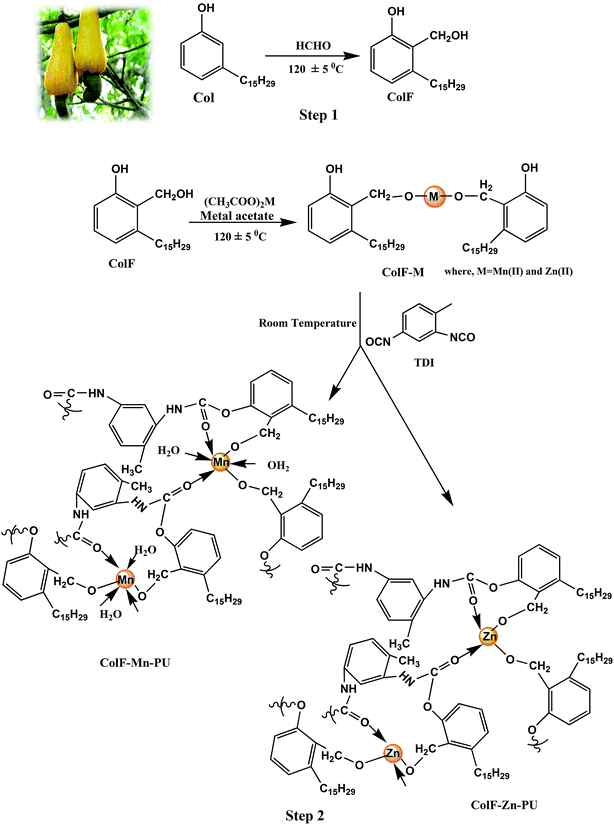
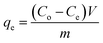Development of bio-derived nanostructured coordination polymers based on cardanol–formaldehyde polyurethanes with ‘d5’ Mn(II) and ‘d10’ Zn(II) metal nodes: synthesis, characterization and adsorption behavior
Shabnam Khan,
Laxmi,
Fahmina Zafar* and
Nahid Nishat*
Inorganic Materials Research Laboratory, Department of Chemistry, Jamia Millia Islamia, New Delhi 110025, India. E-mail: fahmzafar@gmail.com; nishat_nchem08@yahoo.com
First published on 20th April 2016
Abstract
The synthesis of bio-based coordination polyurethanes (PUs) is an alternative route to conventional petro-based PUs for the green and sustainable development of coordination PUs. In the present study, we report the development of bio-based nanostructured coordination PUs with the aid of using cost effective, non-toxic, biodegradable and abundantly available renewable resource such as cardanol (Col) as a starting precursor and divalent transition metal ions Mn(II), ‘d5’ and Zn(II), ‘d10’ as metal centres for their potential use in the adsorption/removal of environmental pollutants, such as Congo red (CR) dye, in industrial waste water treatment. The composition and geometry of coordination PUs were confirmed by spectral techniques (FTIR and UV-visible), elemental analysis and magnetic moment. The curing behavior was investigated using ATR-FTIR technique. Thermal techniques (TGA/DTG/DTA and DSC) indicated their good thermal stability. Morphology (XRD, SEM-EDX, and HR-TEM) techniques indicated their amorphous/semi-crystalline and layered nanostructured and nanoporous patterns. The preliminary adsorption properties towards CR dye of the synthesized coordinated PUs was also investigated by a batch adsorption technique and it was found that the synthesized nanostructured and nanoporous coordination PU could be used as an effective dye adsorbent.
1. Introduction
With the continual alarming rise in the cost and the impending exhaustion of petroleum resources, the production of commercial products from renewable resources has been an area of keen interest for researchers in both academia and industry. The use of renewable raw materials also contributes towards green and sustainable development through the use of these materials in all areas of their application. The most commonly used renewable raw materials include proteins, cellulose, lignin, starch, chitin, chitosan and vegetable oils.1,2 Vegetable oils are one of the cheapest and most abundant biological resources as they are a bio-renewable material and have advantages such as low toxicity, inherent biodegradability and high purity.3 Vegetable oils, such as soybean, tung, linseed, rapeseed, castor and cashew nut shell liquid (CNSL), have a wide range of applications.4 Among all these renewable resources, CNSL, an agro-byproduct of the cashew processing industry has proven to be an important starting precursor for industrial polymer products due to its low cost, abundant availability (450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tonnes per year) and chemically reactive nature, amongst other attributes.5 CNSL is a mixture of cardanol (Col), cardol, anacardic acid and 2-methylcardol. Col obtained via the vacuum distillation of CNSL is considered a very attractive precursor for use in eco-friendly processes for the development of new materials. Col is a unique phenolic compound comprising a 15-carbon alkyl chain at the meta position with a varying degree of unsaturation, which confers it with several attractive properties, such as good processability and high solubility in organic solvents. Due to the three available functional sites, such as phenolic hydroxyl, aromatic ring and unsaturated alkyl long-chain, Col has been modified as monomers and polymers, such as in phenolic resins,6 epoxies,7 vinyl esters,8 phenalkamines,9–11 benzoxazines,12,13 polyurethanes (PUs),14–17 Schiff base,18 etc., which have found use in many diverse applications,19,20 such as surfactants,21 foams,22 coatings,23 adhesives,24 antioxidants,25 drug delivery,26 etc. PUs, due to their wide range of physical and chemical properties, are suitable for foams, thermoplastic elastomers, adhesives, coatings, sealants, fibres and others.14–17 Chintankumar J. Patel and Vijay Mannari synthesized air-drying bio-based PU dispersion coatings from Col with improved mechanical performance and with a resistance towards solvents, water and corrosion.27 Mukesh Kathalewar et al. reported the synthesis of isocyanate-free PUs from CNSL based bis-cyclic carbonate and studied their curing kinetics with diamines (hexamethylene diamine and isophorone diamine), and observed that the coating properties could be enhanced by the appropriate selection of the amine cross-linker.28 Changqing Fu et al. synthesized three Col-based polyols with different hydroxyl values by thiol–ene coupling, which were then polymerized with hexamethylene diisocyanate (HDI) to produce bio-based PUs with good hydrophobic and thermal properties, suggesting their potential use as a hydrophobic material for material engineering.29
000 metric tonnes per year) and chemically reactive nature, amongst other attributes.5 CNSL is a mixture of cardanol (Col), cardol, anacardic acid and 2-methylcardol. Col obtained via the vacuum distillation of CNSL is considered a very attractive precursor for use in eco-friendly processes for the development of new materials. Col is a unique phenolic compound comprising a 15-carbon alkyl chain at the meta position with a varying degree of unsaturation, which confers it with several attractive properties, such as good processability and high solubility in organic solvents. Due to the three available functional sites, such as phenolic hydroxyl, aromatic ring and unsaturated alkyl long-chain, Col has been modified as monomers and polymers, such as in phenolic resins,6 epoxies,7 vinyl esters,8 phenalkamines,9–11 benzoxazines,12,13 polyurethanes (PUs),14–17 Schiff base,18 etc., which have found use in many diverse applications,19,20 such as surfactants,21 foams,22 coatings,23 adhesives,24 antioxidants,25 drug delivery,26 etc. PUs, due to their wide range of physical and chemical properties, are suitable for foams, thermoplastic elastomers, adhesives, coatings, sealants, fibres and others.14–17 Chintankumar J. Patel and Vijay Mannari synthesized air-drying bio-based PU dispersion coatings from Col with improved mechanical performance and with a resistance towards solvents, water and corrosion.27 Mukesh Kathalewar et al. reported the synthesis of isocyanate-free PUs from CNSL based bis-cyclic carbonate and studied their curing kinetics with diamines (hexamethylene diamine and isophorone diamine), and observed that the coating properties could be enhanced by the appropriate selection of the amine cross-linker.28 Changqing Fu et al. synthesized three Col-based polyols with different hydroxyl values by thiol–ene coupling, which were then polymerized with hexamethylene diisocyanate (HDI) to produce bio-based PUs with good hydrophobic and thermal properties, suggesting their potential use as a hydrophobic material for material engineering.29
