond these changes, only a few small vibrations differed in the two spectra, including modes specific to oligomers at 1110 cm−1, 940 cm−1 895 cm−1, 855 cm−1, 782 cm−1 and 766 cm−1, which all proved to be unquantifiable.
C stretching vibration located at 1641 cm−1 did not shift discernibly in the partially-polymerised sample. While there was a decrease in the intensity of this band, the change was not correlated with conversion, since the total double bond concentration depends on both the conversion and the degree of polymerisation (DP). There appeared to be a general decrease in intensity of all peaks with the exception of the methyl hydrogen bending modes between 1415–1550 cm−1 and methylene hydrogen wagging mode, which both appeared to change in shape slightly. Beyond these changes, only a few small vibrations differed in the two spectra, including modes specific to oligomers at 1110 cm−1, 940 cm−1 895 cm−1, 855 cm−1, 782 cm−1 and 766 cm−1, which all proved to be unquantifiable.
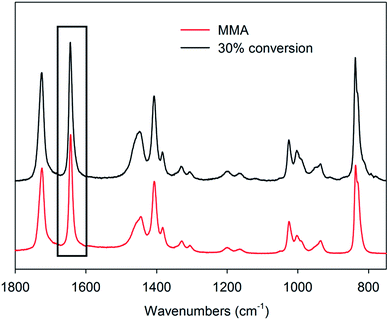 | ||
| Fig. 1 Raman spectra of MMA and a CCT MMA polymerisation at 30% monomer conversion. The rectangular outline highlights the similarity between the peaks at 1641 cm−1. | ||
In an effort to further understand the changes to the spectra on reaction, the MMA dimer (MMA2) and trimer (MMA3) species were separated from the reaction mixtures and their high-resolution Raman spectra were collected (Fig. 2). It was found that a bending mode for hydrogen atoms attached to methyl and methylene moieties in the 1350–1400 cm−1 range disappears in the spectra of the oligomers of methyl methacrylate, while a band due to a similar bending mode at approximately 1445 cm−1 increases in intensity. The lower energy bending mode is likely to be primarily associated with the methylene hydrogen bending, while the modes above 1400 cm−1 are associated with the methyl groups.29 A red shift of 10 cm−1 was noted for the band due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration in the dimeric species when compared to the monomer, however this shift was insufficient to allow resolution of the contributions from the two species. There was also a small blue shift in the band at 833 cm−1 of 4 cm−1, though this was not resolved in the partially converted sample. No additional shifts were observed for the trimer species. As mentioned above, the oligomer molecules included a number of new vibrations and distinct peaks, however these new bands could not be resolved in the spectra of the partially-converted MMA mixture. Therefore conventional peak fitting methodologies cannot be used to deconvolute the monomeric and oligomeric C
C stretching vibration in the dimeric species when compared to the monomer, however this shift was insufficient to allow resolution of the contributions from the two species. There was also a small blue shift in the band at 833 cm−1 of 4 cm−1, though this was not resolved in the partially converted sample. No additional shifts were observed for the trimer species. As mentioned above, the oligomer molecules included a number of new vibrations and distinct peaks, however these new bands could not be resolved in the spectra of the partially-converted MMA mixture. Therefore conventional peak fitting methodologies cannot be used to deconvolute the monomeric and oligomeric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations to allow accurate measurement of monomer conversion.
C vibrations to allow accurate measurement of monomer conversion.
