Facile fabrication of carboxymethyl cellulose sodium/graphene oxide hydrogel microparticles for water purification
Jingjing Liu,
Huijuan Chu,
Hongliang Wei*,
Hongzheng Zhu,
Gang Wang,
Jing Zhu and
Juan He
School of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou 450001, China. E-mail: weihl68@126.com
First published on 17th May 2016
Abstract
Herein, carboxymethyl cellulose sodium (CMCNa)/graphene oxide (GO) hydrogel microparticles (CGs) with diameters of 2.2–3.6 μm were prepared facilely via spray drying. The as-prepared CGs were used as adsorbents for the removal of methylene blue (MB), Eosin Y and various heavy metal ions. The results revealed that the adsorption capacities of the CG with 8 wt% GO increased by 340–354% for the dyes and 350–500% for heavy metal ions, in comparison with the pristine CMCNa reference. Moreover, the microparticle structure also endowed the CGs with high adsorption kinetics as the adsorption equilibrium for all pollutants was commonly reached within 30 min. The adsorption mechanisms for dyes were due to both electrostatic and π–π interactions, while those for heavy metals were the synergistic effect of electrostatic interactions, surface complexation and ion exchange. Further investigation indicated that the adsorption isotherms and kinetics can be well fitted by the Langmuir isotherm model and the pseudo-second-order model, respectively. By adjusting the GO content and solution pH, the adsorption capacities of our CGs can be further improved. The unique adsorption properties of our CGs, together with the spray drying method, make them very promising in the mass production of broad-spectrum adsorbents for water purification.
1. Introduction
The rapid development of petrochemical industries associated with textiles, leather, paper-making and printing, has generated an enormous amount of wastewater that contains toxic dyes, heavy metals and aromatic pollutants. Consequently, a variety of techniques including adsorption, membrane separation, electrocoagulation and so forth, have developed gradually for the removal of the above industrial contaminants.1–3 Due to its low cost, ease of operation and fewer harmful secondary products, adsorption is thought to be the most effective and widely used method. To date, different absorbents, such as activated carbon, wood chip, fly ash and coal, synthetic polymer resin and polysaccharide-based materials, have been prepared for water purification.4–6 However, their drawbacks of low adsorption capacities and efficiencies hardly meet the demands of increasingly serious environmental problems.Hydrogels, one sort of hydrophilic polymers connected by chemical and/or physical crosslinking,7 have 3D network structures and large specific surface area, and thereby are usually employed as superior adsorbents. In the fabrication of such materials, biopolymers are more attractive than synthetic ones, because of their superior biocompatibility and biodegradation.8 As the most abundant nature polymer, cellulose, as well as its derivatives like carboxymethyl cellulose (CMC), have been made into hydrogels for wastewater treatment.9–12 But their adsorption performances are too weak, especially for heavy metals. To further improve the decontamination capacities of hydrogel materials, functional nanofillers began to be introduced into their networks.13–18
Graphene, a new 2D material with sp2-conjugated carbon nanostructure and high specific surface area,19 is able to capture aromatic pollutants via π–π interactions.20–22 Its derivative, graphene oxide (GO), has plenty of oxygen-containing functional groups including epoxy, hydroxyl and carboxyl groups, and shows exceptional adsorption capacities for dyes, heavy metals, organic solvents and oils.23–32 When incorporated into biopolymer-based hydrogels, these carbon fillers can either improve their adsorption ability towards a certain effluent,33–35 or broaden the categories of pollutants.13,36 For example, Chen et al. prepared chitosan (CS)/GO hybrid hydrogels as broad-spectrum adsorbents.37 In aqueous solution, the negatively charged GO is capable of removing cationic methylene blue (MB) via electrostatic interactions, while the positively charged CS is of the adsorption of anionic Eosin Y. As a result, the as-prepared CS/GO hydrogels showed a high adsorption capacity (>300 mg g−1) for both MB and Eosin Y. Moreover, the adsorption capacities of the hydrogels for Cu2+ and Pb2+ were found to be 70 and 90 mg g−1, respectively. According to other reports,10,24,37,38 the underlying mechanisms for the adsorption of heavy metals by CS/GO composites may be assigned to the combination of electrostatic interactions, surface complexation and ion exchange. On the other hand, these nanofillers can be utilized as one sort of reinforcing agents to enhance the mechanical properties of cellulose-based composite.39,40 For instance, Zhang et al. fabricated orderly porous CMC/GO monoliths by an unidirectional freeze-drying method.41 The results revealed that the incorporation of GO not only improved the adsorption capacities of CMC hydrogels for heavy metals, but also significantly enhanced their compression strength. When the content of GO was 5 wt%, the compression strength of the composite reached to ∼41 kPa, much higher than that (∼17 kPa) of the pristine CMC in control. Recently, graphene has also been filled into cellulose and CMC hosts as conductive filler for energy storage and shielding the undesired electromagnetic interference.42,43