The properties of the PUs can further be enhanced for advance applications via metal incorporation or the formation of coordination polymers (CPs). Metal incorporation into the CPs offers several advantages over virgin polymers. Generally, it enhances the porosity, adsorption, thermal stability, fire retardancy, storage, flexibility, solubility and curing, etc.30 CPs can be synthesized from the coordination-directed self-assembly of metal ion nodes with organic linkers. The properties can be tuned by adjustment of these two counterparts. The literature indicates that the different dimensionalities and topological features of these CPs greatly influence their performance in catalysis, luminescence and adsorption.31 Hence, the correct choice of the metal centre and rational design of the ligand system as well as the coordination behaviour and functionality are the major aspects to consider in the synthesis of these CPs. Generally in CPs, the ligands or organic linkers are petroleum-derived (hence from depleting resources), multi-step synthesis processes that use volatile organic solvents and have limited solubility. These problems motivated us to develop CPs from alternative resources and methods with desirable properties.32 Therefore, we selected the Col-derived ligand as an organic linker for the development of our CPs to avoid problems associated with petro-based CPs, and investigated their potential application in the field of waste water treatment, particularly for dye adsorption, which is an issue of great environmental concern. Our research group recently developed nanostructured self-standing and transparent CP films from the oligomer of Col (organic linker) and divalent metal ions [Mn(II) and Co(II)], which showed nanoporous morphology, amorphous behaviour, good thermal stability up to 260–300 °C, moderate antibacterial activity against various bacterial strains and also good antibiofilm activity.33 Literature survey revealed that much work has been done on the synthesis of PUs from Col but there is no work reported on the development of coordination PUs based on Col for application as a dye adsorbent.
With the aim of moving towards the green and sustainable development of CPs by: (i) the use of renewable resources, i.e. Col, (ii) the minimum use of solvents, (iii) in situ monitoring and (iv) their application in waste water treatment, the present work deals with the preparation of Col–formaldehyde (ColF as the organic linker)-based coordination PUs (ColF–M-PU) using transition metals (M = Mn(II), half-filled d-orbitals, ‘d5’, and Zn(II), completely-filled d-orbitals, ‘d10’) as metal centres, for their use in dye adsorption. The effect of these half and completely-filled metal ions on the structure, geometry and curing behaviour of the PUs was determined by FTIR, UV-Vis, elemental analysis, magnetic moment and ATR-FTIR. The thermal behaviour was analyzed by TGA/DTA/DTG/DSC, while the morphological properties were analyzed by XRD, SEM-EDX and HR-TEM. The preliminary adsorption behaviour towards Congo red (CR) dye was investigated by the batch adsorption technique, in order to assess their potential use as dye adsorbents.
2. Experimental
2.1. Materials
Col was provided by Golden Cashew Products (p) LTD, Pondicherry, India [specification: colour: gardeners standard 10; specific gravity at 30 °C: 0.9268; viscosity at 30 °C: 47; iodine value: 235]. Formaldehyde (F), manganese(II) acetatetetrahydrate (mol. wt: 245.09 g mol−1), zinc(II) acetatedihydrate (mol. wt: 219.49 g mol−1), citric acid, toluene diisocyanate (TDI), dibutyl tindilaurate (DBTDL), methanol and xylene were procured from Loba Chemie Pvt. Ltd. and used as received without further purification. CR dye was purchased from Sigma-Aldrich.2.2. Preparation of cardanol–formaldehyde (ColF) ligand
ColF ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 mole ratio) was prepared using citric acid as a catalyst following a method published in the literature.34 Col (1 mole) was taken in a three-necked flask equipped with a Liebig condenser, mechanical stirrer and thermometer. F (0.7 mole) along with a methanolic solution of the catalyst (1% based on Col) was taken in a burette and added drop wise within 1 h to a reaction flask containing Col at 100 ± 5 °C under continuous stirring. After the complete addition of F, the temperature was raised and then maintained at 120 ± 5 °C until completion of the reaction. The progress of the reaction was monitored periodically by pH and thin layer chromatography (TLC). The reaction was stopped at the desired pH value. The initial pH of the reaction mixture was 6.0, which was reduced to 4.8 after 4 h of reaction (yield = 78.86%).
0.7 mole ratio) was prepared using citric acid as a catalyst following a method published in the literature.34 Col (1 mole) was taken in a three-necked flask equipped with a Liebig condenser, mechanical stirrer and thermometer. F (0.7 mole) along with a methanolic solution of the catalyst (1% based on Col) was taken in a burette and added drop wise within 1 h to a reaction flask containing Col at 100 ± 5 °C under continuous stirring. After the complete addition of F, the temperature was raised and then maintained at 120 ± 5 °C until completion of the reaction. The progress of the reaction was monitored periodically by pH and thin layer chromatography (TLC). The reaction was stopped at the desired pH value. The initial pH of the reaction mixture was 6.0, which was reduced to 4.8 after 4 h of reaction (yield = 78.86%).