Spectral normalisation by error minimisation
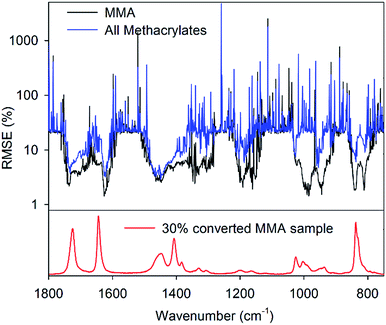
The Raman spectra of the CCT polymerisation mixtures do not contain a unique peak, which is invariant in intensity throughout the polymerisation. The absence of an internal intensity standard during the polymerisation reaction necessitated a method of error reduction to select the point of normalisation. This was achieved by identifying the spectral wavenumber giving the lowest RMSECV value for the various PLSR models. Typically the lowest RMSECV values were obtained when the spectra were normalised on a peak rather than on the baseline (Fig. 3), due to the intensity of baseline noise relative to a zero point. The wavenumbers selected for each of the models were not always located on local peak maxima, indicating either the presence of an isosbestic point or linear correlation of conversion at various points throughout the whole spectrum relative to any change at the normalisation point. Examining the spectra, it is evident that there are no new peaks appearing to generate isosbestic points. This means that normalisation points not located on local maxima are likely to be non-static intensities that result in other intensities changing in a linear fashion when correlated with conversion.For methyl methacrylate, normalising on the region from 800–850 cm−1 containing peaks arising from the C–O–C in-plane bending vibration provided some of the lowest RMSECV values. However, the same region did not provide a suitable normalisation point for models including the monomer HEMA, due to the increased complexity in the C–O–C in-plane bending vibration, as well as additional vibrations such as C–C–O stretching in HEMA (Fig. S4, ESI†).30 Similar effects were observed when normalising between 950–1050 cm−1, where the model for MMA had low error but models for other monomers were not so accurate. For both the individual and combined models, normalisation around the peaks due to methyl and methylene scissoring modes at 1440 cm−1 resulted in low RMSECV values. It is proposed that this region should be used routinely, rather than searching for a normalisation point across the whole spectrum. The latter approach would entail unacceptably long computation times if the calibration had to be performed often. Although this provided the best point of normalisation in the individual MMA models, the optimal point of normalisation in the combined set was located on the peak due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. A low RMSECV was also achieved in the model including all monomers by normalisation around the shoulders of the C
C stretching vibration. A low RMSECV was also achieved in the model including all monomers by normalisation around the shoulders of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peak, despite the intensities of both peaks changing significantly during the polymerisation. This supports the suggestion that peaks which are changing in intensity can provide good normalisation points.
C stretching peak, despite the intensities of both peaks changing significantly during the polymerisation. This supports the suggestion that peaks which are changing in intensity can provide good normalisation points.
The procedure of optimising the normalisation point increased the computation time from an order of minutes to several hours, depending on how many spectra were used to calibrate the model. However, since the process only needs to be undertaken once per monomer system this should not have an effect on the utility of the approach. This method of normalisation also allows for the inclusion of data points that are not linearly related to the perturbation, because points can be linearly related to the normalisation point with respect to the perturbation, where the normalisation point is then related to the perturbation in the same or another manner. Importantly, this method of selecting a normalisation point ultimately proved effective at minimising the error of the models.
Partial-least-squares regression model
The strong overlap of peaks due to monomer and oligomeric products in the Raman spectra of the reaction mixture necessitated the use of PLSR to accurately predict conversion in CCT polymerisations. A number of models were generated from the data sets listed in Table 1 and the results are summarised in Table 2. Models constructed using the least chemically-complex monomer considered here, i.e. MMA, yielded very low RMSECV and high R2 values when using the three data sets (MMA-A to C) independently, where the type and concentration of catalyst was varied. This confirmed the applicability of the PLSR models for one of the most commercially important and widely used monomers. Examining the PLSR calibrations for MMA separately, they all appear to be accurate models with RMSECV values below 1.5%.| PLSR model | Conversion range (%) | Normalisation wavenumber (cm−1) | Latent variables | R2 | RMSE (%) |
|---|---|---|---|---|---|
| a RMSE values reported are RMSECV unless otherwise noted.b Model was calibrated with MMA-A and MMA-B, and tested using MMA-C. R2 values reported are generated by prediction rather than cross validation, and RMSE values are RMSEP. | |||||
| MMA-A | 0–51 | 1459 | 2 | 0.99 | 0.98 |
| MMA-B | 0–30 | 1401 | 2 | 0.99 | 0.56 |
| MMA-C | 0–57 | 1451 | 4 | 0.99 | 1.4 |
| Combined MMA | 0–57 | 1626 | 6 | 0.99 | 1.3 |
| MMA-A + MMA-Bb | 0–51 | 1165 | 3 | 0.97 | 3.2 |
| HEMA | 0–66 | 964 | 2 | 0.99 | 2.3 |
| t-BMA | 0–48 | 1724 | 4 | 0.99 | 1.0 |
| Combined monomers | 0–66 | 1450 | 8 | 0.98 | 3.1 |
When all MMA data sets were incorporated into a single model, the RMSECV increased compared to the MMA-A and MMA-B sets, however the combined data provided a reduced error when compared to the MMA-C set, at 1.3%. As expected the average error of the combined model was lower as a result of the inclusion of the highly-accurate MMA-A and MMA-B sets in the combined MMA model. The low RMSECV and high R2 of the combined MMA calibration are strong evidence for the accuracy of the MMA model, as well as its applicability across a variety of reaction conditions.