Although great progress has been made, there are still some challenges. For the above CS/GO hydrogels, the time required for their adsorption equilibrium are about 58 and 36 h for MB and Eosin Y,37 indicating slow adsorption kinetics. Similar phenomena are also observed in graphene hydrogels and porous CS/gelation/GO monoliths.21,44 One of the possible reasons is believed to be their large volume, which hinders the diffusion of the adsorbate molecules through the hydrogels. Hydrogel microparticles (or microgels) have small volume and large specific surface area,45 and may be a good option for the preparation of high-efficiency adsorbents. In previous report,46 our group reviewed the recent progress in graphene-based hydrogels as well as their wide applications in water purification, supercapacitors, drug delivery, microfluidic switch, catalyst support and self-healing materials. To best of our knowledge, the adsorption behaviors of cellulose/GO or graphene composite hydrogel microparticles for organic dyes and heavy metals still remains unknown. Inspired by these, it is interesting to fabricate CMC/GO hydrogel microparticles and investigate their adsorption behaviors for industrial pollutants.
In this article, CMC/GO hydrogel microparticles (CGs) were prepared simply by spray drying. The structure of microparticle was expected to accelerate the rapid adsorption of pollutants, while the spray drying method may help to realize the mass production of CGs. As a result, the adsorption properties of the CGs for cationic MB, anionic Eosin Y and heavy metals ions were investigated under different conditions: contact time, GO contents and pH, and their adsorption mechanisms were also discussed. Furthermore, the corresponding adsorption isotherm and kinetics were analyzed to better understand the adsorption process.
2. Experimental
2.1. Materials
Graphite oxide was synthesized by a modified Hummers method,47 from natural graphite powders provided by Qingdao Huatai lubricant sealing S&T Co. Ltd., China. Carboxymethyl cellulose sodium (CMCNa), citric acid (CA), MB and Eosin Y were purchased from Sinopharm Chemical Reagent, China. The stock solutions containing metal ions of Pb2+, Cd2+, Cu2+ and Cr6+ (1 mg mL−1) were obtained from Central Iron and Steel Research Institute, Beijing. All the chemicals were used as received without further purification.2.2. Preparation of CMCNa/GO mixtures via solution blending
CGs were fabricated by the combination of solution blending and spray drying methods as shown in Fig. 1. Prior to the spray drying process, homogeneous CMCNa/GO mixtures need to be prepared via simple solution blending. In a typical procedure, graphite oxide (0.50 g) was dispersed in distilled water (250 mL), and fully exfoliated into graphene oxide (GO) with the assistance of ultrasonication for 1 h. On the other hand, CMCNa (1.25 g) was dissolved in distilled water (250 mL) at 60 °C to obtain an aqueous solution. After cooling to room temperature, the aqueous solution was blended with GO suspension under vigorous stirring to form homogeneous mixture. Meanwhile, the cross linker (0.5 g), CA, was added. Similarly, the CMCNa solution with GO content of 0, 2, 4, 6 and 8 wt% were prepared and ready for spray drying.2.3. Fabrication of CGs via spray drying
In this process, CGs were fabricated using a mini spray dryer (BUCHI B-290). To better understand its working principle, a schematic diagram for the apparatus has been given in Fig. 2. As seen, CMCNa/CA/GO aqueous solutions were initially brought to an atomization device in flowing compressed hot air. After spray drying, the solutions were converted into powders that would be further transferred into a sample collector. The resulting powders were then transferred into vacuum oven and dried at 90 °C for 6 h, in order to guarantee the adequate cross-linking of CMCNa. The resultant microparticles are termed as CGs, where the subscript “s” represents the mass fraction of GO. For example, CG2 is the CMCNa hybrid microparticle with 2 wt% GO, while CG0 represents pristine CMCNa microparticle.2.4. Swelling experiments
The swelling experiment was performed by immersing dried CGs into distilled water at room temperature for 4 h. For the evaluation of swelling ratio (SR), the hydrogels needed to be separated by the centrifugation at 4000 rpm for 15 min, and then were weighted after removing the residual water with filter papers. The SR was calculated using the equation as follows:
 | (1) |
2.5. Adsorption experiments
All adsorption experiments were performed in sealed 50 mL glass conical bottles that contained 0.03 g of CGs and 25 mL of standard solutions. The model pollutant in the standard solutions involves both organic dyes (MB and Eosin Y) and heavy metal ions (Pb2+, Cu2+, Cd2+ and Cr6+), and their concentration were precisely tuned to 100 mg L−1 with deionized water. When the experiments began, the bottles needed to be placed in a shaking water bath at room temperature.The adsorption isotherm and kinetic studies for all pollutants was carried out on a representative sample CG8 under the same solution conditions (20 °C for all the model pollutants; pH = 6 for MB and heavy metals, and pH = 2 for Eosin Y). The concentration of dye solutions was measured with UV-vis absorbance, while that of heavy metals was determined by atomic absorption spectrometer.