2.3. Preparation of ColF–M-PU
ColF–M-PU was synthesized by a “one-pot, two-step” reaction. In the first step, freshly prepared ColF (2 mole) was taken in a three-necked flask equipped with a Liebig condenser, mechanical stirrer and thermometer and heated up to 80 ± 5 °C. At this temperature, a methanolic solution of divalent metal [Mn(II) or Zn(II), 1 mole] acetate was added to it within 15–20 min under continuous stirring. After complete addition of the alcoholic solution of the metal salt, the temperature was raised and then maintained at 120 ± 5 °C until a clear solution was obtained, resulting in the formation of ColF–M with an appreciable change in viscosity. The reaction was conducted under vacuum for 15 min. The reaction was monitored by TLC and on the evolution of vinegar-like smells. The synthesis of ColF–M was carried out within 2 h. After completion of the said reaction, the temperature was cooled down to room temperature (28–30 °C). At this temperature, 20 wt% xylene, DBTDL (0.1%) and the calculated amount of TDI [in three different ratios with respect to ColF–M (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.2
1, 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] were added drop wise by using a burette within 15–20 min in the same pot under continuous stirring. The reaction was carried out at the same temperature for 1 h until a clear liquid with optimum viscosity for application was obtained, which was in the ratio of 1
1)] were added drop wise by using a burette within 15–20 min in the same pot under continuous stirring. The reaction was carried out at the same temperature for 1 h until a clear liquid with optimum viscosity for application was obtained, which was in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (ColF–M
1 (ColF–M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TDI) in our case. The reaction was also monitored by TLC at regular intervals and was finally confirmed by FTIR. The synthesized coordination PU was obtained as a clear, free-flowing, reddish brown liquid as ColF–M-PU, where M = Mn(II) and Zn(II) (yield = 85.19%).
TDI) in our case. The reaction was also monitored by TLC at regular intervals and was finally confirmed by FTIR. The synthesized coordination PU was obtained as a clear, free-flowing, reddish brown liquid as ColF–M-PU, where M = Mn(II) and Zn(II) (yield = 85.19%).
2.4. Preparation of ColF-PU
The ColF-PU was prepared for comparison against ColF–M-PU by taking ColF and TDI at an optimum ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of the catalyst DBTDL using a similar procedure as adopted for the synthesis of ColF–M-PU, except for the metal acetate (yield = 82.56%).
1 in the presence of the catalyst DBTDL using a similar procedure as adopted for the synthesis of ColF–M-PU, except for the metal acetate (yield = 82.56%).
2.5. Preparation of the films
To obtain free-standing films, the desired amount of ColF–Mn-PU, ColF–Zn-PU and ColF-PU, respectively, were dissolved in a minimum amount of xylene and poured onto Teflon sheets. They were then kept undisturbed for drying under ambient temperature (28–30 °C) for 24–72 h.2.6. Characterization methods
The solubility of the synthesized materials were tested in various polar and non-polar solvents by dissolving 20 mg of the sample in 10 ml of solvent in a closed test tube and set aside for 24 h at room temperature. The elemental analysis for the estimation of C, H and N percentages was recorded using the Vario Micro Cube Instrument (Elementar Analysensyteme GmbH, Germany). The electrical conductivities were determined using a digital conductivity meter, PICO+ (LabIndia Instruments, Pvt. Ltds.). Fourier transform infrared spectroscopy (FTIR) spectra were recorded in the mid operating range of 4000–500 cm−1 using the IR Affinity-1 CE spectrometer (Shimadzu corporation analytical and measuring instrument division, Kyoto, Japan). The thickness of the films was measured by an Elcometer instrument (Model 345 NT; Elcometer Instruments, Manchester, UK). FTIR spectra of the cured films were recorded using the Attenuated Total Reflectance instrument (ATR, TENSOR 37 spectrophotometer, Germany) in the mid operating range of 4000–500 cm−1 by placing the films onto the Universal Diamond ATR top plate, and data acquisition was carried out using Opus-Spectroscopic software. The electronic spectra of the samples in xylene were recorded using a UV-Vis spectrophotometer (Perkin-Elmer Lambda 40) under ambient conditions. Magnetic susceptibility measurements of the polymeric samples were carried out on a Faraday balance. An Environmental Scanning Electron Microscope, SEM (model FEI Quanta 200F IE 250 X Max 80) along with energy dispersive X-ray spectroscopic, EDX (Oxford-EDS system) attachment was used to analyze the morphology of the samples and its surface chemical composition at the SMITA research lab IIT, Delhi, India. A High Resolution Transmission Electron Microscope (HR-TEM) with TECHNAI 200 kV TEM operating at 200 kV (Fei, Electron Optics), equipped with digital imaging and a 35 mm photography system, was used to analyze the morphology of the materials (individual phases i.e. the inorganic and organic phases) at the All India Institute of Medical Sciences, New Delhi, India. Prior to the analysis, the required amount of sample was ultrasonicated in ethanol for 20 min and dropped on a carbon-coated grid, which was dried in air. The thermal stability of the polymeric films was studied by thermogravimetric (TG)/derivative weight (DTG) analysis by a TA instruments Q-500 Thermal Gravimetric Analyzer under nitrogen atmosphere at a heating rate of 10 °C min−1 from room temperature to 1000 °C. TGA/DTA/DSC (Mettler Toledo AG, Analytical CH-8603, Schwerzenbach, Switzerland) was also used for thermal analysis of the cured samples under a nitrogen atmosphere at a heating rate of 10 °C min−1. The wide angle powder X-ray diffraction (XRD) patterns of the materials were recorded using an X-ray diffractometer (Ultima IV model, Rigaku Cooperation, Japan) with Cu Kα radiation (k 50.15406 nm). The spectra were recorded against 2θ from 10 to 60° at a scan rate of 1° min−1.Adsorption studies of CR dye on ColF–M-PU were carried out by a batch adsorption technique in tightly covered beakers. A stock solution of 500 mg L−1 CR was prepared by the dissolution of solid CR in doubly distilled water. The aqueous CR stock solution was diluted to different concentrations from 10 to 200 mg L−1. The synthesized ColF–Mn-PU and ColF–Zn-PU were dried in a vacuum desiccator prior to adsorption. Initially, 5 mg adsorbent was added in 30 ml dye solution of said concentrations and the mixture was agitated for 12 h at 40 °C. Thereafter, the clear supernatant solution was extracted using a micropipette, and the concentration of residual CR was determined by a Hitachi U3900 UV-Vis spectrophotometer at the calibrated maximum wavelength of 497 nm.