Loadings plots can be used to highlight correlations between spectral intensities and conversion. Examining the loadings plot for the MMA model (Fig. 2) it can be seen that the variance of the peak due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration shifts as expected. This is evidenced by the negative correlation at 1636 cm−1 moving into a positive correlation at 1629 cm−1 for the C
C stretching vibration shifts as expected. This is evidenced by the negative correlation at 1636 cm−1 moving into a positive correlation at 1629 cm−1 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band, resulting from a red shift of the oligomeric C
C band, resulting from a red shift of the oligomeric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching band. Other significant correlations are observed in the range from 820–830 cm−1 arising from vibrational modes associated with the oligomers. The disappearance of a band associated with twisting of the monomer vinyl hydrogen atoms is also apparent in the loadings plot, with a large negative correlation at ∼820 cm−1.31 The alkene moiety is retained on the oligomer molecules, however its concentration is dependent on both DP and conversion. Unexpectedly there was a small positive correlation between intensity of the C
C stretching band. Other significant correlations are observed in the range from 820–830 cm−1 arising from vibrational modes associated with the oligomers. The disappearance of a band associated with twisting of the monomer vinyl hydrogen atoms is also apparent in the loadings plot, with a large negative correlation at ∼820 cm−1.31 The alkene moiety is retained on the oligomer molecules, however its concentration is dependent on both DP and conversion. Unexpectedly there was a small positive correlation between intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch and monomer conversion in the individual MMA models (Fig. S8–10, ESI†). It may be expected that this band would decrease with conversion due to decreasing conjugation. However this observation is consistent with decreasing intensity on the normalisation wavenumber, causing the apparent intensity of the C
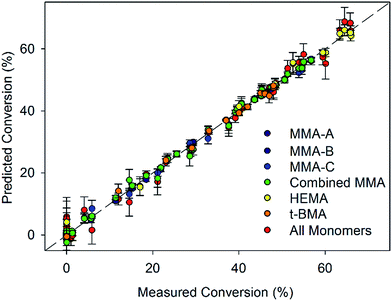
O stretch and monomer conversion in the individual MMA models (Fig. S8–10, ESI†). It may be expected that this band would decrease with conversion due to decreasing conjugation. However this observation is consistent with decreasing intensity on the normalisation wavenumber, causing the apparent intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch to increase with conversion. The correlations revealed by the model are largely consistent with the chemical changes exhibited by the oligomeric MMA molecules, as well as the individual Raman spectra. The model based on MMA-A and MMA-B sets performed well when predicting the conversion from the set of spectra MMA-C with a root-mean-square error of prediction (RMSEP) of 3.4%. The value of RMSEP decreased to 2.5% when the spectrum at zero conversion was omitted. Models based on HEMA and t-BMA were also generated to assess the effect of larger, more chemically complex side groups (Fig. 4). All models displayed low errors of less than 2.5%, and the accuracy was best for MMA, followed by t-BMA and then HEMA. The lower accuracy of the HEMA model was likely due to the higher viscosity of the reaction mixture, which leads to difficulties in sampling, as well as branching due to the presence of ethylene glycol dimethacrylate impurities.32 In the combined monomer model, the RMSECV value was a comparatively low 3.1%, demonstrating the robustness of the approach. From these results it appears that PLS models based on Raman spectra are very accurate in predicting the conversion of CCT polymerisations that proceed to low DP.