The effect of GO content on the adsorption of MB, Eosin Y and the metal ions were investigated, respectively. Afterwards, CG8 was selected as a typical sample and the effect of the solution pH on its adsorption capacity was studied in the pH range of 2–12 for the pollutants. Meanwhile, the solution pH was adjusted with HCl and NaOH solution (0.1 mol L−1).
2.6. Characterization
The Fourier transform infrared (FT-IR) measurements were recorded in KBr Pellets using a Thermo Nicolet 6700 spectrometer. The X-ray diffraction (XRD) was conducted using a Bruker AXS X-ray diffractometer at 40 kV and 40 mA for monochromatized Cu Kα (λ = 0.154178 nm) radiation, and the interlayer spacing (d002) was calculated by Bragg's law: d = λ/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ.48 The Raman spectra were monitored on a Labram spectrometer with a laser of 633 nm. Additionally, the morphology of CGs was also observed with a Hitachi S-4800 filed emission scanning electron microscopy (SEM) at the accelerating voltage of 8 kV. Before the observation, all samples needed to be coated with platinum. Ultraviolet-visible (UV-vis) absorption spectra were recorded with a Shimadzu UV-2401PC spectrophotometer.
θ.48 The Raman spectra were monitored on a Labram spectrometer with a laser of 633 nm. Additionally, the morphology of CGs was also observed with a Hitachi S-4800 filed emission scanning electron microscopy (SEM) at the accelerating voltage of 8 kV. Before the observation, all samples needed to be coated with platinum. Ultraviolet-visible (UV-vis) absorption spectra were recorded with a Shimadzu UV-2401PC spectrophotometer.
3. Results and discussion
3.1. Chemical changes in the fabrication of CGs
In the fabrication of cellulose-based hybrid hydrogels, chemical cross-linking agents in the forms of sodium polyacrylates and formaldehyde compounds are commonly utilized to initialize the cross-linked reactions. However, the fact is that most of the chemical cross-linkers like divinylsulfone (DVS) are highly toxics that may cause significant environmental concerns.49 As to the physical method by electron beam, it generally requires relatively high doses of irradiation and poses a risk of the degradation of CMC.50 Therefore, a green cross-linker, CA, was developed for the cross-linking of CMCNa,51 and a heat treatment over 80 °C was generally required for the process. Based on these, CA was selected as a cross-linker in the present study and the chemical changes in CGs were investigated with FT-IR spectra before and after their fabrication.As shown in Fig. 3a, neat CMCNa presents a characteristic peak at 1595 cm−1, due to the stretching vibration of carboxylate group. Moreover, strong absorption bands are also observed at 3402 cm−1 (O–H stretching vibration), 2912 cm−1 (C–H stretching vibration), 1421 cm−1 (–CH2 scissoring vibration), 1322 cm−1 (–OH bending vibration), 1060 cm−1 (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CH–O–CH2 stretching vibrations), respectively.40 When the sample is heat-treated in the presence of CA, a new peak will appear at 1732 cm−1, which is assigned to the stretching vibration of carbonyl group, suggesting the formation of anhydride (an intermediate necessary for the esterification reaction between CA and the hydroxyl groups of CMCNa).51 After the incorporation of GO, the absorption band around 1732 cm−1 can still be observed (Fig. 3b), further confirming the successful cross-linking of the CMCNa. Moreover, the carbonyl stretch of the carboxylic groups of GO was overlapped with that of carboxylate groups of CMCNa at about 1595 cm−1, indicating the strong interaction between the carboxyl groups of GO and the hydroxyl groups of CMCNa.39
CH–O–CH2 stretching vibrations), respectively.40 When the sample is heat-treated in the presence of CA, a new peak will appear at 1732 cm−1, which is assigned to the stretching vibration of carbonyl group, suggesting the formation of anhydride (an intermediate necessary for the esterification reaction between CA and the hydroxyl groups of CMCNa).51 After the incorporation of GO, the absorption band around 1732 cm−1 can still be observed (Fig. 3b), further confirming the successful cross-linking of the CMCNa. Moreover, the carbonyl stretch of the carboxylic groups of GO was overlapped with that of carboxylate groups of CMCNa at about 1595 cm−1, indicating the strong interaction between the carboxyl groups of GO and the hydroxyl groups of CMCNa.39