The adsorption capacity, qe (mg g−1), of the synthesized coordination PU was determined using a mass-balance relationship, which represents the amount of dye adsorbed per unit weight of adsorbent:
The % dye adsorption was calculated as:
3. Results and discussion
3.1. Chemistry of ColF–M-PU formation
Scheme 1 represents the chemical reaction route involved in the synthesis of ColF–M-PU. The two major steps involved in the synthesis are briefly discussed below:(1) The first step involved the synthesis of ColF ligand by the condensation reaction, which was carried out between Col and F in the presence of an acidic catalyst. This step results in the substitution of the methylol or –CH2OH group (or formylation) at the ortho position, as confirmed by FTIR and UV (discussed in Sections 3.3 and 3.5).
(2) In the second step, the synthesis of ColF–M-PU from ColF was carried out through: (i) metallation by the condensation reaction of the primary –OH group of ColF, (ii) followed by urethanation through the addition polymerization reaction between the phenolic –OH of ColF–M with the –NCO group of TDI in the presence of DBTDL as the catalyst. These reactions (metallation and urethanation) forming ColF–M-PU were carried out in the same pot, that is in a “one-pot, two-step” synthesis.
In the synthesis of ColF–M-PU, TDI and ColF–M were taken in three different ratios, i.e. 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.2
1, 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, to obtain the optimum ratio. It was observed that the product, ColF–M-PU, obtained in the ratio of 0.8
1, respectively, to obtain the optimum ratio. It was observed that the product, ColF–M-PU, obtained in the ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 did not show curing, even after 72 h of drying under ambient conditions; whereas, ColF–M-PU in the ratio of 1
1 did not show curing, even after 72 h of drying under ambient conditions; whereas, ColF–M-PU in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in the formation of free-standing films within 15–24 h under ambient temperature. On the other hand, the product ColF–M-PU in the ratio of 1.2
1 resulted in the formation of free-standing films within 15–24 h under ambient temperature. On the other hand, the product ColF–M-PU in the ratio of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 tended to form a gel within the reaction vessel. Hence, we obtained ColF–M-PU in the ratio 1
1 tended to form a gel within the reaction vessel. Hence, we obtained ColF–M-PU in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the optimum ratio for the present case for the preparation of free-standing films and further characterization.
1 as the optimum ratio for the present case for the preparation of free-standing films and further characterization.
The synthesized ColF–M-PUs obtained were completely soluble in DMSO, ethyl methyl ketone and xylene, but insoluble in common organic solvents, such as water, ethanol and methanol.
The elemental analysis data of ColF-PU and ColF–M-PU are presented in Table 1, and were found to be in good agreement with the proposed stoichiometry of the ColF–M-PU. It was also revealed that there was a coordination of two water molecules in ColF–Mn-PU, which was further corroborated by FTIR and TGA analysis, which are discussed further.
| Abbreviation | Empirical formula | Calculated (observed)% | Elec. conductance S cm−1 × 10−6 | ||
|---|---|---|---|---|---|
| C | H | N | |||
| ColF–Mn-PU | [C53H80N2O8Mn]n | 68.44 (68.85) | 8.89 (7.65) | 3.01 (2.74) | 4.576 |
| ColF–Zn-PU | [C53H76N2O6Zn]n | 70.37 (72.37) | 8.69 (8.12) | 3.10 (2.96) | 6.348 |
| ColF-PU | [C32H45N2O4]n | 73.39 (75.16) | 9.05 (8.21) | 5.35 (4.15) | 1.206 |
3.2. Electrical conductivity
Electrical conductivity measurements provide a method of testing the degree of ionization of complexes via the molecular ions (generally anions present outside the coordination sphere) released by complexes in solution. The higher the degree of ionization, the higher will be its conductivity, and vice versa.35 The electrical conductivity values of ColF-PU and ColF–M-PU solutions in xylene were analyzed and are tabulated in Table 1. These reveal that the electrical conductivity slightly increases by the incorporation of a metal in ColF–M-PU, as compared to ColF-PU, with the values being in the order of magnitude of 10−6 S cm−1, suggesting a semi-conducting behaviour.363.3. FTIR spectral analysis
FTIR spectra of Col, ColF, ColF–Mn-PU and ColF–Zn-PU are shown in Fig. 1a–d.These spectra showed the following characteristic absorption peaks:
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 3010.23 cm−1 (C
C–H ν), 3010.23 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 2934.89 and 2849.92 cm−1 (asymm and symm CH2/CH3 ν), 1593 cm−1 (C
C–H ν), 2934.89 and 2849.92 cm−1 (asymm and symm CH2/CH3 ν), 1593 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, ν), 1268.42 cm−1 (phenolic C–O ν) and 778 cm−1 (C–H out of plane δ).
C, ν), 1268.42 cm−1 (phenolic C–O ν) and 778 cm−1 (C–H out of plane δ).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 3008.65 cm−1 (C
C–H ν), 3008.65 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 2933.91 and 2847.77 cm−1 (asymm and symm CH2/CH3 ν), 1583.91 cm−1 (C
C–H ν), 2933.91 and 2847.77 cm−1 (asymm and symm CH2/CH3 ν), 1583.91 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ν), 1279.37 cm−1 (phenolic C–O ν), 1097.37 cm−1 (C–O ν of CH2OH) and 778 cm−1 (C–H out of plane δ) and 743 cm−1 (ortho substitution at benzene ring).