O stretch to increase with conversion. The correlations revealed by the model are largely consistent with the chemical changes exhibited by the oligomeric MMA molecules, as well as the individual Raman spectra. The model based on MMA-A and MMA-B sets performed well when predicting the conversion from the set of spectra MMA-C with a root-mean-square error of prediction (RMSEP) of 3.4%. The value of RMSEP decreased to 2.5% when the spectrum at zero conversion was omitted. Models based on HEMA and t-BMA were also generated to assess the effect of larger, more chemically complex side groups (Fig. 4). All models displayed low errors of less than 2.5%, and the accuracy was best for MMA, followed by t-BMA and then HEMA. The lower accuracy of the HEMA model was likely due to the higher viscosity of the reaction mixture, which leads to difficulties in sampling, as well as branching due to the presence of ethylene glycol dimethacrylate impurities.32 In the combined monomer model, the RMSECV value was a comparatively low 3.1%, demonstrating the robustness of the approach. From these results it appears that PLS models based on Raman spectra are very accurate in predicting the conversion of CCT polymerisations that proceed to low DP.
The ability to predict conversion of monomer for each oligomer is essential for optimisation of reaction conditions when specific target DPs are required. Using the combined MMA sets, the specific conversions to dimer and trimer were successfully predicted using a PLSR model (Table 3). Importantly, because several calibration sets were used, all containing different ratios of dimer to trimer, as well as higher molecular weight products, the model did rely on a scaled total conversion to calculate oligomer concentration. Using samples with varied composition to calibrate the model ensures it can predict specific oligomer concentrations in systems with varying average molecular weights. Differences between the models were identified by examining the loadings plots of the dimer and trimer models (Fig. 5). Both plots follow the same general trends, however differences were noted, such as the broader region of correlation in the range 1400–1500 cm−1 for MMA2. Additionally, for the MMA2 model, the shift in the carbonyl stretching vibration leads to a negative correlation, whereas the MMA3 model has a positive correlation across this region. Prediction of dimer and trimer concentrations made with an RMSECV of 1.0% and 1.2% respectively. These values correspond to concentrations being within 5% and 10% of the total concentration range for the dimer and trimer respectively. To put this into context R2 values here were 0.99 for the dimer and 0.95 for the trimer compared with other reported models with good predictive capacity having R2 values of 0.75–0.86.22 These results reported here indicate the applicability and robustness of these models to accurately predict specific oligomer concentrations in MMA CCT polymerisations. The RMSEP values for the model predicting conversion to trimer for the MMA-C set, using a model based on MMA-A and MMA-B indicated good accuracy of the model, however the RMSEP values for prediction of conversion to dimer using the same sets was significantly higher. This may be due to the changed ratio of oligomers in the MMA-C set, and the training set not being able to account for such effects. For example, when MMA-A and MMA-C sets were used to generate a model for prediction of conversion to dimer, and the MMA-B set was used as an external validation set, the RMSEP was low at 1.1% (Fig. 6).