 | ||
| Fig. 3 (a) FT-IR spectra of CMCNa and CG0; (b) FT-IR spectra of CGs; (c) XRD patterns of graphite, GO and CG8; (d) Raman spectra of graphite, GO and CG8. | ||
Aside from the cross-linking of CMCNa, the heat treatment process also brings about the partially reduction of GO, as proved by the XRD diffraction pattern shown in Fig. 3c. The interlayer spacing (d002) of CG8 was calculated using Bragg's law to be about 0.55 nm, corresponding to the diffraction peak at 16.1°. This value is much lower than the 0.89 nm for GO (2θ = 10.3°), while slightly higher than the 0.34 nm for graphite (2θ = 26.6°), indicating that the embedded GO has been partially reduced.25 Moreover, the Raman spectra in Fig. 3d reveal that the G-band of CG8 locates in the domain of 1579–1604 cm−1, the peak position of graphite and GO, respectively. And the intensity ratio of the D-band to G-band (ID/IG), inverse to the average size of sp2-conjugated domains in graphene,48 also exhibited an increasing tendency from graphite, to CG8 and GO, further affirming the partial reduction of GO.
3.2. Morphology of CGs
Fig. 4 gives the microstructure of CGs. As seen, CG0 is one sort of round or oval gel which owns an average diameter of 2.2 μm. In the heat-treated process, pronounced shrinkage occurred, resulting in the rough surface of CG0. Moreover, tenuous cracks are also observed obviously on its surface, due to the weak mechanical properties. After the addition of GO, the size of the particle increases to about 3.6 μm, and the shrinkage of the samples are largely suppressed because of the rigid structure of GO. More importantly, no obvious cracks are found on the surface of the CG8, possibly because of the reinforcement effect of GO on CMCNa matrix.39,403.3. Swelling behaviors of CGs
As we know, swelling properties are critical to hydrogel materials. The swelling ratios of our CGs are shown in Fig. 5. It is found that all samples exhibit high swelling rates within the initial 30 min, and then little change is found in their values with time prolonging. As a result, the equilibrium swelling ratios exhibit an increasing tendency with GO content increasing. Similar swelling behaviors were also reported in poly(N-isopropylacrylamide)/GO and poly(vinyl alcohol)/GO composite hydrogels,52,53 where the swelling ratio increment for composite hydrogels were ascribed to the hydrogen bond between GO and water molecules that is not existent in the pure polymeric hydrogel systems. Furthermore, they also pointed out that excessive GO content could cause the predominance of the hydrogen bond between GO and the polymers that have a competitive relationship with the bond between GO and water molecules, consequently leading to the increase in the cross-linking density and some decrease in the swelling ratio. Based on these information, we think that the swelling properties of our CGs may be possibly enhanced by the hydrogen bond between GO and water molecules. Generally, the more the GO content, the more obvious enhancement effect in the swelling ratio. At the same time, the hydrogen bond between GO and CMCNa proved by El Achaby, et al. and Yadav et al. in the studies on CMC/GO composite films,39,40 should not be neglected. The competition in the hydrogen bonds between GO with water molecules and GO with CMCNa may result in the increase in the swell ratio, but the decrease in the increasing rate.3.4. Adsorption of dyes
The adsorption properties of CGs for cationic MB and anionic Eosin Y were investigated. In Fig. 6a, the pure MB (pH = 6) and Eosin Y (pH = 2) aqueous solutions are in blue and orange colors, respectively. With the addition of CGs, the colors fade by degrees, suggesting the elimination of the dissolved dyes. Moreover, the sample with more GO possesses much higher adsorption capacity. The UV-vis absorption spectra in Fig. 6b and c further confirm this observation, for the characteristic absorption intensity of MB at 664 nm and Eosin Y at 516 nm decreases noticeably with GO content increasing. Consequently, over 95% dyes were captured by CG8 within 1 h. To better understand the adsorption process of the dyes, the adsorption isotherms and kinetics of CGs were researched using CG8 as a representative sample.