C ν), 1279.37 cm−1 (phenolic C–O ν), 1097.37 cm−1 (C–O ν of CH2OH) and 778 cm−1 (C–H out of plane δ) and 743 cm−1 (ortho substitution at benzene ring).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–}, 3075.81 cm−1 (Ar C
O)–O–}, 3075.81 cm−1 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 3010.23 cm−1 (C
C–H ν), 3010.23 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 2911.85 and 2847.14 cm−1 (asymm and symm CH2/CH3 ν), 2258.62 cm−1 (–NCO ν), 1714.12 cm−1 (>C
C–H ν), 2911.85 and 2847.14 cm−1 (asymm and symm CH2/CH3 ν), 2258.62 cm−1 (–NCO ν), 1714.12 cm−1 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν, bonded), 1614.54 cm−1 (>C
O ν, bonded), 1614.54 cm−1 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν, free), 1561.39 cm−1 {–NH δ vibrations of –NHC(
O ν, free), 1561.39 cm−1 {–NH δ vibrations of –NHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–}, 516–557 cm−1 (Mn–O ν).38
O)–O–}, 516–557 cm−1 (Mn–O ν).38![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–}, 3073.78 cm−1 (Ar C
O)–O–}, 3073.78 cm−1 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 3009.43 cm−1 (C
C–H ν), 3009.43 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν), 2923.92 and 2849.41 cm−1 (asymm and symm CH2/CH3 ν), 2261.85 cm−1 (–NCO ν), 1716.62 cm−1 (>C
C–H ν), 2923.92 and 2849.41 cm−1 (asymm and symm CH2/CH3 ν), 2261.85 cm−1 (–NCO ν), 1716.62 cm−1 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν, bonded), 1620.10 cm−1 (>C
O ν, bonded), 1620.10 cm−1 (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν, free), 1598.94 cm−1 (–NH δ vibrations of –NHC(
O ν, free), 1598.94 cm−1 (–NH δ vibrations of –NHC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–), 520–563 cm−1 (Zn–O ν).38
O)–O–), 520–563 cm−1 (Zn–O ν).38It was observed that the spectra of ColF showed all the characteristic peaks of Col,33 along with some additional peaks at 1015–1092 cm−1 (C–O ν of CH2OH), a sharp peak at 743 cm−1 and the persistence of a peak at 1583.91 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ν). These results indicate that the formylation reaction has taken place through substitution at the ortho position and not at the double bond in the side chain of Col.37 The FTIR spectra of ColF–Mn-PU (Fig. 1c) and ColF–Zn-PU (Fig. 1d) showed almost similar results. They showed some additional peaks in comparison to ColF at 3315–3327 cm−1, 1568–1581 cm−1, 2240.41–2266.68 cm−1, 1671–1680 cm−1, 1714–1730 cm−1 and 510–566 cm−1 due to –NH ν and –NH δ vibrations of the urethane group, free –NCO groups, >C
C ν). These results indicate that the formylation reaction has taken place through substitution at the ortho position and not at the double bond in the side chain of Col.37 The FTIR spectra of ColF–Mn-PU (Fig. 1c) and ColF–Zn-PU (Fig. 1d) showed almost similar results. They showed some additional peaks in comparison to ColF at 3315–3327 cm−1, 1568–1581 cm−1, 2240.41–2266.68 cm−1, 1671–1680 cm−1, 1714–1730 cm−1 and 510–566 cm−1 due to –NH ν and –NH δ vibrations of the urethane group, free –NCO groups, >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν (free), >C
O ν (free), >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ν (bonded) and M–O ν vibrations,38 respectively. Along with these additional peaks, the spectra shows a disappearance of C–O ν peaks at 1050–1260 cm−1, which can be correlated to the metallation between –CH2OH and metal acetate followed by the urethanation reaction between the phenolic –OH and –NCO of TDI. The spectra of ColF–Mn-PU also exhibited a somewhat broad band at 3326 cm−1, as compared to ColF–Zn-PU, which overlapped with –NH ν, which indicates the presence of a coordinated crystalline water molecule,39,40 along with bands at 994 cm−1 and 852 cm−1 (rocking and wagging modes of coordinated water).41 The presence of coordinated water in the case of ColF–Mn-PU was also confirmed by TG analysis (Section 3.6. and Fig. 4b).
O ν (bonded) and M–O ν vibrations,38 respectively. Along with these additional peaks, the spectra shows a disappearance of C–O ν peaks at 1050–1260 cm−1, which can be correlated to the metallation between –CH2OH and metal acetate followed by the urethanation reaction between the phenolic –OH and –NCO of TDI. The spectra of ColF–Mn-PU also exhibited a somewhat broad band at 3326 cm−1, as compared to ColF–Zn-PU, which overlapped with –NH ν, which indicates the presence of a coordinated crystalline water molecule,39,40 along with bands at 994 cm−1 and 852 cm−1 (rocking and wagging modes of coordinated water).41 The presence of coordinated water in the case of ColF–Mn-PU was also confirmed by TG analysis (Section 3.6. and Fig. 4b).
3.4. Curing of ColF-PU and ColF–M-PU films
Films of ColF-PU, ColF–Mn-PU and ColF–Zn-PU were allowed to cure at room temperature. The free-standing films of ColF–Mn-PU and ColF–Zn-PU were obtained at room temperature in 15 h and 24 h, respectively, while ColF-PU films were obtained in 48 h. The thickness of the films was found to be between 100 and 150 μm. It was observed that ColF–M-PU films (ColF–Mn-PU > ColF–Zn-PU) cured faster than the ColF-PU film.The curing of ColF-PU is a two-step process, i.e. physical and chemical. The physical process involves the evaporation of the solvent, leading to polymer chain entanglement. The chemical process usually involves the fast curing reactions through the interaction of free –NCO and atmospheric oxygen or through slow autoxidation, resulting in cross-linking of the polymeric chains.42 In ColF–M-PU curing, the coordination involved between M(II) and the ligand along with the aforementioned process and the presence of M(II) also catalyze the autoxidation of unsaturated alkyl chains under ambient conditions. It is reported that metal ions (Co2+ and Mn2+, etc.) can be used to fasten the autoxidation process, due to the catalytic effect of the metal itself.42 The curing mechanisms of ColF-PU and ColF–M-PU were confirmed by using ATR-FTIR (Fig. 2a–c) with the cured films and compared with FTIR spectra of the uncured samples (Fig. 1). The spectrum revealed a broadening and shifting of the peak due to –NH stretching. The disappearance of free –NCO peaks in the cured samples indicated its possible involvement in the curing process.29 There was also a disappearance of the peak at around 3011 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H ν) in ColF–M-PU, indicating autoxidation of the long unsaturated alkyl chain in Col with atmospheric oxygen, further resulting in cross-linking of the polymeric chains, except in the case of ColF-PU, in which a decrease in peak intensity was observed.
C–H ν) in ColF–M-PU, indicating autoxidation of the long unsaturated alkyl chain in Col with atmospheric oxygen, further resulting in cross-linking of the polymeric chains, except in the case of ColF-PU, in which a decrease in peak intensity was observed.
The shorter curing time in ColF–Mn-PU as compared to ColF–Zn-PU can be corroborated to the difference in their electronic configuration. Mn(II) having half-filled ‘d’-orbitals (‘d5’) with unpaired electrons contributes towards its high reactivity in comparison to Zn(II) with completely-filled d-orbitals (‘d10’), thus lowering the curing time. Hence, the curing time follows the trend ColF-PU > ColF–Zn-PU > ColF–Mn-PU.