| Oligomer | Conversion range (%) | Normalisation wavenumber (cm−1) | Latent variables | R2 | RMSE (%) |
|---|---|---|---|---|---|
| a RMSE values reported are RMSECV unless otherwise noted.b Model was calibrated with MMA-A and MMA-B, and tested using MMA-C. R2 values reported are generated by prediction rather than cross validation, and RMSE values are RMSEP. | |||||
| MMA2 | 0–27 | 985 | 3 | 0.99 | 1.0 |
| MMA3 | 0–18 | 1165 | 3 | 0.95 | 1.2 |
| MMA2b | 0–26 | 1627 | 3 | 0.85 | 3.4 |
| MMA3b | 0–13 | 1165 | 3 | 0.90 | 1.8 |
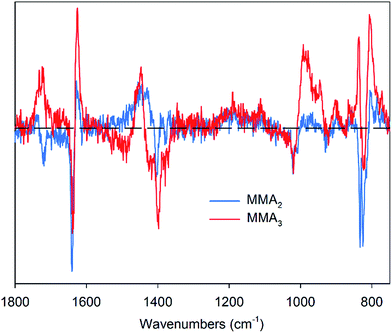 | ||
| Fig. 5 Loadings plots for the MMA2 (blue line) and MMA3 (red line) models. The horizontal dashed line indicates the zero value of correlation. | ||
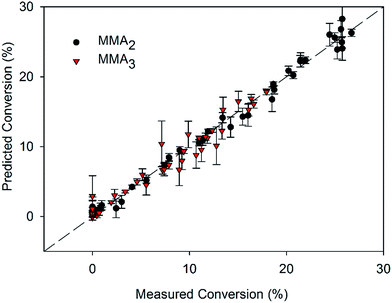 | ||
| Fig. 6 Calibration plot for conversion to oligomer for the CCT polymerisation of MMA using the combined MMA calibration set. | ||
Conclusions
A range of PLSR models were created for the prediction of conversion of CCT polymerisation reactions, using a variety of methacrylate monomers and catalyst systems. The models allowed for accurate prediction of conversion across all tested systems using a universal model, combining all monomers, with relatively low RMSECV values and very good predictive capability. It also proved possible to predict conversion during the polymerisation of a variety of methacrylate monomers, providing good predictive power with low RMSECV. Specific concentrations of dimer and trimer units in CCT polymerisations of MMA were able to be predicted in similarly high accuracies, which allows for the targeting of specific DP oligomers in CCT polymerisations that are applicable for a variety of applications. In summary, this level of accuracy allows for optimisation of CCT reactions and therefore targeting and production of specific alkene-terminated, oligomeric materials.Acknowledgements
The authors would like to acknowledge the advice provided by Dr Kevin Adlington (University of Nottingham) in the field of the in situ catalysts. The authors thank the Australian Research Council for Funding (FT110100284, DP110104299, DP130103774, DP140100951). This work was performed in part at the Queensland node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano- and micro-fabrication facilities for Australia's researchers. This research was conducted and funded in part by the ARC Centre of Excellence in Convergent Bio-Nano Science and Technology (CE140100036).References
- J. P. A. Heuts and N. M. B. Smeets, Polym. Chem., 2011, 2, 2407–2423 RSC.
- K. Adlington, A. Green, W. Wang, S. M. Howdle and D. J. Irvine, Dalton Trans., 2013, 42, 127–136 RSC.
- K. Adlington, G. J. Jones, J. El Harfi, G. Dimitrakis, A. Smith, S. W. Kingman, J. P. Robinson and D. J. Irvine, Macromolecules, 2013, 46, 3922–3930 CrossRef CAS.
- K. Adlington, R. McSweeney, G. Dimitrakis, S. W. Kingman, J. P. Robinson and D. J. Irvine, RSC Adv., 2014, 4, 16172–16180 RSC.
- B. S. Tovrog, D. J. Kitko and R. S. Drago, J. Am. Chem. Soc., 1976, 98, 5144–5153 CrossRef CAS.
- L. R. Melby, A. H. Janowicz and S. D. Ittel, EP 0196783 A1, 1987.
- C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1996, 29, 7717–7726 CrossRef CAS.
- M. Embiruçu, E. L. Lima and J. C. Pinto, Polym. Eng. Sci., 1996, 36, 433–447 Search PubMed.
- P. Cacioli, D. G. Hawthorne, R. L. Laslett, E. Rizzardo and D. H. Solomon, J. Macromol. Sci., Part A: Pure Appl. Chem., 1986, 23, 839–852 CrossRef.
- G. C. Sanders, T. J. J. Sciarone, H. M. L. Lambermont-Thijs, R. Duchateau and J. P. A. Heuts, Macromolecules, 2011, 44, 9517–9528 CrossRef CAS.
- K.-D. Hungenberg, U. Nieken, K. Zöllner, J. Gao and A. Szekely, Ind. Eng. Chem. Res., 2004, 44, 2518–2524 CrossRef.