 | (2) |
Fig. 6d indicates that the adsorption capacity of CG8 increases sharply with their equilibrium concentration of dyes, and then starts to level off. The increasing driving force derived from the concentration gradient of the dyes is usually considered to be a reasonable explanation,20 which accelerates the diffusion of the dyes on CG8. To simulate the experimental data, Langmuir and Freundlich models are the most commonly used two.
The Langmuir isotherm model is suitable for the monolayer adsorption occurring on a homogeneous surface where no subsequent interaction between the adsorbed species occurs. The equation can be expressed as:20
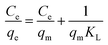 | (3) |
As to the Freundlich model, it is an empirical model based on multilayer adsorption on heterogeneous surfaces, and can be described using the equation as follows:20
 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce.
Ce.
The isotherm parameters of both models are summarized in Table 1. By comparing the values of the correlation coefficient R2, we believed that the Langmuir model fits the adsorption data better than the Freundlich does. It demonstrates that the adsorption of MB and Eosin Y occurs in a monolayer manner.
| Isotherm models | Parameters | Dyes | Metal ions | ||||
|---|---|---|---|---|---|---|---|
| MB | Eosin Y | Pb2+ | Cd2+ | Cu2+ | Cr6+ | ||
| Langmuir | KL | 5.4 | 3.6 | 0.3 | 0.4 | 1.3 | 0.6 |
| qm | 58.24 | 65.70 | 19.19 | 27.01 | 28.41 | 27.81 | |
| R2 | 0.9815 | 0.9837 | 0.9858 | 0.9944 | 0.9626 | 0.9846 | |
| Freundlich | KF | 29.17 | 33.50 | 5.85 | 10.98 | 11.75 | 9.87 |
| n | 3.63 | 3.19 | 3.32 | 4.08 | 3.25 | 3.34 | |
| R2 | 0.8729 | 0.8630 | 0.9075 | 0.6689 | 0.9120 | 0.9064 | |
The pseudo-first-order model can be expressed as:54
 | (5) |
The pseudo-second-order model contains different kinds of adsorptions including external film diffusion, adsorption and internal particle diffusion. Its equation can be given as:21
 | (6) |
After simulation, all parameters are listed in Table 2. Based on the correlation coefficient R2 of the both models, it is evident that the pseudo-second-order model is more suitable for the adsorption by our CGs, further confirming that the adsorption of the dyes is dominated by chemisorption.
| Kinetic models | Parameters | Dyes | Heavy metal ions | ||||
|---|---|---|---|---|---|---|---|
| MB | Eosin Y | Pb2+ | Cd2+ | Cu2+ | Cr6+ | ||
| Pseudo-first-order | k1 × 10−2 | 10.09 | 10.57 | 4.12 | 5.09 | 5.57 | 5.14 |
| R2 | 0.9967 | 0.9850 | 0.9150 | 0.8807 | 0.8643 | 0.8755 | |
| Pseudo-second-order | k2 × 10−3 | 4.10 | 3.55 | 7.18 | 3.90 | 2.29 | 3.91 |
| R2 | 0.9999 | 0.9978 | 0.9984 | 0.9882 | 0.9719 | 0.9825 | |
Additionally, the pH of the solution is another controlling factor that may exert a critical influence on the adsorption process. Therefore, the effect of pH on the adsorption of both dyes was studied over the pH range of 2–12. As shown in Fig. 6i, the adsorption curve of MB increases firstly and then declines. The adsorption of Eosin Y is quite different, the curve shows a decreasing tendency with the increase in the solution pH. It has been said that the pKa of carboxylic groups is around 4.6.55 Below this value, the –COO− groups in CMCNa and GO will be protonated to –COOH groups, which makes CGs almost uncharged and benefits the adsorption of cationic MB and anionic Eosin Y via electrostatic attraction. When the pH increases above 4.6, ionization occurs to the –COOH groups, yielding the negatively charged CGs. Electrostatic repulsion will be the predominated force between Eosin Y and CGs, while for MB and CGs, electrostatic attraction will be enhanced. As a result, the maximum adsorption capacity of MB is obtained at pH = 6. Beyond this value, a screening effect will be formed by the excessive produced Na+,55 causing the decrease in the adsorption of MB.