3.5. UV-visible and magnetic moment measurements
Fig. 3 shows the UV-Vis absorption spectra of Col, ColF and ColF–Mn-PU taken in xylene. The spectrum of Col (Fig. 1a) reveals two absorption bands. The first band at 226 nm might be attributed to the K-band of the aromatic ring and the absorption of double bonds in the olefinic side chain of Col. The second main absorption band at 287 nm is attributed to the B-band of the benzene ring.43 The B-band in the absorption spectra of ColF (Fig. 3b) is shifted to a longer wavelength at 299 nm, due to the bathochromic shift imparted by the –CH2OH group at the ortho position present in the ligand.44 The spectrum of ColF–Mn-PU (Fig. 3c) shows three absorption bands at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 726 cm−1, 21
726 cm−1, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1 and 33
500 cm−1 and 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 cm−1, corresponding to 4T1g (G) ← 6A1g, 4T2g (G) ← 6A1g and 4Eg (D) ← 6A1g transitions, respectively, correlated to an octahedral geometry. The magnetic moment value of ColF–Mn-PU was found to be 2.88 B.M., which indicates that the coordination PU involves a low-spin idealized t2g,5 with an octahedral geometry.45 No such d–d transitions were observed for ColF–Zn-PU due to the diamagnetic nature of Zn(II), suggesting a tetrahedral structure of the same.46
100 cm−1, corresponding to 4T1g (G) ← 6A1g, 4T2g (G) ← 6A1g and 4Eg (D) ← 6A1g transitions, respectively, correlated to an octahedral geometry. The magnetic moment value of ColF–Mn-PU was found to be 2.88 B.M., which indicates that the coordination PU involves a low-spin idealized t2g,5 with an octahedral geometry.45 No such d–d transitions were observed for ColF–Zn-PU due to the diamagnetic nature of Zn(II), suggesting a tetrahedral structure of the same.46
3.6. Thermal stability
The TGA and the derivative (DTG and DTA) curves of ColF-PU, ColF–Mn-PU and ColF–Zn-PU are illustrated in Fig. 4a–c, respectively, while the thermal data, such as initial decomposition temperature (IDT), % weight loss at different temperatures and % weight loss at 800 °C, are tabulated in Table 2. The relatively low thermal stability of PUs, depending on the diisocyanate and polyols employed decomposing below 300 °C because of the presence of labile urethane groups, have already been reported.47 In the present work, ColF-PU and ColF–M-PU proceeded through more than one thermal degradation step, which can be clearly seen in the TGA/DTG/DTA thermograms as two well defined peaks in all cases except for ColF–Mn-PU. In the TGA–DTG thermogram of ColF–Mn-PU (Fig. 4b), initial weight losses of 7% and 10% at 70 °C and 130 °C can be observed, which could be corroborated to the presence of lattice water and coordinated water, respectively. On the other hand, in the case of the TGA–DTA thermogram of ColF–Zn-PU (Fig. 4c), no such weight loss was observed in the temperature range of 50–150 °C, indicating the absence of water molecules (lattice or coordinated). The initial degradation step, which was observed at approximately 220–350 °C, corresponded to the decomposition of labile urethane linkages.29 The final degradation step in the temperature range of 420–530 °C might correspond to the scission of the hydrocarbon moiety present in the Col.29 | ||
| Fig. 4 (a) TGA–DTA thermograms of ColF-PU, (b) TGA–DTG thermograms of ColF–Mn-PU, and (c) TGA–DTA thermograms of ColF–Zn-PU. | ||
| Polymer | IDT (°C) | Temperature at weight loss (°C) | Weight loss at 800 °C (%) | ||
|---|---|---|---|---|---|
| 30% | 50% | 70% | |||
| ColF–Mn-PU | 220 | 370 | 450 | 620 | 75 |
| ColF–Zn-PU | 280 | 400 | 490 | 522 | 78 |
| ColF-PU | 282 | 370 | 440 | 500 | 92 |
Another change observed in ColF-PU and ColF–M-PU (Fig. 4 and Table 2) revealed that the IDTs of ColF–M-PU are in the range 220–280 °C, which are lower than that of ColF-PU. The IDTs of ColF–M-PU decrease in the order Zn > Mn. However, the decomposition of ColF-PU was found to be higher than ColF–M-PU, and followed the order ColF-PU > ColF–Zn-PU > ColF–Mn-PU. It is well documented in the literature that the decomposition of metal-containing polymers is less than that of virgin polymers.48 In the present case, metal enhances the decomposition in the initial stage, but then further reduces the decomposition at higher temperature. The observed trend for decomposition can be correlated to the fact that the metal acts as a catalyst facilitating the first stage decomposition but retards the decomposition in the later stages.49
3.7. DSC
The DSC thermograms of ColF-PU and ColF–Zn-PU are shown in Fig. 5a and b. They reveal two broad endotherms both for ColF-PU and ColF–Zn-PU. The Tg for ColF-PU was observed at 80 °C, while the Tg for ColF–Zn-PU was observed at 115 °C. The higher Tg in the case of ColF–Zn-PU can be correlated to the presence of metal. It was also observed that both the DTA and DSC thermograms correlate to each other in the temperature range up to 400 °C. The 2nd endotherm ranging from 280 to 340 °C might correspond to the decomposition of the urethane linkage in both ColF-PU and ColF–Zn-PU.3.8. FE-SEM
FE-SEM micrographs of ColF–Mn-PU and ColF–Zn-PU at different magnifications (Fig. 6 and 7) revealed a layered morphology along with pores in the nanometre range, i.e. 400–900 nm and 69–200 nm, respectively, distributed uniformly all over the surface. ColF–Mn-PU can be seen to be somewhat more porous than the other one in the micrograph; however, smaller pores are observed in case of ColF–Zn-PU. Hence, it is clear in both cases that ColF–M-PU agglomerated to form a nanoporous layered morphology with a smooth surface.3.9. SEM-EDX
The elemental compositions of ColF–Mn-PU and ColF–Zn-PU were determined with the aid of EDX spectroscopy, as represented in Fig. 6d and 7d, respectively. The presence of metal was confirmed by EDX analysis, along with other elements, such as carbon, oxygen and nitrogen. EDX surface scanning of ColF–Mn-PU and ColF–Zn-PU presents the atomic percentage of Mn (1.40%) and Zn (4.63%), respectively.3.10. HR-TEM