- E. Gulari, K. McKeigue and K. Y. S. Ng, Macromolecules, 1984, 17, 1822–1825 CrossRef CAS.
- J. C. Santos, M. M. Reis, R. A. F. Machado, A. Bolzan, C. Sayer, R. Giudici and P. H. H. Araújo, Ind. Eng. Chem. Res., 2004, 43, 7282–7289 CrossRef CAS.
- T. Özpozan, B. Schrader and S. Keller, Spectrochim. Acta, Part A, 1997, 53, 1–7 CrossRef.
- B. K. Lavine and J. Workman, Anal. Chem., 2012, 85, 705–714 CrossRef PubMed.
- P. Geladi and B. R. Kowalski, Anal. Chim. Acta, 1986, 185, 1–17 CrossRef CAS.
- A. F. T. Moore, H. C. Goicoechea, F. Barbosa and A. D. Campiglia, Anal. Chem., 2015, 87, 5232–5239 CrossRef CAS PubMed.
- M. Abou Fadel, X. Zhang, A. de Juan, R. Tauler, H. Vezin and L. Duponchel, Anal. Chem., 2015, 87, 3929–3935 CrossRef CAS PubMed.
- B. Li, A. Calvet, Y. Casamayou-Boucau, C. Morris and A. G. Ryder, Anal. Chem., 2015, 87, 3419–3428 CrossRef CAS PubMed.
- J. J. Kelly, C. H. Barlow, T. M. Jinguji and J. B. Callis, Anal. Chem., 1989, 61, 313–320 CrossRef CAS.
- J. B. Cooper, K. L. Wise, J. Groves and W. T. Welch, Anal. Chem., 1995, 67, 4096–4100 CrossRef CAS.
- M. V. Schulmerich, M. K. Gelber, H. M. Azam, S. K. Harrison, J. McKinney, D. Thompson, B. Owen, L. S. Kull and R. Bhargava, Anal. Chem., 2013, 85, 11376–11381 CrossRef CAS PubMed.
- T. G. Levitskaia, J. M. Peterson, E. L. Campbell, A. J. Casella, D. R. Peterman and S. A. Bryan, Ind. Eng. Chem. Res., 2013, 52, 17607–17617 CrossRef CAS.
- I. H. Boyaci, H. T. Temiz, H. E. Genis, E. Acar Soykut, N. N. Yazgan, B. Guven, R. S. Uysal, A. G. Bozkurt, K. Ilaslan, O. Torun and F. C. Dudak Seker, RSC Adv., 2015, 5, 56606–56624 RSC.
- U. A. Barbosa, I. F. dos Santos, S. L. C. Ferreira and A. M. P. dos Santos, RSC Adv., 2015, 5, 54046–54052 RSC.
- B. Panneton, J.-M. Roger, S. Guillaume and L. Longchamps, Appl. Spectrosc., 2008, 62, 747–752 CrossRef CAS PubMed.
- S. Wold, Technometrics, 1978, 20, 397–405 CrossRef.
- Q.-S. Xu and Y.-Z. Liang, Chemom. Intell. Lab. Syst., 2001, 56, 1–11 CrossRef CAS.
- H. G. M. Edwards, A. F. Johnson, I. R. Lewis, N. J. Ward and S. Wheelwright, J. Raman Spectrosc., 1993, 24, 495–500 CrossRef CAS.
- O. Belaidi, M. Adjim, T. Bouchaour and U. Maschke, Spectrochim. Acta, Part A, 2015, 148, 396–404 CrossRef CAS PubMed.
- T. R. Manley and C. G. Martin, Spectrochim. Acta, Part A, 1976, 32, 357–368 CrossRef.
- J.-P. Montheard, M. Chatzopoulos and D. Chappard, J. Macromol. Sci., Part C: Polym. Rev., 1992, 32, 1–34 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Conversion information for the calibration samples, RMSECV plots for varying normalisation, loadings plots for all of the models and MATLAB scripts. See DOI: 10.1039/c6ra06462k |
| This journal is © The Royal Society of Chemistry 2016 |