3.5. Adsorption of heavy metals
The adsorption of heavy metals was performed by immersing CGs in the desired standard solutions (100 mg L−1) that were prepared by diluting the stork solutions with distilled water. Similar to the organic dyes, the analysis on the adsorption of heavy metals is also composed of three sections: adsorption isotherms, kinetics and effect of GO content and pH.When simulated with the two isotherm models, it is found that the adsorption behaviors of the metals are better fitted by the Langmuir model. And Fig. 7b gives the linear relationship of t/qt versus t. The corresponding correlation coefficients R2 in Table 1 suggest that the whole adsorption process of the metal ions is a kind of monolayer adsorption. This result is similar to the adsorption by GO sheets.29,30
Moreover, the simulation using the pseudo-first-order and pseudo-second-order equations demonstrate that the latter is found to be more suitable for the adsorption behaviors of our CGs for heavy metals. The associated parameters are given in Table 2 and the linear plot for the calculation of the pseudo-second-order model is also shown in Fig. 7d. All these results indicated the chemisorption-dominated process.
As to the effect of pH, it is well known to us that the existence form of metal ions in aqueous solution can be significantly affected by the pH value. The reaction scheme for the hydroxide formation of divalent metal ions (Me2+) can be expressed as follows:29
| Me2+ ↔ Me(OH)+ ↔ Me(OH)02 ↔ Me(OH)3− ↔⋯ |
Previous report demonstrated that, at low pH < 6, the predominant metal species in aqueous solution is Me2+ and the ionization of the oxygen-containing groups of GO makes electrostatic attraction to be the main mechanism for the adsorption of heavy metals by GO.29,30 For the pH = 6–8, hydroxide complex Me(OH)+ starts to form.30 Further increasing the pH to 8–12, the main species in the solution gradually become to Me(OH)02, Me(OH)3−, Me(OH)42−, etc., and the precipitation of Me(OH)2(s) coupled with adsorption of other metal hydroxyl complex ions may occur. Similar phenomenon was also observed by Lu et al. in the adsorption of zinc ion with purified carbon nanotube.56
To reduce the measurement error brought by metal hydroxide precipitations, the effect of pH was investigated in acidic pH rang of 2–6 with CG8. As shown in Fig. 7f, the adsorption capacities of the four metal ions are highly affected by the solution pH, and exhibit an increasing tendency with pH increasing. This result is closely related to the ionization degree of carboxyl groups in GO and CMCNa, which consequently may exert a significant influence on the complicated interactions between the metal ions and CG8.
4. Conclusions
In summary, CGs were prepared simply in this work via a spray drying method. Meanwhile, hydrogen bonds were formed among the oxygen-containing groups in GO sheets and CMCNa matrix, which enhanced not only the mechanical strength of the microparticles, but also their swelling properties. Moreover, the introduction of GO also significantly improved the adsorption performances of CGs towards dyes and heavy metals. With the addition of 8 wt% GO, the adsorption capacities of the sample increased by 340–354% for dyes and 350–500% for heavy metal ions. Thanks to the microscale structure, CGs exhibited high adsorption rates and the adsorption equilibrium was obtained for all pollutants within 30 min. Further analysis on the adsorption isotherms and kinetics revealed that the adsorption of the pollutants can be well described by the Langmuir isotherm model and the pseudo-second-order model, respectively. The adsorption mechanism for dyes includes both electrostatic and π–π interactions, while for heavy metals, the mechanism consists of electrostatic interactions, surface complexation and ion exchange. Additionally, the adsorption behaviors of our CGs can be tuned easily by the content of GO as well as the solution pH.Acknowledgements
The work was supported by the Henan Province University Innovation Talents of Science and Technology Support Program (2012HASTIT017), the Science and Technology Department of Henan Province (142300410011, 102102210131 and 152102110073), the Education Department of Henan Province (2010A430002), and Henan University of Technology (2012JCYJ07).References
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- G. McKay, M. S. Otterburn and A. G. Sweeney, Water Res., 1980, 14, 15–20 CrossRef CAS.