HR-TEM was performed in order to get a good insight into the structural features of the ColF–M-PU at the atomic scale. TEM images of the samples ColF–Mn-PU and ColF–Zn-PU at different resolutions are shown in Fig. 8 and 9, respectively. The images clearly show the light and dark phase corresponding to the organic (ligand) and inorganic (metal ion) moieties, respectively. A close examination of the TEM micrograph also reveals the uniform pore distribution in the nanometre range. Hence, the synthesized coordination PU is aggregated to give a nanostructured morphology with uniform repeating light and dark phases.3.11. XRD
It has been reported in the literature that TDI-based polymers are generally amorphous in nature.49 From the XRD patterns shown in Fig. 10, the synthesized ColF–Mn-PU seems to be amorphous in nature, as indicated by the appearance of a broad peak at 2θ value of 20° (Fig. 10b). However, ColF–Zn-PU shows a semi-crystalline behaviour, as indicated by the appearance of some sharp peaks, along with broad peaks (Fig. 10c). During fast curing, the cross-linking between segments of the molecules takes place in a disordered manner and, hence, leads to improper alignment of the molecules, resulting in the formation of an amorphous region.50 The difference in the XRD pattern between ColF–Mn-PU and ColF–Zn-PU can be correlated to the curing time, i.e. the time taken by the polymeric material to arrange itself. As already mentioned, the curing time of ColF–Zn-PU is somewhat greater as compared to ColF–Mn-PU, contributing towards more time for the proper alignment, which result in the semi-crystalline nature of the former.3.12. Adsorption studies
CR is one of the most important azo dyes and secondary diazo dyes. It is highly soluble in water and relentless in the environment as a complex pollutant, and hence it has long been neglected due to its toxic behaviour. The present study deals with the utilization of synthesized ColF–Mn-PU and ColF–Zn-PU as adsorbents for the removal of CR dye from an aqueous solution.The process of dye adsorption is related to the following equilibrium:
| Dye in bulk + solid adsorbent ⇄ dye adsorbed on surface of adsorbent |
The effect of dye concentration on the adsorption process can be clearly seen in Fig. 11a and b. The decrease in the % dye removal (Fig. 12a) was from 25% to 12.5% in ColF–Mn-PU and 50% to 13.75% in the case of ColF–Zn-PU, with an increase in concentration from 10 mg L−1 to 200 mg L−1, while the actual dye adsorbed (qe, mg g−1) was increased in both ColF–Mn-PU and ColF–Zn-PU. The observed trend in % dye removal can be correlated to the fact that an increase in the dye concentration over the fixed adsorption sites of CP can lead to a decrease in the % dye removal. On the other hand, the optimum adsorption capacities (Fig. 12b) of 150 mg g−1 and 165 mg g−1 for ColF–Mn-PU and ColF–Zn-PU were observed at higher dye concentrations. An increase in the dye adsorption capacity can be correlated to the forward shift in equilibrium with the increasing dye concentration of CR.51
 | ||
| Fig. 11 (a) UV-visible spectral changes of CR and ColF–Mn-PU at different dye concentrations. (b) UV-visible spectral changes of CR and ColF–Zn-PU at different dye concentrations. | ||
 | ||
| Fig. 12 (a) Effect of dye concentration on % dye removal in ColF–Mn-PU and ColF–Zn-PU. (b) Effect of dye concentration on the adsorption capacities of ColF–Mn-PU and ColF–Zn-PU. | ||
It was also observed that ColF–Zn-PU tends to show a higher % dye removal as well as adsorption capacity in comparison to ColF–Mn-PU, which can be correlated to its smaller pore sizes and, hence, greater surface area available for adsorption of the former (Section 3.8.). Thus, both the synthesized coordination polymers show optimum adsorption tendency for dye removal and can be employed for the same.
4. Conclusion
In a nutshell, bio-based nanostructured coordination PUs derived from the renewable resource CNSL/Col were successfully synthesized by an efficient, inexpensive, one-pot environment-friendly route. The curing behaviour of free-standing films of the synthesized coordination PUs was also simultaneously discussed. The effect of transition metal ions with half-filled [Mn(II)] and completely-filled [Zn(II)] d-orbitals on the geometry revealed octahedral and tetrahedral geometries, respectively. The synthesized materials tended to adopt an amorphous/crystalline behaviour and layered morphology with nanopores distributed all over the surface with improved thermal stability. The adsorption of CR dye on the developed PUs was also determined and they were proven to be efficient adsorbents for dye removal from industrial waste water. The metal coordination PUs with other metals in the 3d transition series with improved adsorption capacity and also their application in the biomedical field will be investigated and reported in future publications.Acknowledgements
Author Shabnam Khan wishes to acknowledge University Grants Commission, New Delhi, India UGC for Maulana Azad National Fellowship, Ref # F1-17.1/2014-15/MANF-2014-15-MUS-UTT-36965/(SA-III/Website). Laxmi would like to thank UGC (New Delhi, India) for Non-NET fellowships. Dr Fahmina Zafar is thankful to UGC (New Delhi, India) for Dr D S Kothari Post Doctoral Fellowship, Ref. # F.4/2006(BSR)/13-986/2013(BSR). Authors are also thankful to the Head, Department of Chemistry, Jamia Millia Islamia, for providing facilities to carry out the research work. Authors also thanks to All India Institute of Medical Sciences for HR TEM micrographs and Center Instrumentation Facilities (CIF), Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia for ATR-FTIR.References
- L. M. de Espinosa and M. A. R. Meier, Eur. Polym. J., 2011, 47, 837–852 CrossRef.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- S. Pathan and S. Ahmad, ACS Sustainable Chem. Eng., 2013, 1(10), 1246–1257 CrossRef CAS.
- U. Biermann, U. Bornscheuer, M. A. R. Meier, J. O. Metzger and H. J. Schafer, Angew. Chem., Int. Ed., 2011, 50, 3854–3871 CrossRef CAS PubMed.
- C. K. S. Pillai, Des. Monomers Polym., 2010, 13, 87–121 CrossRef CAS.
- R. Yadav, A. Devi, G. Tripathi and D. Srivastava, Eur. Polym. J., 2007, 43, 3531–3537 CrossRef CAS.
- S. Kanehashi, K. Yokoyama, R. Masuda, T. Kidesaki, K. Nagai and T. Miyakoshi, J. Appl. Polym. Sci., 2013, 130, 2468–2478 CrossRef CAS.
- M. Sultania, J. S. P. Rai and D. Srivastava, Eur. Polym. J., 2010, 46, 2019–2032 CrossRef CAS.
- Y. Liu, J. Wang and S. Xu, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 472–480 CrossRef CAS.
- S. K. Pathak and B. S. Rao, J. Appl. Polym. Sci., 2006, 102, 4741–4748 CrossRef CAS.
- K. Huang, Y. Zhang, M. Li, J. W. Lian, X. H. Yang and J. L. Xia, Prog. Org. Coat., 2012, 74, 240–247 CrossRef CAS.
- X. Li, X. Luo and Y. Gu, Phys. Chem. Chem. Phys., 2015, 17, 19255–19260 RSC.
- S. Li and S. Yan, RSC Adv., 2015, 5, 61808–61814 RSC.
- K. I. Suresh and V. S. Kishanprasad, Ind. Eng. Chem. Res., 2005, 44, 4504–4512 CrossRef CAS.
- C. V. Mythili, A. M. Retna and S. Gopalakrishnan, Bull. Mater. Sci., 2004, 27, 235–241 CrossRef CAS.
- H. P. Bhunia, G. B. Nando, T. K. Chaki, A. Basak, S. Lenka and P. L. Nayak, Eur. Polym. J., 1999, 35, 1381–1391 CrossRef CAS.
- M. Kathalewar, A. Sabnis and D. D'Melo, Prog. Org. Coat., 2014, 77, 616–626 CrossRef CAS.
- C. I. S. Raj, M. Christudhas and G. A. G. Raj, J. Chem. Pharm. Res., 2011, 3, 127–135 CAS.
- C. Voirin, S. Caillol, N. V. Sadavarte, B. V. Tawade, B. Boutevinab and P. P. Wadgaonkar, Polym. Chem., 2014, 5, 3142–3162 RSC.
- D. Balgude and A. S. Sabnis, J. Coat. Technol. Res., 2014, 11, 169–183 CrossRef CAS.
- S. I. Kattimuttathu, G. Foerst, R. Schubert and E. Bartsch, J. Surfactants Deterg., 2012, 15, 207–215 CrossRef.
- K. I. Suresh, ACS Sustainable Chem. Eng., 2013, 1, 232–242 CrossRef CAS.
- E. Darroman, N. Durand, B. Boutevin and S. Caillol, Prog. Org. Coat., 2015, 83, 47–54 CrossRef CAS.
- M. C. Lubi and E. T. Thachil, Des. Monomers Polym., 2000, 3, 123–153 CrossRef CAS.
- M. A. D. S. Rios, S. N. Santiago, A. A. L. S. Lopes and S. E. Mazzetto, Energy Fuels, 2010, 24, 3285–3291 CrossRef CAS.
- D. Mahata, S. M. Mandal and G. B. Nando, RSC Adv., 2014, 4, 48559–48562 RSC.
- C. J. Patel and V. Mannari, Prog. Org. Coat., 2014, 77, 997–1006 CrossRef CAS.
- M. Kathalewar, A. Sabnis and D. D'Mello, Eur. Polym. J., 2014, 57, 99–108 CrossRef CAS.
- C. Fu, J. Liu, H. Xia and L. Shen, Prog. Org. Coat., 2015, 83, 19–25 CrossRef CAS.
- R. Jayakumar, S. Nanjundan and M. Prabaharan, J. Macromol. Sci., Part C: Polym. Rev., 2005, 45, 231–261 CrossRef.
- S. Bhattacharya, S. Bala and R. Mondal, RSC Adv., 2016, 6, 25149–25158 RSC.
- I. Imaz, M. Rubio-Martıínez, J. An, I. Solé-Font, N. L. Rosi and D. Maspoch, Chem. Commun., 2011, 47, 7287–7302 RSC.
- F. Zafar, M. Azam, E. Sharmin, H. Zafar, Q. M. R. Haque and N. Nishat, RSC Adv., 2016, 6, 6607–6622 RSC.
- A. Devi and D. Srivastava, J. Appl. Polym. Sci., 2000, 102, 2730–2737 CrossRef.
- M. S. Refat, J. Mol. Struct., 2007, 842, 24–37 CrossRef CAS.
- A. Gallego, O. Castillo, C. J. Goómez-García, F. Zamora and S. Delgado, Inorg. Chem., 2012, 51, 718–727 CrossRef CAS PubMed.
- D. Tiwari, A. Devi and R. Chandra, Int. J. Drug Dev. Res., 2011, 3, 171–175 CAS.
- N. Nishat, R. Rasool, S. Parveen and S. A. Khan, J. Appl. Polym. Sci., 2011, 122, 2756–2764 CrossRef CAS.
- R. S. Azarudeen, M. A. R. Ahamed and A. R. Burkanudeen, J. Polym. Res., 2011, 18, 1331–1341 CrossRef CAS.
- A. Burkanudeen and M. Karunakaran, Orient. J. Chem., 2003, 19, 225–228 Search PubMed.
- J. Fuzita, K. Nakamoto and M. Kobayashi, J. Am. Chem. Soc., 1956, 78, 3963–3965 CrossRef.
- F. Zafar, H. Zafar, E. Sharmin, S. M. Ashraf and S. Ahmad, J. Inorg. Organomet. Polym., 2011, 21, 646–654 CrossRef CAS.
- W. Bai, X. Xiao, Q. Chen, Y. Xu, S. Zheng and J. Lin, Prog. Org. Coat., 2012, 75, 184–189 CrossRef CAS.
- A. Knowles and C. Burgess, Practical Absorption Spectrometers, Chapman and Hall, London, 1984 Search PubMed.
- A. Saha, P. Majumdar and S. Goswami, Dalton Trans., 2000, 1703–1708 RSC.
- S. Hasnain and N. Nishat, Spectrochim. Acta, Part A, 2012, 95, 452–457 CrossRef CAS PubMed.
- Z. S. Petrović, A. Guo, I. Javni, I. Cvetković and D. P. Hong, Polym. Int., 2008, 57, 275–281 CrossRef.
- P. Rajalingam and G. Radhakrishnan, Polymer, 1992, 10, 2214–2216 CrossRef.
- R. Jayakumar, Y.-S. Lee and S. Nanjundan, React. Funct. Polym., 2003, 55, 267–276 CrossRef CAS.
- P. Basak and B. Adhikari, Mater. Sci. Eng., C, 2012, 32, 2316–2322 CrossRef CAS.
- A. Ghosal, J. Shah, R. K. Kotnalab and S. Ahmad, J. Mater. Chem. A, 2013, 1, 12868–12878 CAS.
| This journal is © The Royal Society of Chemistry 2016 |