- L. Wang, J. Hazard. Mater., 2009, 171, 577–581 CrossRef CAS PubMed.
- G. F. Liu, J. Ma, X. C. Li and Q. D. Qin, J. Hazard. Mater., 2009, 164, 1275–1280 CrossRef CAS PubMed.
- G. S. Gupta, G. Prasad and V. H. Singh, Water Res., 1990, 24, 45–50 CrossRef CAS.
- L. Dehabadi and L. D. Wilson, Carbohydr. Polym., 2014, 113, 741–749 CrossRef PubMed.
- H. L. Wei, Z. Yang, H. J. Chu, J. Zhu, Z. C. Li and J. S. Cui, Polymer, 2010, 51, 1694–1702 CrossRef CAS.
- X. Vecino, R. Devesa-Rey, J. M. Cruz and A. B. Moldes, Carbohydr. Polym., 2015, 115, 129–138 CrossRef CAS PubMed.
- D. Zhou, L. Zhang and S. L. Guo, Water Res., 2005, 39, 3755–3762 CrossRef CAS PubMed.
- D. Zhou, L. N. Zhang, J. P. Zhou and S. L. Guo, Water Res., 2004, 38, 2643–2650 CrossRef CAS PubMed.
- L. Yan, Q. Shuai, X. Gong, Q. Gu and H. Yu, Clean: Soil, Air, Water, 2009, 37, 392–398 CrossRef CAS.
- C. Chang and L. Zhang, Carbohydr. Polym., 2011, 84, 40–53 CrossRef CAS.
- X. Wang, Z. Liu, X. Ye, K. Hu, H. Zhong, J. Yu, M. Jin and Z. Guo, Appl. Surf. Sci., 2014, 308, 82–90 CrossRef CAS.
- T. Jiao, H. Guo, Q. Zhang, Q. Peng, Y. Tang, X. Yan and B. Li, Sci. Rep., 2015, 5, 11873 CrossRef CAS PubMed.
- T. Jiao, H. Zhao, J. Zhou, Q. Zhang, X. Luo, J. Hu, Q. Peng and X. Yan, ACS Sustainable Chem. Eng., 2015, 3, 3130–3139 CrossRef CAS.
- X. Rui-Hong, R. Peng-Gang, H. Jian, R. Fang, R. Lian-Zhen and S. Zhen-Feng, Carbohydr. Polym., 2016, 138, 222–228 CrossRef PubMed.
- H. Zhao, T. Jiao, L. Zhang, J. Zhou, Q. Zhang, Q. Peng and X. Yan, Sci. China Mater., 2015, 58, 811–818 CrossRef.
- H. H. Najafabadi, M. Irani, L. R. Rad, A. H. Haratameh and I. Haririan, RSC Adv., 2015, 5, 16532–16539 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- J. Xu, L. Wang and Y. Zhu, Langmuir, 2012, 28, 8418–8425 CrossRef CAS PubMed.
- J. N. Tiwari, K. Mahesh, N. H. Le, K. C. Kemp, R. Timilsina, R. N. Tiwari and K. S. Kim, Carbon, 2013, 56, 173–182 CrossRef CAS.
- G. Zhao, L. Jiang, Y. He, J. Li, H. Dong, X. Wang and W. Hu, Adv. Mater., 2011, 23, 3959–3963 CrossRef CAS PubMed.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- H. P. Cong, X. C. Ren, P. Wang and S. H. Yu, ACS Nano, 2012, 6, 2693–2703 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- Y. Xu, Q. Wu, Y. Sun, H. Bai and G. Shi, ACS Nano, 2010, 4, 7358–7362 CrossRef CAS PubMed.
- X. Yang, C. Chen, J. Li, G. Zhao, X. Ren and X. Wang, RSC Adv., 2012, 2, 8821–8826 RSC.
- G. K. Ramesha, A. Vijaya Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270–277 CrossRef CAS PubMed.
- L. Liu, S. Liu, Q. Zhang, C. Li, C. Bao, X. Liu and P. Xiao, J. Chem. Eng. Data, 2013, 58, 209–216 CrossRef CAS.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Dalton Trans., 2013, 42, 5682–5689 RSC.
- G. Zhao, J. Li, X. Ren, C. Chen and X. Wang, Environ. Sci. Technol., 2011, 45, 10454–10462 CrossRef CAS PubMed.
- H. Wang, X. Yuan, Y. Wu, H. Huang, G. Zeng, Y. Liu, X. Wang, N. Lin and Y. Qi, Appl. Surf. Sci., 2013, 279, 432–440 CrossRef CAS.
- Y. Wan, F. Zhang, C. Li, G. Xiong, Y. Zhu and H. Luo, J. Mater. Chem. A, 2015, 3, 24389–24396 CAS.
- X. Liu, Y. Zhou, W. Nie, L. Song and P. Chen, J. Mater. Sci., 2015, 50, 6113–6123 CrossRef CAS.
- H. Wei, L. Han, Y. Tang, J. Ren, Z. Zhao and L. Jia, J. Mater. Chem. B, 2015, 3, 1646–1654 RSC.
- H. Gao, Y. Sun, J. Zhou, R. Xu and H. Duan, ACS Appl. Mater. Interfaces, 2013, 5, 425–432 CAS.
- Y. Chen, L. Chen, H. Bai and L. Li, J. Mater. Chem. A, 2013, 1, 1992–2001 CAS.
- V. Chandra, J. Park, Y. Chun, J. W. Lee, I.-C. Hwang and K. S. Kim, ACS Nano, 2010, 4, 3979–3986 CrossRef CAS PubMed.
- M. El Achaby, N. El Miri, A. Snik, M. Zahouily, K. Abdelouahdi, A. Fihri, A. Barakat and A. Solhy, J. Appl. Polym. Sci., 2016, 133, 42356 CrossRef.
- M. Yadav, K. Y. Rhee, I. H. Jung and S. J. Park, Cellulose, 2013, 20, 687–698 CrossRef CAS.
- Y. Zhang, Y. Liu, X. Wang, Z. Sun, J. Ma, T. Wu, F. Xing and J. Gao, Carbohydr. Polym., 2014, 101, 392–400 CrossRef CAS PubMed.
- W. Ouyang, J. Sun, J. Memon, C. Wang, J. Geng and Y. Huang, Carbon, 2013, 62, 501–509 CrossRef CAS.
- H. Zhang, D. Zhai and Y. He, RSC Adv., 2014, 4, 44600–44609 RSC.
- N. Zhang, H. Qiu, Y. Si, W. Wang and J. Gao, Carbon, 2011, 49, 827–837 CrossRef CAS.
- H. Z. Zhu, L. Q. You, H. L. Wei, G. F. Wang, H. J. Chu, J. Zhu and J. He, Polym. Eng. Sci., 2015, 55, 2775–2782 CAS.
- J. J. Liu, H. J. Chu, H. L. Wei, H. Z. Zhu, J. Zhu and J. He, Prog. Chem., 2015, 27, 1591–1603 Search PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Y. Li, B. Shen, X. L. Pei, Y. G. Zhang, D. Yi, W. T. Zhai, L. H. Zhang, X. C. Wei and W. G. Zheng, Carbon, 2016, 100, 375–385 CrossRef CAS.
- G. Marci, G. Mele, L. Palmisano, P. Pulito and A. Sannino, Green Chem., 2006, 8, 439–444 RSC.
- S. M. Ibrahim, K. M. El Salmawi and A. H. Zahran, J. Appl. Polym. Sci., 2007, 104, 2003–2008 CrossRef CAS.
- C. Demitri, R. Del Sole, F. Scalera, A. Sannino, G. Vasapollo, A. Maffezzoli, L. Ambrosio and L. Nicolais, J. Appl. Polym. Sci., 2008, 110, 2453–2460 CrossRef CAS.
- X. Ma, Y. Li, W. Wang, Q. Ji and Y. Xia, Eur. Polym. J., 2013, 49, 389–396 CrossRef CAS.
- H. Bai, C. Li, X. Wang and G. Shi, Chem. Commun., 2010, 46, 2376–2378 RSC.
- X. Huang, X. Liao and B. Shi, J. Hazard. Mater., 2009, 170, 1141–1148 CrossRef CAS PubMed.
- M. F. Abou Taleb, H. L. A. El-Mohdy and H. A. A. El-Rehim, J. Hazard. Mater., 2009, 168, 68–75 CrossRef CAS PubMed.
- C. Y. Lu and H. S. Chiu, Chem. Eng. Sci., 2006, 61, 1138–1145 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |






